Abstract
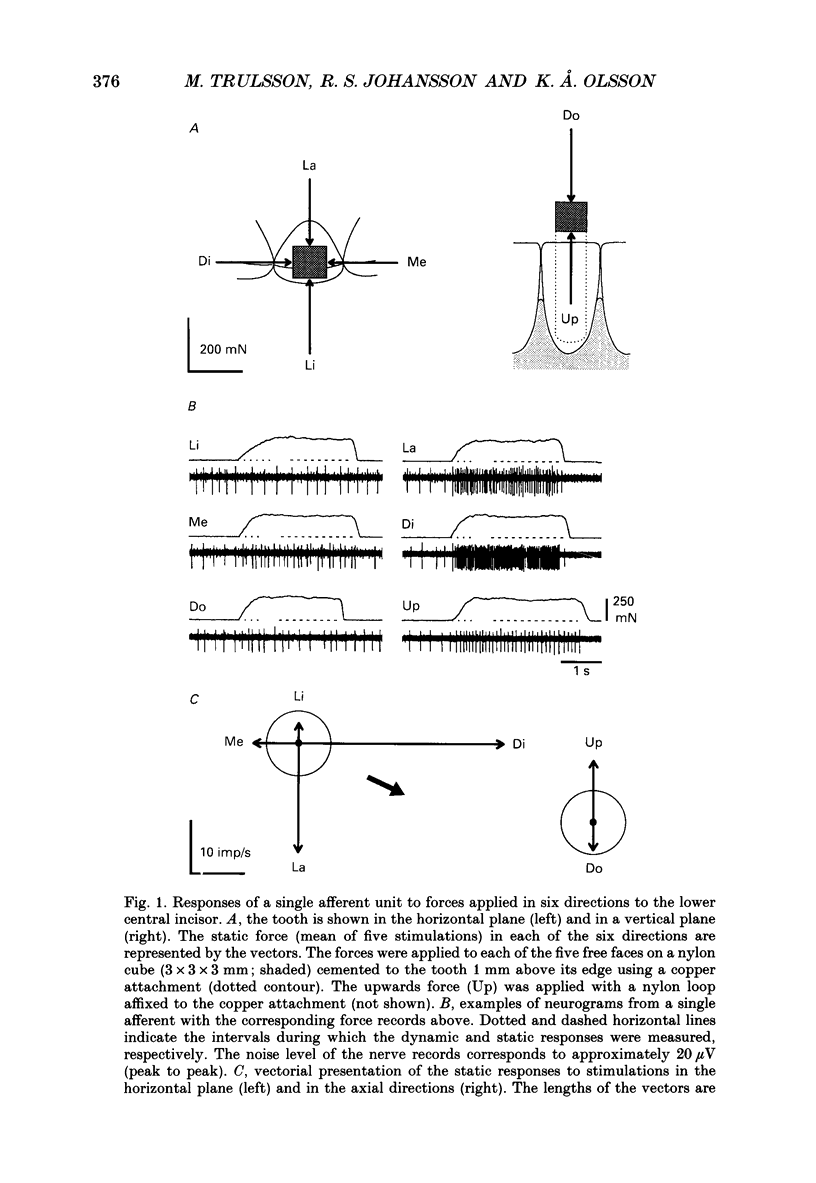
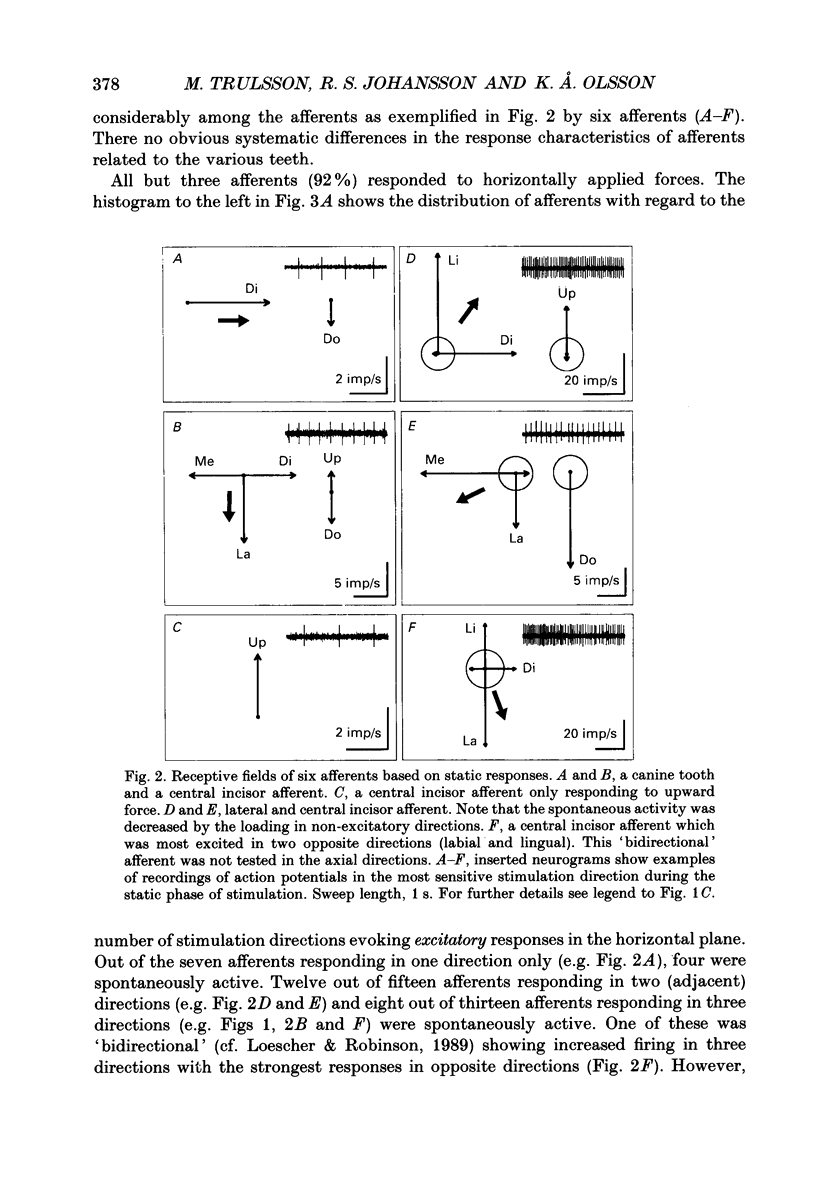
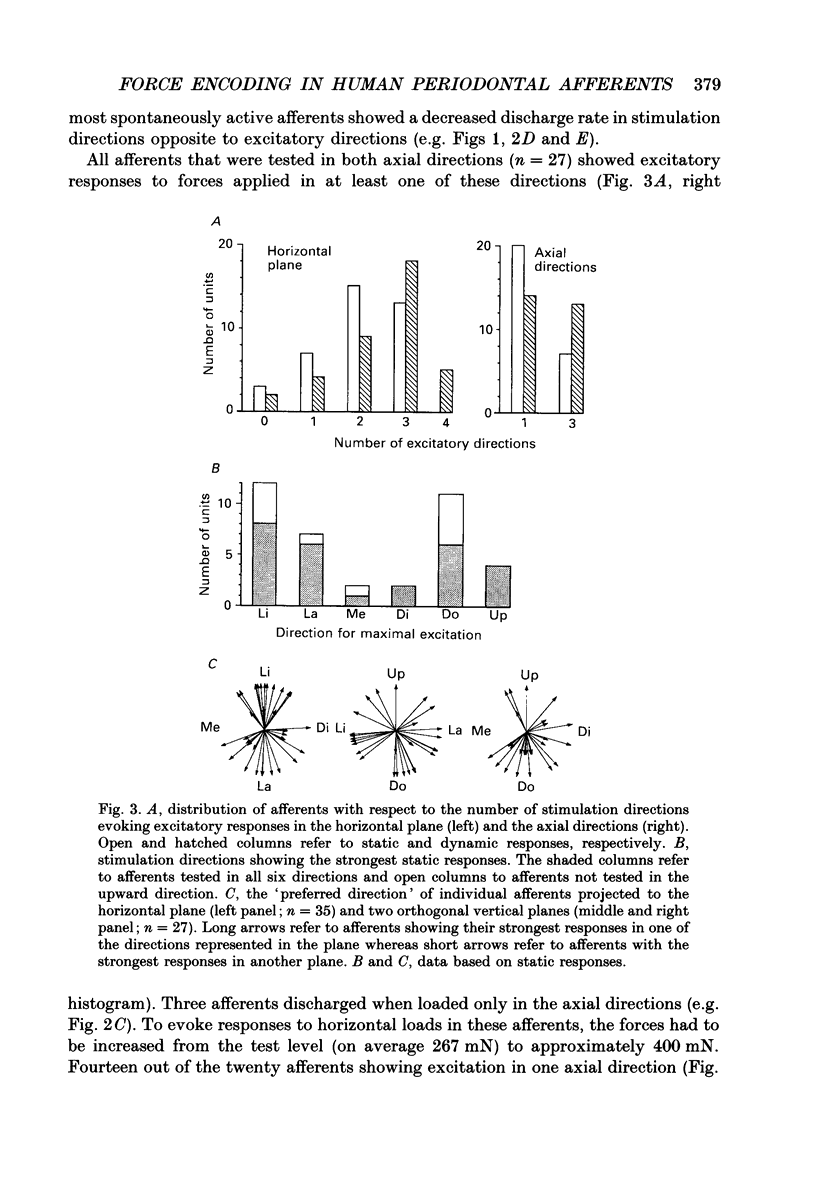
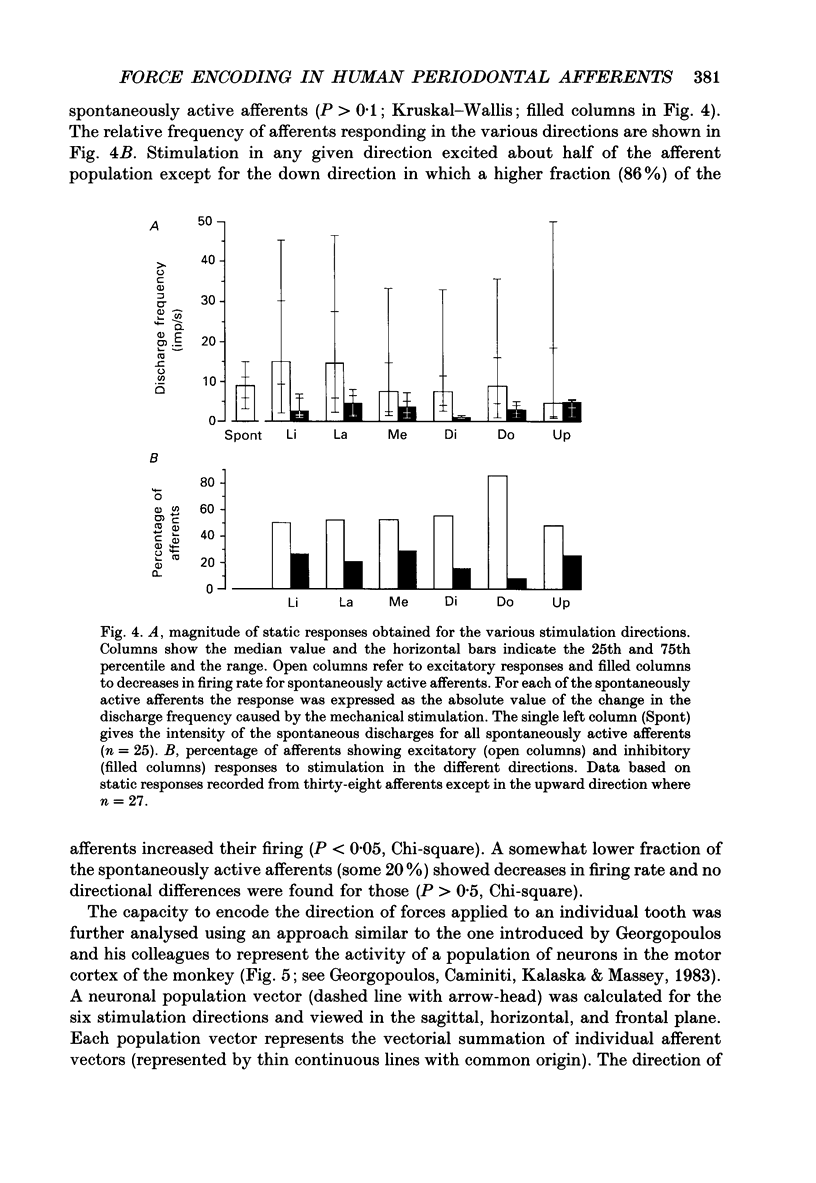
1. Single-unit impulse activity from thirty-eight mechanoreceptive afferent fibres was recorded in the human inferior alveolar nerve using tungsten microelectrodes. All afferents responded to mechanical stimulation of the teeth and most likely supplied periodontal mechanoreceptors. 2. All afferents showed their highest sensitivity to forces applied to a particular tooth (the lower incisors, the canine or the first premolar). Forces with 'ramp-and-hold' shaped profiles of similar magnitudes were applied to that tooth in the following six directions: lingual, labial, mesial and distal in the horizontal plane, and up and down in the axial direction of the tooth. Both static and dynamic response components were analysed. 3. All afferents were 'slowly adapting' since they discharged continuously in response to static forces in at least one stimulation direction. Twenty-five afferents (66%) were spontaneously active in the sense that they showed an on-going discharge in the absence of external stimulation. 