Abstract
Background
Antimicrobial resistance remains a worldwide health problem with serious societal and economical repercussions. Multidrug resistant and Extended-Spectrum β-Lactamase producing-Enterobacterales (ESBL-E) are pathogens of critical public health priority that urgently require the research and development of new drugs. This study aims to determine the prevalence and characterize the genes conferring resistance to β-lactams among Escherichia coli and Klebsiella pneumoniae isolated from patients with urinary tract infections (UTIs) in the West region, Cameroon.
Methods
A cross-sectional study was conducted among two healthcare facilities during a four-month period from February to May, 2023. All mid-stream urine samples were collected from UTIs patients. The Escherichia coli and K. pneumoniae strains were identified using Enterosystem 18R kit following the manufacturer’s instructions. The antimicrobial susceptibility test (AST) was performed using the Kirby-Bauer disk diffusion method. The screening of ESBL production was done using ESBL ChromAgar medium combined with the double-disk synergy test (DDT). Antimicrobial resistance genes were detected using polymerase chain methods. The data analysis was performed using Excel 2016 and IBM SPSS version 20.
Results
A total of 215 urine samples were collected and analyzed during the study period. A 31.62% (68/215) prevalence of Enterobacterales was detected with prevalence of 79.41% (54/68) and 14.70% (10/68) for Escherichia coli and Klebsiella pneumoniae respectively. The overall prevalence of ESBL-Enterobacterales was 64.70% (44/68). About 82% (36/44) of isolates were MDR and high resistance level was observed for amoxicillin + clavulanic acid and ceftazidime. The resistance genes detected were blaCTX−M, and blaTEM respectively.
Conclusion
The findings of this study highlight the high burden of MDR and ESBL-E. coli and K. pneumoniae isolates from UTIs. The study emphasizes the necessity of routine screening and monitoring of antimicrobial resistance in healthcare facilities and community settings. It is therefore crucial to implement antimicrobial stewardship programs in the country and infection prevention and control (IPC) measures in hospital settings.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-025-10483-8.
Keywords: UTIs, Escherichia coli, K. pneumoniae, ESBL, CTX-M
Introduction
Hospital and community-acquired urinary tract infections (UTIs) are amongst the most common infections caused by Enterobacterales affecting especially pediatric patients and women worldwide [1, 2]. High frequency of Enterobacteriaceae involved in urinary tract infections have been reported in several Africa countries with 89.17% in Nigeria, 39.13% in Uganda, 10.1% in Ghana and 21.2% among children in Gambia [3]. These reports shown that most species belonged to Enterobacterales including Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis were associated with numerous risk factors such as age, poor economic status, poor hygiene measure, hospitalization, catheterization, sexual activities, pregnancy and diabetes mellitus [3–5].
Emergence and escalation of extended-spectrum β-lactamase-producing E. coli and K. pneumoniae is aggravating the global concern of urinary tract infections. They are commonly associated with increased length of hospital stay, use of last-resort and often expensive drug and increase mortality [6]. Numerous studies reported high prevalence of UTI caused by ESBL-E. coli and K pneumoniae [2, 7–9].
Extended-spectrum β-lactamase (ESBL) enzymes are capable of hydrolyzing penicillin, broad-spectrum cephalosporins and monobactams leading to use carbapenems and quinolone as a last resort treatment option [10]. Its emergence is generally derived from blaCTX−M, blaSHV and blaTEM expression [10]. It is well known that blaCTX−M among Enterobacterales disseminate worldwide including in sub-Saharan Africa [6, 10]. However, there is still a paucity of data regarding the prevalence of β-lactams encoding genes among E. coli and K. pneumoniae in UTI patients in Cameroon and particularly in the Western region. This study aims at determining the prevalence, phenotypic and genotypic characteristics of ESBL-producing E. coli and K. pneumoniae isolated from urine of hospitalized and community patients at Bafoussam Regional Hospital and Dschang Regional Annex Hospital in West region, Cameroon.
Methods
Study settings and design
This cross-sectional analysis study was conducted over a four-month period from February to May 2023 in two biggest public hospitals in the Western region of Cameroon. All urine samples from patients presenting signs and symptoms of hospital-acquired infections (HA-UTI) and/or community-acquired infection (CA-UTI) were collected at the medical laboratory of the Dschang Regional Annex Hospital and Bafoussam Regional Hospital. Demographic data were recorded using a case report form and questionnaire.
