Abstract
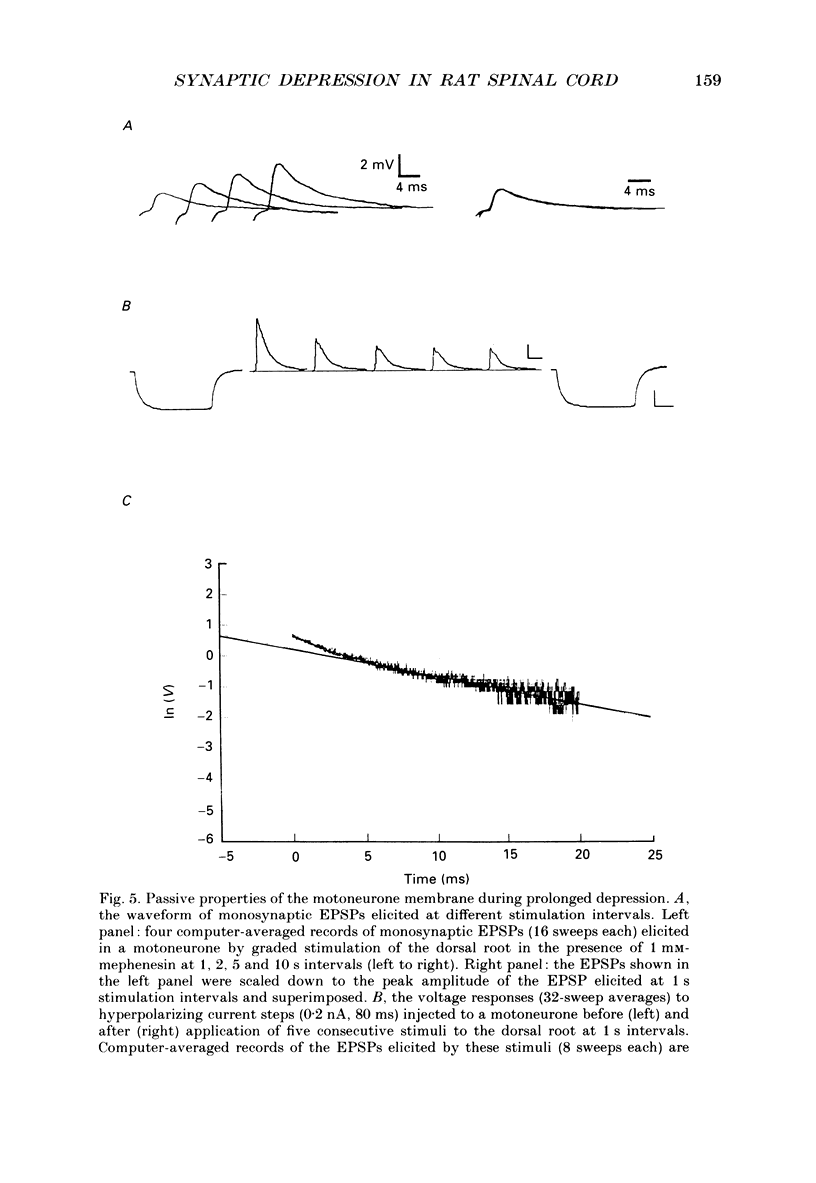
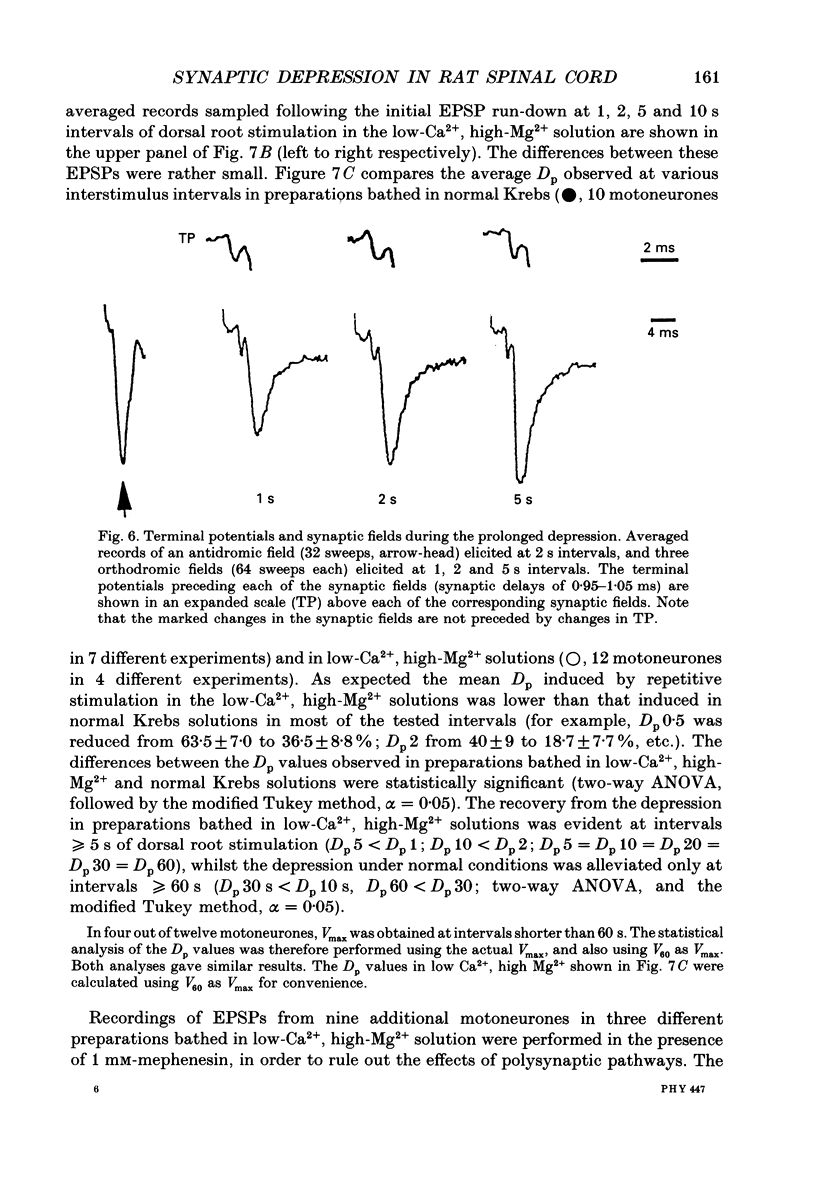
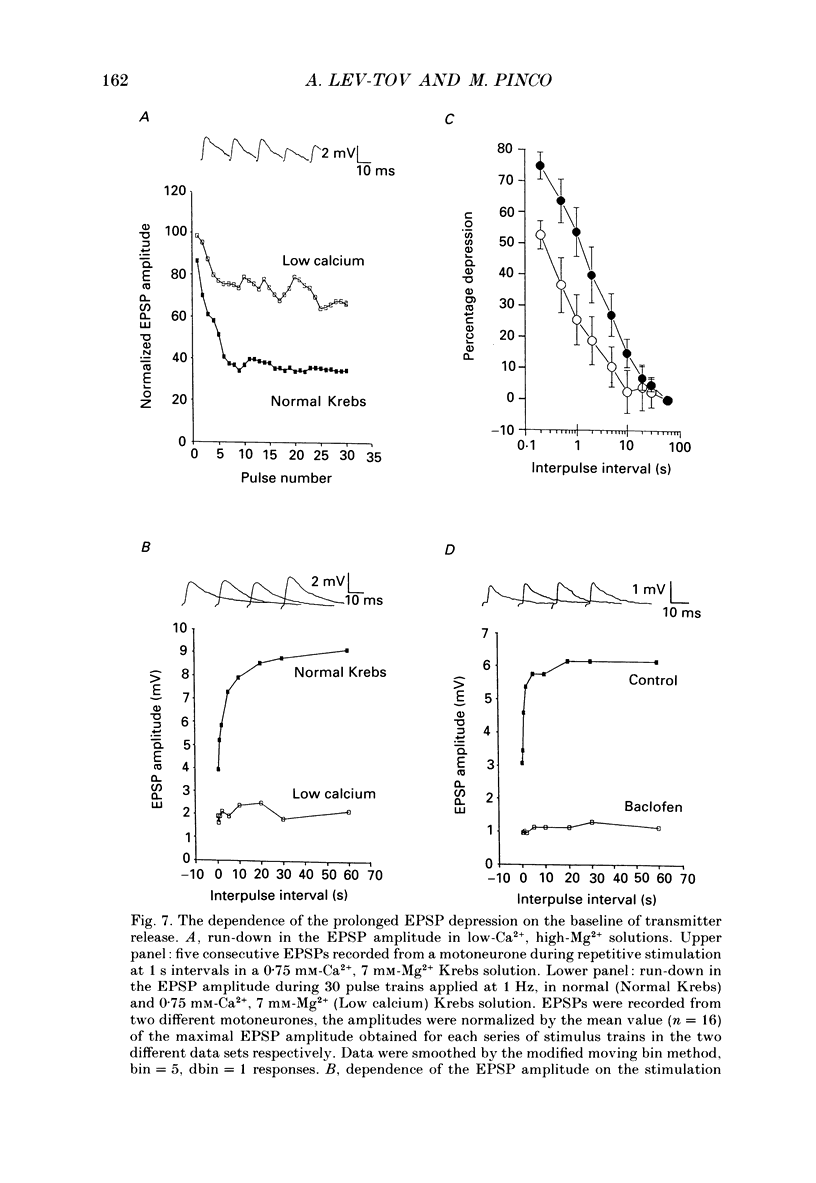
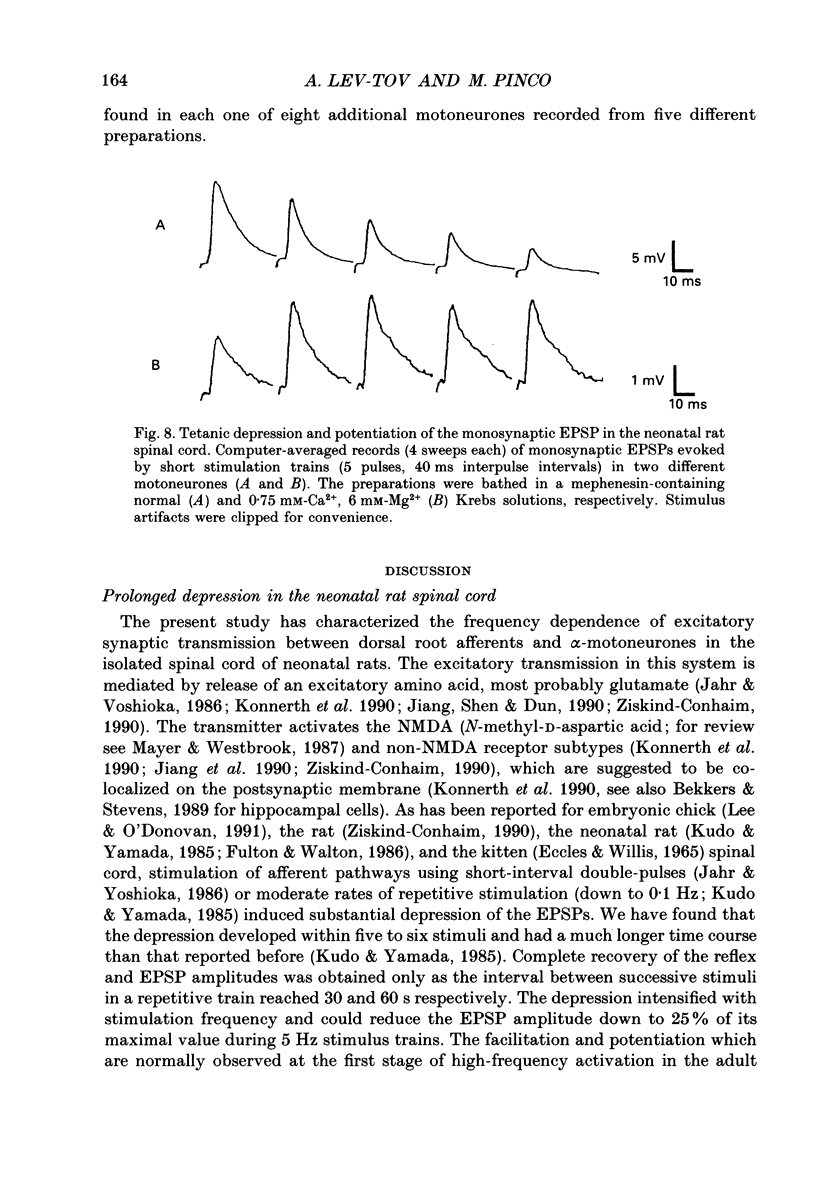
1. Synaptic transmission between dorsal root afferents and alpha-motoneurones was studied in the in vitro hemisected spinal cord preparation isolated from neonatal rats. 2. Repetitive stimulation of the dorsal roots depressed the monosynaptic reflex recorded from the homologous ventral roots. The depression developed within the first five to six pulses in a stimulus train and stabilized at a plateau-like level for many seconds of stimulation. 3. The magnitude of the reflex depression depended on the stimulation interval and was capable of reducing the reflex to 17% of its undepressed control during 5 Hz stimulus trains. Complete recovery from depression was obtained at stimulation intervals greater than or equal to 30 s. 4. Monosynaptic excitatory postsynaptic potentials (EPSPs) were recorded intracellularly after reduction of the activity in polysynaptic pathways by addition of mephenesin to the bathing media. These EPSPs exhibited a prolonged, frequency-dependent synaptic depression. The depression reduced the amplitude of the EPSP to 25% of the undepressed control during 5 Hz stimulus trains, and was alleviated completely at stimulus interval greater than or equal to 60 s. 5. The prolonged EPSP depression was not altered by blockade of glycinergic and type-A