4. Diverse receptive fields were observed. Most afferents (74%) responded to static forces in two or three of the four horizontal directions. Likewise, all units showed excitatory responses to axial loading with a majority (74%) responding in one of the two axial directions and the remainder in both axial directions. Spontaneously active afferents generally decreased their discharge rate when stimulated in directions opposite to the directions exciting the afferent. With regard to population responses, approximately half of the afferents showed excitatory responses to each stimulus direction except for downwards, in which 86% responded. 5. Twenty-three afferents (61%) exhibited the strongest response to forces in one of the horizontal directions. Of those, a majority were most responsive to the lingual direction (52%) and some to the labial direction (30%). Accordingly, the discharge rates during force application averaged over the whole afferent sample were highest in these directions. Of the remaining afferents, most responsive to one of the axial directions, 60% showed their strongest responses to forces in the downward direction. 6. Forty-five per cent of the afferents showed wider receptive fields to the dynamic component of the force stimulation than to the static. The direction of maximal sensitivity, however, remained the same with few exceptions. 7. It was demonstrated that even though individual periodontal mechanoreceptive afferents provide ambiguous information regarding the direction of a force applied to a tooth, populations of such afferents are well suited to give detailed directional information. It is suggested that such information may play an important role for the control of mastication.
Full text
PDF




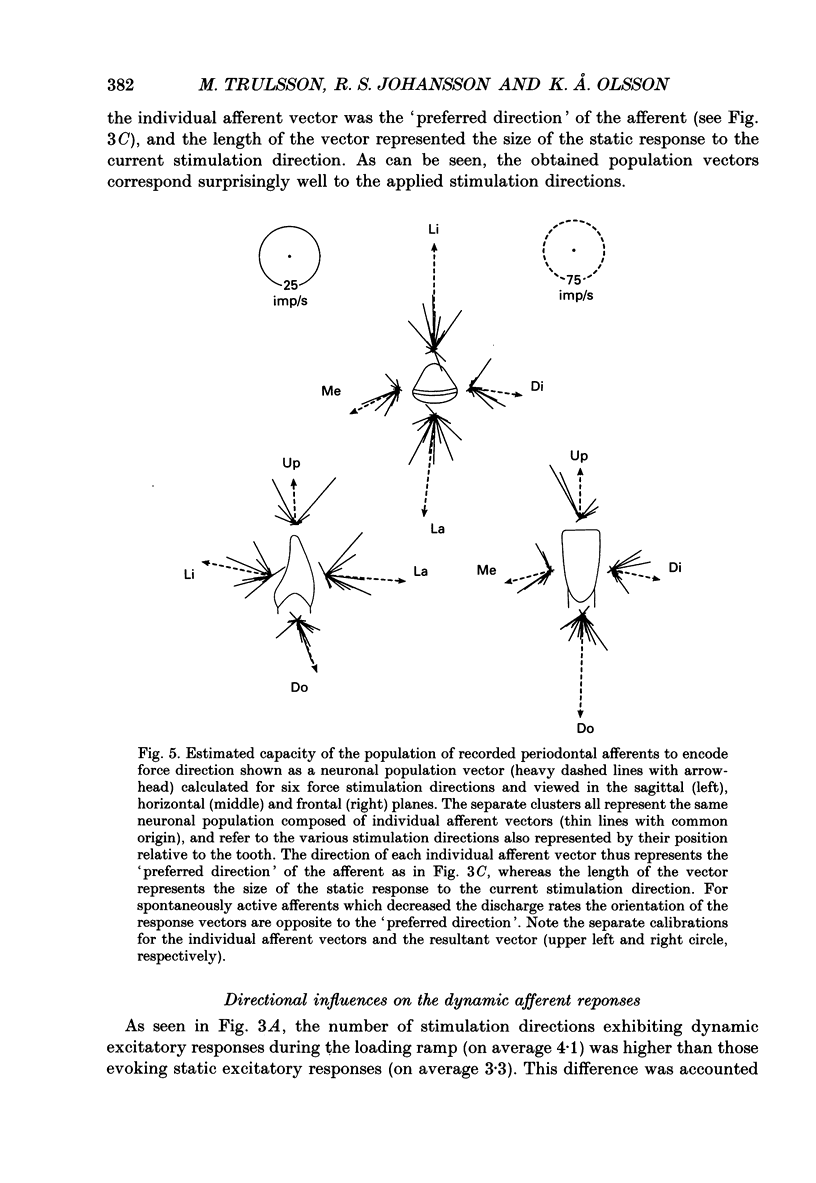






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
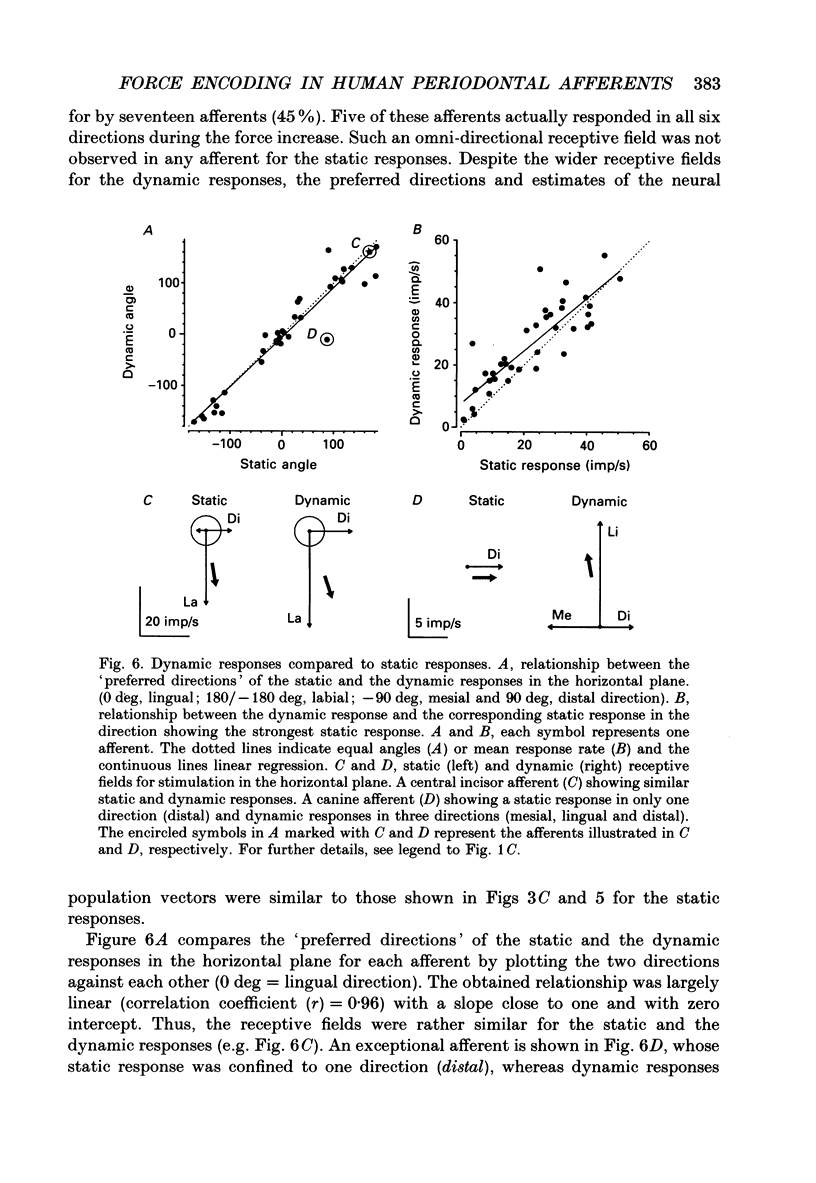
- Byers M. R., Dong W. K. Comparison of trigeminal receptor location and structure in the periodontal ligament of different types of teeth from the rat, cat, and monkey. J Comp Neurol. 1989 Jan 1;279(1):117–127. doi: 10.1002/cne.902790110. [DOI] [PubMed] [Google Scholar]
- Byers M. R. Sensory innervation of periodontal ligament of rat molars consists of unencapsulated Ruffini-like mechanoreceptors and free nerve endings. J Comp Neurol. 1985 Jan 22;231(4):500–518. doi: 10.1002/cne.902310408. [DOI] [PubMed] [Google Scholar]
- Cash R. M., Linden R. W. The distribution of mechanoreceptors in the periodontal ligament of the mandibular canine tooth of the cat. J Physiol. 1982 Sep;330:439–447. doi: 10.1113/jphysiol.1982.sp014350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin B. B., Bäckström P. A., Bäckström L. O. Single unit retrieval in microneurography: a microprocessor-based device controlled by an operator. J Neurosci Methods. 1988 Jun;24(2):137–144. doi: 10.1016/0165-0270(88)90057-x. [DOI] [PubMed] [Google Scholar]