Culture and identification
Clean catch mid-stream urines were cultured on Cysteine Lactose Electrolytes Deficient (CLED) agar using a calibrated loop 0.001 ml, and incubated in presence of oxygen at 37 °C for 24 h. All growing colonies were counted and leukocytes were enumerated with a Malassez cell. UTI was defined based on pyuria (≥ 104 leukocyte/mL of urine) and positive culture (≥ 105 colony-forming units) diffusion method. Only bacteria that grew at a significant rate (Kass criteria) were identified by their morphological and biochemical characters using Enterosystem 18R according to the manufacturer’s instructions.
Antimicrobial susceptibility testing (AST) and ESBL screening methods
Antimicrobial Susceptibility Testing (AST) was performed using Kirby-Bauer disc diffusion method on muller Hinton agar according to the Antibiogram Committee of the French Society of Microbiology (CA-SFM) guidelines. A panel of 12 antibiotics belonging to six different families were tested including: amoxicillin + clavulanic acid (AMC; 10 μg), cefoxitin (CFX; 30 μg), ceftazidime (CAZ; 30 μg), ceftriaxone (CRO; 30 μg), cefotaxime (CTX; 30 μg), imipenem (IMP; 30 μg), aztreonam (AZT; 30 μg), gentamicin (CN; 30 μg), amikacin (AN; 30 μg), ciprofloxacin (CIP; 5 μg), fosfomycin (FOS; 30 μg), nitrofurantoin (NIT; 30 μg). The production of ESBL was detected through culture on medium namely CHROMagar™ ESBL (CHROMagar, Paris - France). The samples were then stored at -20 °C in cryotubes containing trypticase soya broth supplemented with 20% glycerol for future use. Escherichia coli ATCC25922 and K. pneumoniae ATCC700603 were used to assess the quality of the media and antibiotic discs. The inhibition zone diameters were measured and interpreted according to the criteria defined by CA-SFM 2022. A participant was considered positive to ESBL-E when at least one ESBL colony was detected. The isolates being resistant to at least one antibiotic of three or more families of antibiotics tested were considered as multi-drug resistant bacteria (MDR).
Conventional and multiplex polymerase chain reaction (PCR)
Genomic DNA was extracted from all ESBL-E. coli and K. pneumoniae isolates by boiling method as previously described [11]. Detection of blaCTX−M,blaTEM and blaOXA−48 genes was carried out by multiplex-PCR method using a BIO-RAD thermal cycler (Bio-Rad Laboratories, Mames-la coquette, France). The reaction was carried out in a 10 μL reaction mixture consisting of 5 μL of Dream Taq green Polymerase Master Mix 2x (New England Biolabs, Ipswich, MA, USA); 2.6 μL of nuclease-free water, 0.1 μL of each forward and reverse primers (10 μM) and 2 μL of DNA. Thermal cycler program included initial denaturation (95 °C for 3 min), 30 cycles of denaturation at 95 °C for 4s, annealing at 46.9 °C for 40s, elongation at 72 °C for 50s and final elongation at 72 °C for 5 min. In addition, amplification of blaSHV gene occurs in a 10 μL reaction mixture consisting of 5 μL Dream Taq Green Polymerase Master Mix 2x (ThermoFisher Scientic, Vilnius, Lithuania), 2.8 μL nuclease-free water, 0.1 μL each primer direct and reverse [10 μM] and 2 μl of DNA with approximately the same condition.
Agarose electrophoresis and gel visualization
PCR products were subjected to electrophoresis analysis performed on an agarose gel of 1.5% (w/v) that was run at 90 V for 45 min along with a 100 bp molecular ladder (New England Biolabs, MA, USA). After electrophoresis, the gel was stained in an ethidium bromide solution (0.5 μg/mL) for 15 min and briefly unstained with water. PCR products were then visualized under UV light using a gel documentation system G-BOX Chemi-XL (Syngene, Cambridge, UK). An internal quality control for bla genes was assessed using a K. pneumoniae ATCC700603 and internal strains previously sequenced (Unpublished result).
Statistical analysis
Data analysis was performed using Excel 2016 and IBM SPSS Statistics 20. Proportions were compared using the Fischer exact test and chi-square test as appropriate. A p-value < 0.05 was considered statistically significant.