gamma-aminobutyric acid (GABAA-ergic) receptors underlying postsynaptic inhibition in the spinal cord. Injection of current steps to motoneurones before and during the prolonged depression revealed similar values of the membrane time constant and input resistance. These excluded changes in the passive properties of the motoneurone membrane as an explanation for the observed synaptic depression. 6. Extracellular recordings of terminal potentials and their accompanying synaptic fields from motor nuclei in the ventrolateral cord revealed that the frequency-dependent depression in the synaptic fields was not preceded by any detectable changes in the amplitude or the shape of the terminal potential, suggesting that the depression cannot be attributed to impairment of action potential invasion to the afferent terminals. 7. Reduction of the basic level of transmitter release in the spinal cord by increasing the Mg2+/Ca2+ ratio of the bathing solution or by application of 2 microM of L(-)baclofen markedly diminished the synaptic potential depression at all the stimulation intervals tested in this study. Recovery from depression was evident for stimulation intervals greater than or equal to 5 s. Under these conditions, short tetanic trains (5 pulses at 25 Hz) revealed a substantial facilitation and potentiation of the EPSPs. 8. We suggest that prolonged depression of synaptic potentials in the neonatal rat reflects decreased transmitter output from the activated afferent terminals.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

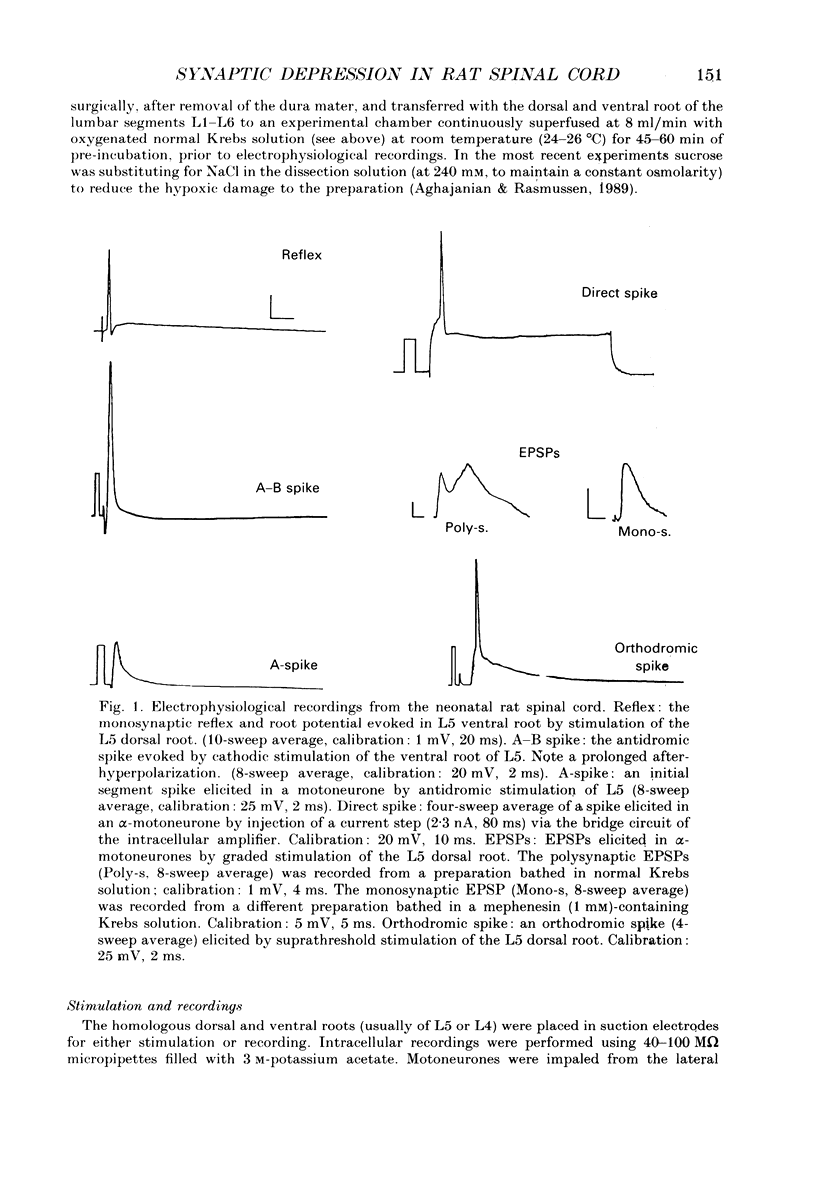


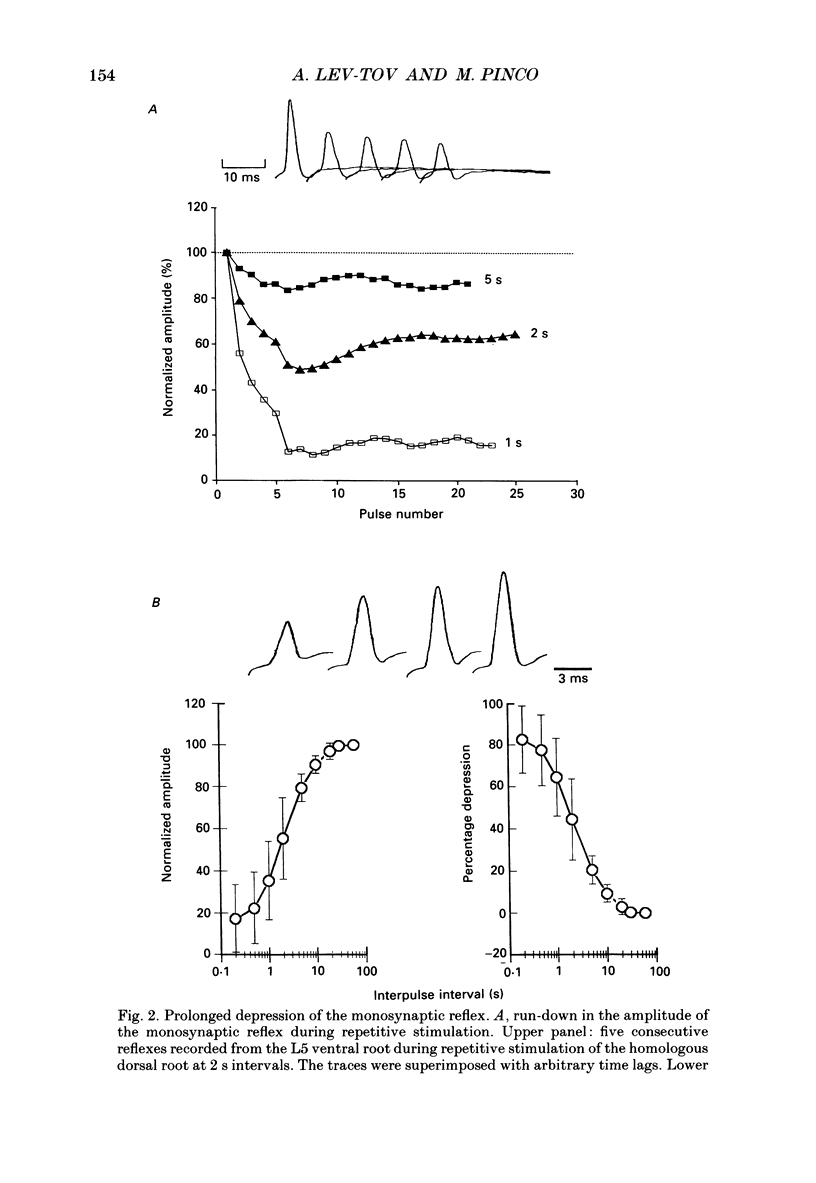

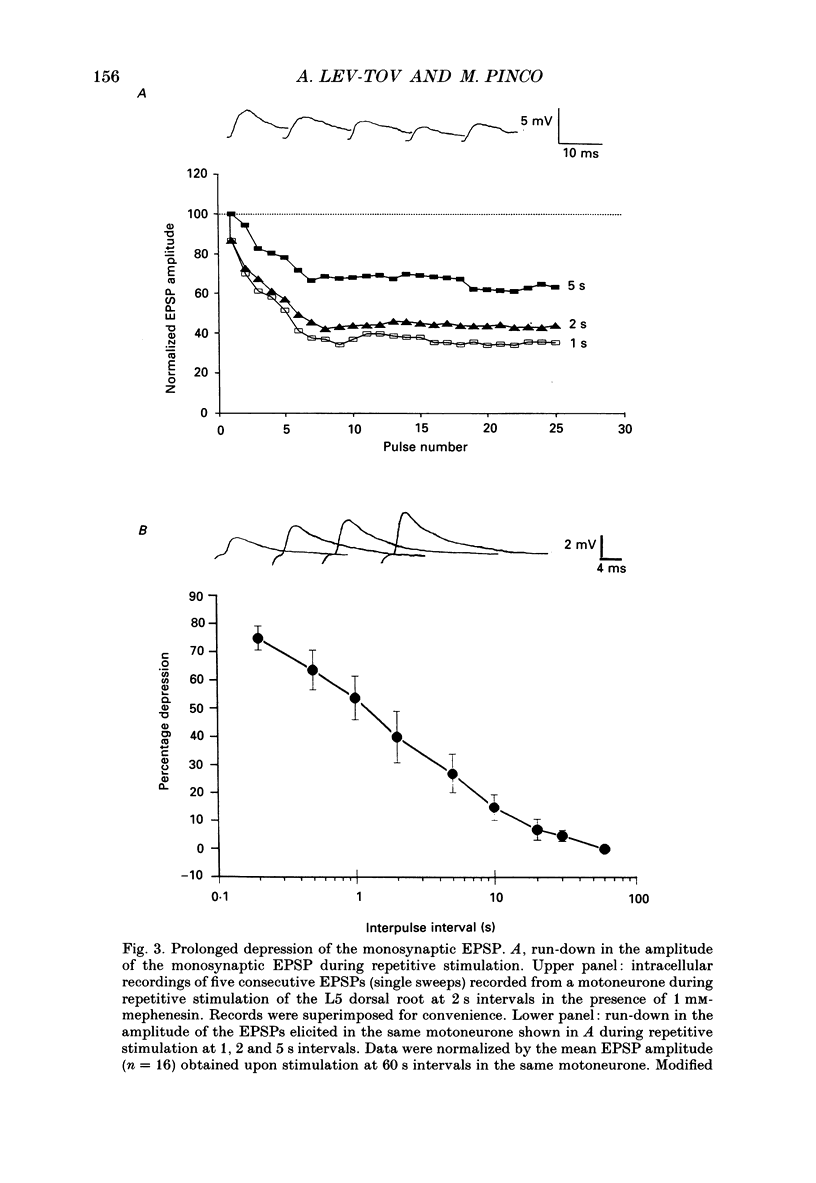

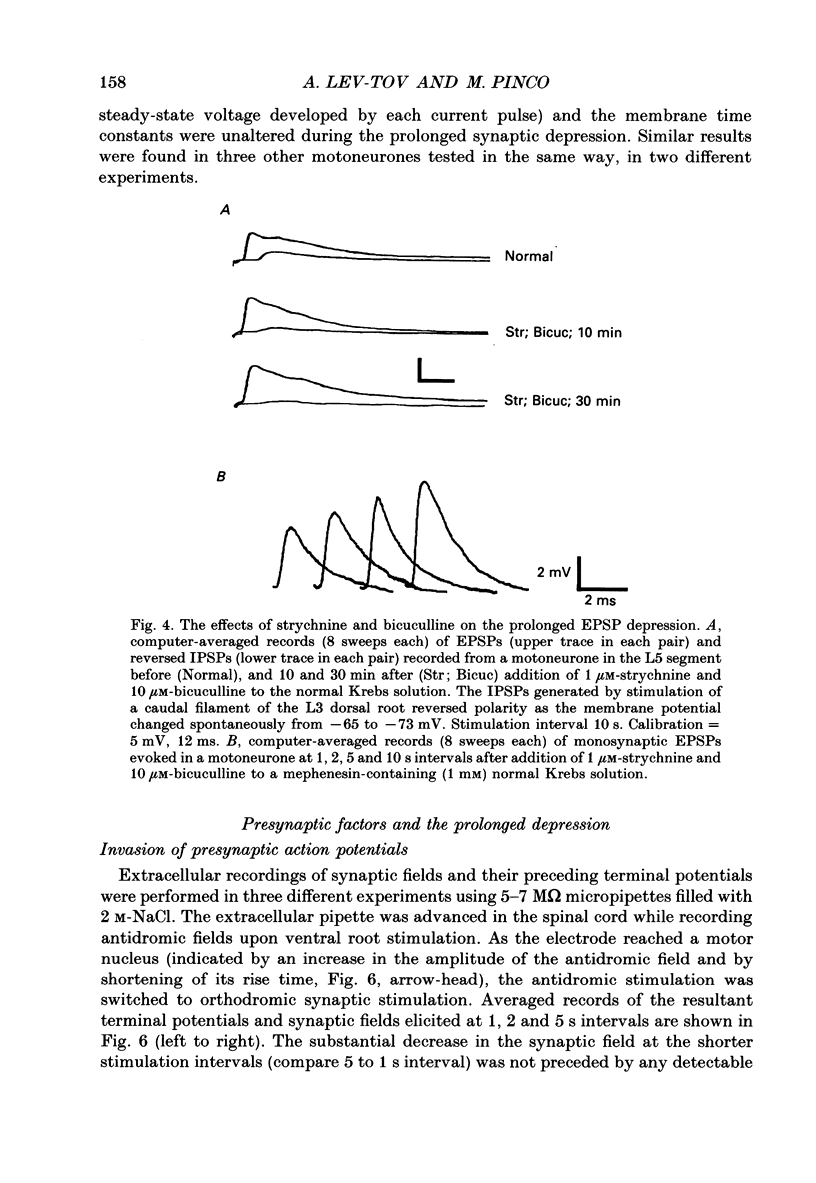







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3(4):331–338. doi: 10.1002/syn.890030406. [DOI] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989 Sep 21;341(6239):230–233. doi: 10.1038/341230a0. [DOI] [PubMed] [Google Scholar]
- Betz W. J. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol. 1970 Mar;206(3):629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988 Mar;11(3):112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- Bowery N. GABAB receptors and their significance in mammalian pharmacology. Trends Pharmacol Sci. 1989 Oct;10(10):401–407. doi: 10.1016/0165-6147(89)90188-0. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. Synaptic action during and after repetitive stimulation. J Physiol. 1960 Feb;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins W. F., 3rd, Honig M. G., Mendell L. M. Heterogeneity of group Ia synapses on homonymous alpha-motoneurons as revealed by high-frequency stimulation of Ia afferent fibers. J Neurophysiol. 1984 Nov;52(5):980–993. doi: 10.1152/jn.1984.52.5.980. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Inhibition of calcium currents in cultured rat dorsal root ganglion neurones by (-)-baclofen. Br J Pharmacol. 