- Gracco V. L., Abbs J. H. Dynamic control of the perioral system during speech: kinematic analyses of autogenic and nonautogenic sensorimotor processes. J Neurophysiol. 1985 Aug;54(2):418–432. doi: 10.1152/jn.1985.54.2.418. [DOI] [PubMed] [Google Scholar]
- Hannam A. G. Receptor fields of periodontal mechanosensitive units in the dog. Arch Oral Biol. 1970 Oct;15(10):971–978. doi: 10.1016/0003-9969(70)90092-0. [DOI] [PubMed] [Google Scholar]
- Hannam A. G. The response of periodontal mechanoreceptors in the dog to controlled loading of the teeth. Arch Oral Biol. 1969 Jul;14(7):781–791. doi: 10.1016/0003-9969(69)90169-1. [DOI] [PubMed] [Google Scholar]
- Inoue T., Kato T., Masuda Y., Nakamura T., Kawamura Y., Morimoto T. Modifications of masticatory behavior after trigeminal deafferentation in the rabbit. Exp Brain Res. 1989;74(3):579–591. doi: 10.1007/BF00247360. [DOI] [PubMed] [Google Scholar]
- Johansson R. S., Olsson K. A. Microelectrode recordings from human oral mechanoreceptors. Brain Res. 1976 Dec 17;118(2):307–311. doi: 10.1016/0006-8993(76)90716-2. [DOI] [PubMed] [Google Scholar]