Results
Demographic characteristics
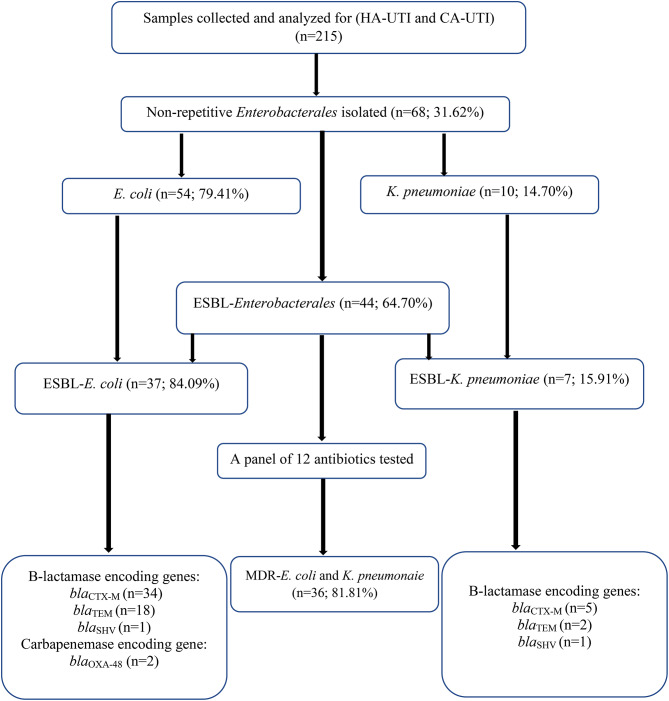
A total of 215 urine samples were collected from Dschang Regional Annex Hospital (n = 107) and Bafoussam Regional Hospital laboratories (n = 108), with 68 (31.62%) of these being positives with at least one Enterobacterales (Fig. 1). Women were more represented than men (65.11%, 140/215 vs. 34.88%, 75/215). The age of participants varies between 1 year and 49 years with a median age of 25 years. Patients aged between 20 and 29 years constitute the most common age group (30.7%) of the population infected by Enterobacterales.
Fig. 1.
Flowchart of participant isolates. MDR-E. coli and K. pneumoniae = Multidrug Resistance E. coli and K. pneumoniae, ESBL-E = Extended -Spectrum β-lactamase producing-Enterobacterales, n = number
Prevalence of ESBL-producing E. coli and K. pneumoniae
Out of the 68 Enterobacterales isolated, the most represented species were E. coli 54 (79.41%) and K. pneumoniae 10 (14.70%). The overall prevalence of ESBL- producing Enterobacterales was 44 (64.70%) with ESBL-E. coli 37 (84.09%) and ESBL-K. pneumoniae 7 (15.91%) (Table 1).
Table 1.
Distribution of Enterobacterales isolated from urinary tract infections according to the socio-demographic characteristics
| Variables | Isolates n (%) | Total | |||
|---|---|---|---|---|---|
| E. coli | K. pneumoniae | P. mirabilis | C. freundii | ||
| Sex | |||||
| Female | 44 (81.48) | 8 (80.0) | 2 (66,66) | 1 (100) | 140 (65.11) |
| Male | 10 (18.51) | 2 (20.0) | 1 (33,33) | 0 (0) | 75 (34.88) |
| MDR | |||||
| MDR Positive | 42 (84) | 8 (16) | 0(0) | 0(0) | 50 |
| MDR Negative | 13 (72.22) | 2 (11.11) | 2 (11.11) | 1 (5.56) | 18 |
| ESBL | |||||
| ESBL Positive | 37 (84.09) | 7 (15.91) | 0(0) | 0(0) | 44 (100) |
| ESBL Negative | 18 (75) | 3 (12.50) | 2 (8.33%) | 1 (4.17) | 20 (100) |
| Resistance genes | |||||
| bla CTX−M | 35 (94.89) | 7 (71.42) | 0(0) | 0(0) | 42 (95.5) |
| bla TEM | 18 (48.64) | 2 (28.57) | 0(0) | 0(0) | 24 (54.5) |
| bla SHV | 1 (2.70) | 1 (14.28) | 0(0) | 0(0) | 2 (4.5) |
| bla OXA−48 | 2 (5.40) | 0(0) | 0(0) | 0(0) | 2 (4.5) |
Antibiotic resistance profile of ESBL-E. coli and K. pneumoniae
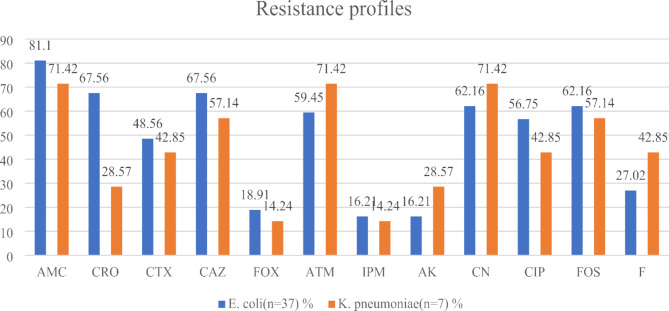
All ESBL-E. coli isolates tested displayed a high level of resistance to amoxicillin-clavulanic acid (30/37; 81.1%), ceftriaxone (25/37; 67.56%), ceftazidime (25/37; 67.56%), gentamicin (23/37; 62.16%), fosfomycin (23/37; 62.16%) and ciprofloxacin (21/37; 56.75%). In addition, ESBL-K. pneumoniae have shown a high-level resistance to amoxicillin clavulanic acid (5/7; 71.42%), aztreonam (5/7; 71.42%) and gentamicin (5/7; 71.42%) (Fig. 2). However, ESBL-E. coli and ESBL-K. pneumoniae displayed high susceptibility for both imipenem and amikacin.