1986 May;88(1):213–220. doi: 10.1111/j.1476-5381.1986.tb09489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J., Franke C., Hatt H. Rapid activation, desensitization, and resensitization of synaptic channels of crayfish muscle after glutamate pulses. Biophys J. 1990 Mar;57(3):533–545. doi: 10.1016/S0006-3495(90)82569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981 Aug;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Harrison P. J., Jack J. J., Kullmann D. M. Reduction by baclofen of monosynaptic EPSPs in lumbosacral motoneurones of the anaesthetized cat. J Physiol. 1989 Sep;416:539–556. doi: 10.1113/jphysiol.1989.sp017776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Stimulation of spinal motoneurones with intracellular electrodes. J Physiol. 1956 Nov 28;134(2):451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton B. P., Walton K. Electrophysiological properties of neonatal rat motoneurones studied in vitro. J Physiol. 1986 Jan;370:651–678. doi: 10.1113/jphysiol.1986.sp015956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Y., Parnas I., Spira M. E. Differential conduction block in branches of a bifurcating axon. J Physiol. 1979 Oct;295:283–305. doi: 10.1113/jphysiol.1979.sp012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatt H., Smith D. O. Synaptic depression related to presynaptic axon conduction block. J Physiol. 1976 Jul;259(2):367–393. doi: 10.1113/jphysiol.1976.sp011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Redman S. J., Wong K. Post-tetanic potentiation and facilitation of synaptic potentials evoked in cat spinal motoneurones. J Physiol. 1981 Dec;321:97–109. doi: 10.1113/jphysiol.1981.sp013973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Yoshioka K. Ia afferent excitation of motoneurones in the in vitro new-born rat spinal cord is selectively antagonized by kynurenate. J Physiol. 1986 Jan;370:515–530. doi: 10.1113/jphysiol.1986.sp015948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z. G., Shen E., Dun N. J. Excitatory and inhibitory transmission from dorsal root afferents to neonate rat motoneurons in vitro. Brain Res. 1990 Dec 3;535(1):110–118. doi: 10.1016/0006-8993(90)91829-6. [DOI] [PubMed] [Google Scholar]
- KUNO M. MECHANSIM OF FACILITATION AND DEPRESSION OF THE EXCITATORY SYNAPTIC POTENTIAL IN SPINAL MOTONEURONES. J Physiol. 1964 Dec;175:100–112. doi: 10.1113/jphysiol.1964.sp007505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A., Keller B. U., Lev-Tov A. Patch clamp analysis of excitatory synapses in mammalian spinal cord slices. Pflugers Arch. 1990 Nov;417(3):285–290. doi: 10.1007/BF00370994. [DOI] [PubMed] [Google Scholar]
- Kudo N., Yamada T. Development of the monosynaptic stretch reflex in the rat: an in vitro study. J Physiol. 1985 Dec;369:127–144. doi: 10.1113/jphysiol.1985.sp015892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONGO V. G. Effects of mephenesin on the repetitive discharge of spinal cord interneurones. Arch Int Pharmacodyn Ther. 1961 Jun 1;132:222–236. [PubMed] [Google Scholar]