- Kapur K. K., Garrett N. R., Fischer E. Effects of anaesthesia of human oral structures on masticatory performance and food particle size distribution. Arch Oral Biol. 1990;35(5):397–403. doi: 10.1016/0003-9969(90)90187-f. [DOI] [PubMed] [Google Scholar]
- Karita K., Tabata T. Response fields of the periodontal mechanosensitive units in the superior alveolar nerve of the cat. Exp Neurol. 1985 Dec;90(3):558–565. doi: 10.1016/0014-4886(85)90153-0. [DOI] [PubMed] [Google Scholar]
- Kawamura Y., Nishiyama T. Projection of dental afferent impulses to the trigeminal nuclei of the cat. Jpn J Physiol. 1966 Oct 15;16(5):584–597. doi: 10.2170/jjphysiol.16.584. [DOI] [PubMed] [Google Scholar]
- Lavigne G., Kim J. S., Valiquette C., Lund J. P. Evidence that periodontal pressoreceptors provide positive feedback to jaw closing muscles during mastication. J Neurophysiol. 1987 Aug;58(2):342–358. doi: 10.1152/jn.1987.58.2.342. [DOI] [PubMed] [Google Scholar]
- Loescher A. R., Robinson P. P. Receptor characteristics of periodontal mechanosensitive units supplying the cat's lower canine. J Neurophysiol. 1989 Oct;62(4):971–978. doi: 10.1152/jn.1989.62.4.971. [DOI] [PubMed] [Google Scholar]
- Lund J. P., Rossignol S., Murakami T. Interactions between the jaw-opening reflex and mastication. Can J Physiol Pharmacol. 1981 Jul;59(7):683–690. doi: 10.1139/y81-104. [DOI] [PubMed] [Google Scholar]