Fig. 2.
Resistance proportions of ESBL-E. coli and K. pneumoniae. AMC: Amoxicillin + Clavulanic acid, FOX: Cefoxitin, CAZ: Ceftazidime, CRO: Ceftriaxone, CTX: Cefotaxime, IPM: Imipenem, ATM: Aztreonam, CN: Gentamicin, AK: Amikacin, CIP: Ciprofloxacin, FOS: Fosfomycin, F: Nitrofurantoin
Multidrug resistance of ESBL-producing E. coli and K. pneumoniae
Majority of ESBL-producing Enterobacterales were multi-drug resistant (42/44; 95.45%) with ESBL-E. coli as 85.71% (n = 36/42) and ESBL-K. pneumoniae as 14.26% (n = 6/42) isolates. The most prevalent phenotypic profile was AMC-CRO-CTX-CAZ-F-ATM-AK-FOS-CIP-CN (3/42; 7,14%) including 10 antibiotics from 5 different family (Table 2).
Table 2.
Resistant patterns of ESBL-E. coli and K. pneumoniae
| Phenotypic profiles | Number of antibiotics | Number of antibiotic families | Frequency of isolates (%) |
|---|---|---|---|
| AMC-CRO-CTX-CAZ | 4 | 1 | 2(5.55) |
| AMC-ATM-FOS | 3 | 2(5.55) | |
| AMC-CN | 2 | 3 | 1(2.77) |
| AMC-CRO-CTX-CAZ-FOS | 5 | 1(2.77) | |
| AMC-CRO-CTX-FOX-AK-FOS-CN-IPM | 8 | 2(5.55) | |
| AMC-CRO-CTX-CAZ-F-ATM-CIP | 7 | 2(5.55) | |
| AMC-CRO-AK-FOS | 4 | 3 | 1(2.77) |
| AMC-CRO-CAZ-FOX-F-AK-CN | 7 | 1(2.77) | |
| AMC-CRO-CTX-CAZ-FOX-F-AK | 7 | 1(2.77) | |
| AMC-CRO-CAZ-F-FOS-CIP | 6 | 1(2.77) | |
| AMC-CRO-CTX-CAZ-FOS-CIP-CN | 7 | 2(5.55) | |
| AMC-CRO-FOX-F-FOS-CN | 6 | 2(5.55) | |
| AMC-CRO-CTX-FOX-F-FOS-CIP-PM | 8 | 1(2.77) | |
| AMC-CRO-CAZ-FOX-F-ATM-AK-FOS | 8 | 2(5.55) | |
| AMC-CRO-CAZ-ATM-AK-FOS-CIP-CN | 8 | 4 | 2(5.55) |
| AMC-CAZ-F-AK-FOS-CN | 6 | 1(2.77) | |
| AMC-CRO-CTX-CAZ-ATM-FOS-CIP-CN | 8 | 1(2.77) | |
| AMC-CRO-ATM-AK-FOS-CIP | 6 | 1(2.77) | |
| AMC-CRO-CTX-CAZ-F-ATM-AK-FOS-IPM | 9 | 2(5.55) | |
| AMC-CRO-ATM-AK-FOS-CIP-CN | 7 | 1(2.77) | |
| AMC-CRO-CTX-CAZ-F-ATM-AK-FOS-CIP-CN | 10 | 3(8.33) | |
| AMC-CRO-CTX-F-AK-FOS-CIP-CN | 8 | 2(5.55) | |
| AMC-CRO-CAZ-F-ATM-AK-FOS-CN | 8 | 2(5.55) | |
| AMC-F-ATM-AK-FOS-CIP-CN | 7 | 1(2.77) | |
| AMC-CAZ-FOX-F-AK-FOS-CIP | 7 | 5 | 1(2.77) |
| AMC-CRO-CAZ-FOX-F-FOS-CIP-CN | 8 | 2(5.55) | |
| AMC-CRO-CTX-F-ATM-AK-FOS-CIP-CN | 9 | 1(2.77) | |
| AMC-CRO-CTX-CAZ-F-ATM-FOS-CIP-CN | 9 | 1(2.77) |
Characterization of resistance determinants
The overall prevalence of genes was blaCTX−M (42/44; 95.5%), blaTEM (24/44; 54.5%), blaSHV (2/44; 4.5%) and blaOXA48 (2/44; 4.5%). A high prevalence of blaCTX−M was observed among ESBL-E. coli (34/37; 91.89%) and ESBL-K. pneumoniae (5/7; 71.42%). Also, a high prevalence of blaTEM was observed among ESBL-E. coli compare to ESBL-K. pneumoniae with 48.64% (18/37) and 28.57% (2/7) respectively.
Discussion