- Lee M. T., O'Donovan M. J. Organization of hindlimb muscle afferent projections to lumbosacral motoneurons in the chick embryo. J Neurosci. 1991 Aug;11(8):2564–2573. doi: 10.1523/JNEUROSCI.11-08-02564.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Tov A., Pinter M. J., Burke R. E. Posttetanic potentiation of group Ia EPSPs: possible mechanisms for differential distribution among medial gastrocnemius motoneurons. J Neurophysiol. 1983 Aug;50(2):379–398. doi: 10.1152/jn.1983.50.2.379. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. Composite EPSPs in motoneurons of different sizes before and during PTP: implications for transmission failure and its relief in Ia projections. J Neurophysiol. 1983 Jan;49(1):269–289. doi: 10.1152/jn.1983.49.1.269. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Mendell L. M. Modifiability of spinal synapses. Physiol Rev. 1984 Jan;64(1):260–324. doi: 10.1152/physrev.1984.64.1.260. [DOI] [PubMed] [Google Scholar]
- Munson J. B., Sypert G. W. Properties of single central Ia afferent fibres projecting to motoneurones. J Physiol. 1979 Nov;296:315–327. doi: 10.1113/jphysiol.1979.sp013007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. Presynaptic inhibition: transmitter and ionic mechanisms. Int Rev Neurobiol. 1979;21:217–258. doi: 10.1016/s0074-7742(08)60639-x. [DOI] [PubMed] [Google Scholar]