- Mei N., Hartmann F., Roubien R. Caractéristiques fonctionnelles des mécanorécepteurs des ligaments dentaires chez le chat. J Biol Buccale. 1975 Mar;3(1):29–39. [PubMed] [Google Scholar]
- Morimoto T., Inoue T., Masuda Y., Nagashima T. Sensory components facilitating jaw-closing muscle activities in the rabbit. Exp Brain Res. 1989;76(2):424–440. doi: 10.1007/BF00247900. [DOI] [PubMed] [Google Scholar]
- NESS A. R. The mechanoreceptors of the rabbit mandibular incisor. J Physiol. 1954 Dec 10;126(3):475–493. doi: 10.1113/jphysiol.1954.sp005223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson K. A., Sasamoto K., Lund J. P. Modulation of transmission in rostral trigeminal sensory nuclei during chewing. J Neurophysiol. 1986 Jan;55(1):56–75. doi: 10.1152/jn.1986.55.1.56. [DOI] [PubMed] [Google Scholar]
- Sakada S., Kamio E. Receptive fields and directional sensitivity of single sensory units innervating the periodontal ligaments of the cat mandibular teeth. Bull Tokyo Dent Coll. 1971 Feb;12(1):25–43. [PubMed] [Google Scholar]
- Sato O., Maeda T., Kobayashi S., Iwanaga T., Fujita T., Takahashi Y. Innervation of periodontal ligament and dental pulp in the rat incisor: an immunohistochemical investigation of neurofilament protein and glia-specific S-100 protein. Cell Tissue Res. 1988 Jan;251(1):13–21. doi: 10.1007/BF00215442. [DOI] [PubMed] [Google Scholar]