Urinary tract infections caused by ESBL-producing E. coli and K. pneumoniae have been widely reported worldwide and represent a critical public health challenge. The alarming resistance observed in sub-Saharan Africa by Murray et al. (2022) urge the importance of surveillance and monitoring efforts for better prevention and containment measures as well as evidence-based decision [12]. This study aimed to determine the prevalence and characterize phenotypically and genotypically the ESBL-E. coli and ESBL-K. pneumoniae in the West region of Cameroon.
The findings showed that E. coli and K. pneumoniae were the major Enterobacterales species involved in urinary tract infections in the West region of Cameroon. The overall prevalence of Enterobacterales species was 31.62%. This result is higher than those obtained in Madagascar (12,9%), Gambia (12.8%) and Ghana (10%) [3, 13, 14]. However, it is lower than those reported in Cameroon (59.6%) by Nzalie et al. (2016), where the authors investigated UTIs among the two biggest hospital settings in Yaoundé and shown that E. coli (50,9%) and K. pneumoniae (16.4%) were the most important bacteria involved in UTI [15, 16]. This result showed that UTI prevalence’s depend on the geographical location in Africa. The result is also lower than that reported in Tanzania (41%) in 2022 [15].
Our findings revealed that E. coli and K. pneumoniae were the most common Enterobacterales species implicated in UTI which affected predominantly women. This could be explained by the anatomy of urinary tract of women which is generally ascending and colonize by Enterobacterales especially E. coli coming from intestinal tract origin. This is in agreement with numerous studies conducted in Africa where the proportion of E. coli and K. pneumoniae isolated in Tanzania, Nigeria and Republic of Djibouti were 40% and 28%, 31.7% and 17.5%, and 82.39% and 9.86% respectively among hospital-and community-acquired urinary tract infections [17].
The high prevalence of ESBL-E. coli in our study is alarming but similar to those obtained in India in 2023 (82.5%) [6]. However, this finding is higher than the results obtained in Iraq, Iran, Republic of Djibouti, Algerie, Tunisie, Northern Ethiopia and Morocco where the prevalences were 71.7%, 52%, 41%, 37.1%, 30.8%, 27.8% and 12.2% respectively [1, 18–20]. In addition, the prevalence of ESBL-K. pneumoniae is lower than those reported in India (74.3%) and Ethiopia (33.8%) [20] but higher than that obtained in the Republic of Djibouti (7%) [17, 18]. The results observed among ESBL-E. coli could be explained by the fact that these countries have implemented the antimicrobial stewardship program.