- Peng Y. Y., Frank E. Activation of GABAB receptors causes presynaptic inhibition at synapses between muscle spindle afferents and motoneurons in the spinal cord of bullfrogs. J Neurosci. 1989 May;9(5):1502–1515. doi: 10.1523/JNEUROSCI.09-05-01502.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond S. A. Effects of nerve impulses on threshold of frog sciatic nerve fibres. J Physiol. 1979 May;290(2):273–303. doi: 10.1113/jphysiol.1979.sp012771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman S. J., McLachlan E. M., Hirst G. D. Nonuniform passive membrane properties of rat lumbar sympathetic ganglion cells. J Neurophysiol. 1987 Mar;57(3):633–644. doi: 10.1152/jn.1987.57.3.633. [DOI] [PubMed] [Google Scholar]
- Redman S. Junctional mechanisms at group Ia synapses. Prog Neurobiol. 1979;12(1):33–83. doi: 10.1016/0301-0082(79)90010-8. [DOI] [PubMed] [Google Scholar]
- Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev. 1990 Jan;70(1):165–198. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- Robertson B., Taylor W. R. Effects of gamma-aminobutyric acid and (-)-baclofen on calcium and potassium currents in cat dorsal root ganglion neurones in vitro. Br J Pharmacol. 1986 Dec;89(4):661–672. doi: 10.1111/j.1476-5381.1986.tb11170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira M. E., Yarom Y., Parnas I. Modulation of spike frequency by regions of special axonal geometry and by synaptic inputs. J Neurophysiol. 1976 Jul;39(4):882–899. doi: 10.1152/jn.1976.39.4.882. [DOI] [PubMed] [Google Scholar]