- Sloan P. Collagen fibre architecture in the periodontal ligament. J R Soc Med. 1979 Mar;72(3):188–191. doi: 10.1177/014107687907200308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T., Karita K. Response properties of periodontal mechanosensitive fibers in the superior dental nerve of the cat. Exp Neurol. 1986 Dec;94(3):469–478. doi: 10.1016/0014-4886(86)90230-x. [DOI] [PubMed] [Google Scholar]
- Taira K. Characteristics of periodontal mechanosensitive neurons in the first somatosensory cortex of the cat. Brain Res. 1987 Apr 14;409(1):52–61. doi: 10.1016/0006-8993(87)90740-2. [DOI] [PubMed] [Google Scholar]
- Thexton A. J., Hiiemae K. M., Crompton A. W. Food consistency and bite size as regulators of jaw movement during feeding in the cat. J Neurophysiol. 1980 Sep;44(3):456–474. doi: 10.1152/jn.1980.44.3.456. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B., Hagbarth K. E. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp Neurol. 1968 Jul;21(3):270–289. doi: 10.1016/0014-4886(68)90041-1. [DOI] [PubMed] [Google Scholar]
- Yokota T., Koyama N., Nishikawa Y., Hasegawa A. Dual somatosensory representation of the periodontium in nucleus ventralis posteromedialis of the cat thalamus. Brain Res. 1988 Dec 13;475(1):187–191. doi: 10.1016/0006-8993(88)90217-x. [DOI] [PubMed] [Google Scholar]