In this study, ESBL-E. coli were more resistant to amoxicillin + clavulanic acid (81.1%), ceftazidime (67.56%), gentamicin (62,16%), fosfomycin (62.16%) and aztreonam (59.45%). In addition, ESBL-Kp were more resistant to amoxicillin + clavulanic acid (71.42%), aztreonam (71.42%), gentamicin (71.42%), ceftazidime (57.14%) and fosfomycin (57.14%). A moderate susceptibility to quinolone and β-lactams families have been observed, despite the broadly prescriptions of theses antibiotics commonly recommended by the physicians to treat UTIs caused by ESBL-Ec and ESBL-Kp in our context, as described [3, 15, 21]. Despite the high resistance levels observed, these isolates were relatively susceptible to nitrofurantoin, imipenem and amikacin. These findings are consistent with a report revealed that ESBL-Ec and ESBL-Kp are more susceptible to nitrofurantoin, imipenem and amikacin [15]. Our findings corroborate with a Cameroonian study carried out in Garoua and where ESBL-Enterobacterales isolates in UTIs were susceptible to amikacin and imipenem [19]. This finding could be explained by the fact that imipenem is not routinely used in the treatment of UTIs in our context, suggesting that it could be the best alternative therapeutic option. This could further be explained by the non-existence of antimicrobial stewardship program in the country, self-medication and over-the-counter supply of antibiotics, over-reliance on antibiotics from physicians, sub-optimal diagnostic and antimicrobial susceptibility testing prior prescription that all contribute to the selective pressure on the microbiome and increasing antimicrobial resistance [22]. The lack of infection prevention and control measures and programs contribute to the fluidity of resistant bacteria between patients in hospitals and communities. The overall prevalence of MDR-E coli and MDR-K pneumoniae was observed which agreed with the results already reported [23]. This high level of MDR-E coli and MDR-K pneumoniae reported could be explained by the excessive and inappropriate use of antibiotics in West region in Cameroon, where antibiotics are easily accessible over the counter without a prescription.
Among the different ESBLs genes tested, blaCTX−M and blaTEM genes were the most frequently detected. This is an agreement with previous studies which showed that blaCTX−M was the most common ESBLs gene carried among Enterobacterales responsible for UTIs [17]. These findings disagree with the results of Zemtsa et al. (2022) where the high prevalence of blaTEM and blaCTX−M among HIV patients in Yaoundé in 2022 was 72% and 48%, respectively [24]. This result can be explained by the fact that in our context, the prescription of third generation cephalosporins is commonly observed over the counter, and this behavior led to increase the resistance to β-lactams and consequently increase the widespread of blaCTX−M encoding for its resistance.
Conclusion
This study shows the high prevalence of MDR-E. coli and K. pneumoniae implicated in urinary tract infections, intimating the need for rational use of antibiotics to treat UTIs. It revealed that imipenem and amikacin remain antibiotics with good activities against ESBL-E. coli and K. pneumoniae. This study highlights the routine screening of ESBL-E. coli and K. pneumoniae and antimicrobial stewardship program implementation across the country. It emphasizes the urgent need for a national action plan at the regional and local levels to fight against AMR.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all staff members of two healthcare structures and all participants who agreed to participate to this study.
Abbreviations
- CAUTI
Community Acquired Urinary Tract Infections
- ESBL-E
Extended Spectrum β-lactamase producing-Enterobacterales
- HAUTI
Hospital Acquired Urinary Tract Infections
- MDR
Multidrug resistance
- UTI
Urinary Tract Infection
Author contributions
Conceptualization: SB, RCF and NM; Methodology: RCF, LLF; Software: SB; Validation: RCF, LLF, FTT and MN; Formal analysis: SB, LLF, OAN and RCF; Investigation: SB, BDD; Resources: RCF, LLF and NM; Writing original draft: SB, OAN, LLF and RCF; Review and editing: RCF, FTT and LLF; Visualization: SB, RCF; Supervision: RCF, LL, FTT and MN; Project administration: RCF and LLF. All authors have reviewed and agreed to the published version of the manuscript.
Funding
Dr Raspail Carrel Founou received funding from the Mérieux Institute, Lyon France for the CAREFOOD project. CAREFOOD project had supported all the molecular aspects of this study. Dr Luria Founou received funding from the Thrasher Research Fund through the Thrasher Early Career Award (Award number 01364). This work was also supported by the Research Institute of Centre of Expertise and Biological Diagnostic of Cameroon (CEDBCAM-RI). The funders had no role in the study design, nor the decision to submit the work for publication. Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the organizations or agencies that provided support for the project.