- Sypert G. W., Munson J. B., Fleshman J. W. Effect of presynaptic inhibition on axonal potentials, terminal potentials, focal synaptic potentials, and EPSPs in cat spinal cord. J Neurophysiol. 1980 Oct;44(4):792–803. doi: 10.1152/jn.1980.44.4.792. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Fischbach G. D. Glutamate receptor desensitization and its role in synaptic transmission. Neuron. 1989 Aug;3(2):209–218. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- WRIGHT E. B. Effect of mephenesin and other depressants on spinal cord transmission in frog and cat. Am J Physiol. 1954 Nov;179(2):390–401. doi: 10.1152/ajplegacy.1954.179.2.390. [DOI] [PubMed] [Google Scholar]
- Wang M. Y., Dun N. J. Phaclofen-insensitive presynaptic inhibitory action of (+/-)-baclofen in neonatal rat motoneurones in vitro. Br J Pharmacol. 1990 Feb;99(2):413–421. doi: 10.1111/j.1476-5381.1990.tb14718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werman R., Davidoff R. A., Aprison M. H. Inhibitory of glycine on spinal neurons in the cat. J Neurophysiol. 1968 Jan;31(1):81–95. doi: 10.1152/jn.1968.31.1.81. [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L. NMDA receptors mediate poly- and monosynaptic potentials in motoneurons of rat embryos. J Neurosci. 1990 Jan;10(1):125–135. doi: 10.1523/JNEUROSCI.10-01-00125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