Data availability
The data are available upon request in accordance with confidentiality and privacy regulations from the corresponding author.
Declarations
Ethics approval and consent to participate
This research was approved by the Regional Ethics Committee for Research in Human Health, West, Cameroon N°393/31/05/2023/CE/CRERSH-OU/VP. In addition, it was approved by the Research institute of the Centre of Expertise and Biological Diagnostic of Cameroon (CEDBCAM-RI) under the number (N° 002/02/22/LA/CEDBCAM-RI/DG). Written informed consent to participate in this study was provided by the participants or the legal guardian/nearest relative for minor. The study was conducted in accordance with the declaration of Helsinki. In addition, the research authorizations of the various healthcare structures have been granted. All methods and protocols used were approved by the CEDBCAM-RI in accordance with the relevant national and international guidelines and regulations for research laboratory ethics.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Afsharikhah S, Ghanbarpour R, Mohseni P, Adib N, Bagheri M, Jajarmi M. High prevalence of β-lactam and fluoroquinolone resistance in various phylotypes of Escherichia coli isolates from urinary tract infections in Jiroft city, Iran. BMC Microbiol. 2023;23(1):114. 10.1186/s12866-023-02860-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohapatra S, Ghosh D, Vivekanandan P, Chunchanur S, Venugopal S, Tak V, et al. Genome profiling of uropathogenic E. Coli from strictly defined community-acquired UTI in paediatric patients: a multicentric study. Antimicrob Resist Infect Control. 2023;12(1):36. 10.1186/s13756-023-01233-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kebbeh A, Dsane-Aidoo P, Sanyang K, Darboe SMK, Fofana N, Ameme D, Anto F. Antibiotics susceptibility patterns of uropathogenic bacteria: a cross-sectional analytic study at Kanifing General Hospital, the Gambia. BMC Infect Dis. 2023;23(1):723. 10.1186/s12879-023-08373-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaboré B, Ouédraogo GA, Cissé H, Ouédraogo HS, Sampo E, Zongo KJ, et al. (GTG)(5)-PCR fingerprinting of multi-drug resistant Escherichia coli bacteria isolates from hospital in Ouagadougou, Burkina Faso. BMC Microbiol. 2022;22(1):118. 10.1186/s12866-022-02537-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kebede A, Aragie S, Shimelis T. The common enteric bacterial pathogens and their antimicrobial susceptibility pattern among HIV-infected individuals attending the antiretroviral therapy clinic of Hawassa university hospital, southern Ethiopia. Antimicrob Resist Infect Control. 2017;6:128. 10.1186/s13756-017-0288-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma S, Kalyan RK, Gupta P, Khan MD, Venkatesh V. Molecular characterization of extended spectrum β-Lactamase producing Escherichia coli and Klebsiella pneumoniae isolates and their antibiotic resistance profile in health care-associated urinary tract infections in North India. J Lab Physicians. 2023;15(2):194–201. 10.1055/s-0042-1757416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mouanga NY, Onanga R, Kassa RF, Bignoumba M, Mbehang NPP, Gafou A, et al. Epidemiology of community origin Escherichia coli and Klebsiella pneumoniae uropathogenic strains resistant to antibiotics in Franceville, Gabon. Infect Drug Resist. 2021;14:585–94. 10.2147/idr.S296054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seid L, Stokes W, Bayih AG, Getie S, Abere A, Tesfa H, Pillai DR. Molecular detection of enteropathogens from diarrheic stool of HIV positive patients in Gondar, Ethiopia. BMC Infect Dis. 2018;18(1):354. 10.1186/s12879-018-3265-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teferi S, Sahlemariam Z, Mekonnen M, Tamrat R, Bekana T, Adisu Y, Darge T. Uropathogenic bacterial profile and antibiotic susceptibility pattern of isolates among gynecological cases admitted to Jimma Medical Center, South West Ethiopia. Sci Rep. 2023;13(1):7078. 10.1038/s41598-023-34048-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernando MM, Luke WA, Miththinda JK, Wickramasinghe RD, Sebastiampillai BS, Gunathilake, et al. Extended spectrum beta lactamase producing organisms causing urinary tract infections in Sri Lanka and their antibiotic susceptibility pattern -A hospital based cross sectional study. BMC Infect Dis. 2017;17(1):138. 10.1186/s12879-017-2250-y [DOI] [PMC free article] [PubMed]
- 11.Dimani BD, Founou RC, Zemtsa JR, Mbossi A, Koudoum PL, Founou LL, et al. Faecal carriage of multidrug-resistant and extended-spectrum β-lactamase-producing enterobacterales in people living with HIV in Yaoundé. Cameroon J Glob Antimicrob Resist. 2023;35:26–34. 10.1016/j.jgar.2023.07.021 [DOI] [PubMed] [Google Scholar]
- 12.Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donkor ES, Horlortu PZ, Dayie NT, Obeng-Nkrumah N, Labi AK. Community acquired urinary tract infections among adults in Accra, Ghana. Infect Drug Resist. 2019;12:2059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rakotondrasoa A, Andrianonimiadana LM, Rahajandraibe S, Razafimahatratra S, Andrianaivoarimanana V, Rahelinirina S, et al. Characterization of Klebsiella pneumoniae isolated from patients suspected of pulmonary or bubonic plague during the Madagascar epidemic in 2017. Sci Rep. 2022;12(1):6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mlugu EM, Mohamedi JA, Sangeda RZ, Mwambete KD. Prevalence of urinary tract infection and antimicrobial resistance patterns of uropathogens with biofilm forming capacity among outpatients in morogoro, Tanzania: a cross-sectional study. BMC Infect Dis. 2023;23(1):660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nzalie RN, Gonsu HK, Koulla-Shiro S. Bacterial etiology and antibiotic resistance profile of community-acquired urinary tract infections in a Cameroonian City. Int J Microbiol. 2016;2016:3240268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed HS, Houmed Aboubaker M, Dumont Y, Didelot MN, Michon AL, Galal L et al. Multidrug-resistant enterobacterales in community-acquired urinary tract infections in Djibouti, Republic of Djibouti. Antibiotics. 2022;11(12). [DOI] [PMC free article] [PubMed]
- 18.Abubaker KT, Anwar KA. Antimicrobial susceptibility and integrons detection among extended-spectrum β-lactamase producing enterobacteriaceae isolates in patients with urinary tract infection. PeerJ. 2023;11:e15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djim-Adjim-Ngana K, Mbiakop BW, Oumar LA, Munshili Njifon HL, Tchinda Fossi C, Enyegue ELE, et al. Phenotypic characterization and epidemiology of extended-spectrum β-lactamase-producing enterobacteriaceae strains from urinary tract infections in Garoua, Cameroon. Front Public Health. 2023;11:1187934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gebremariam G, Legese H, Woldu Y, Araya T, Hagos K, GebreyesusWasihun A. Bacteriological profile, risk factors and antimicrobial susceptibility patterns of symptomatic urinary tract infection among students of Mekelle University, northern Ethiopia. BMC Infect Dis. 2019;19(1):950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khatoon I, Khanam S, Azam A, Qadeer S, Naz S, Hassan NU. Incidence pattern, antibiotic susceptibility pattern and associated risk factors of bacterial uropathogens among general population of Pakistan. Infect Drug Resist. 2023;16:4995–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nkengkana OA, Founou RC, Founou LL, Dimani BD, Koudoum PL, Zemtsa JR, et al. Phenotypic and genotypic characterization of multidrug resistant and extended-spectrum β-lactamase-producing enterobacterales isolated from clinical samples in the western region in Cameroon. BMC Infect Dis. 2023;23(1):819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seid M, Markos M, Aklilu A, Manilal A, Zakir A, Kebede T, et al. Community-acquired urinary tract infection among sexually active women: risk factors, bacterial profile and their antimicrobial susceptibility patterns, Arba Minch, Southern Ethiopia. Infect Drug Resist. 2023;16:2297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zemtsa RJ, Noubom M, Founou LL, Dimani BD, Koudoum PL, Mbossi AD et al. Multidrug-resistant and extended-spectrum β-Lactamase (ESBL) - producing enterobacterales isolated from carriage samples among HIV infected women in Yaoundé, Cameroon. Pathogens. 2022;11(5). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available upon request in accordance with confidentiality and privacy regulations from the corresponding author.