Abstract
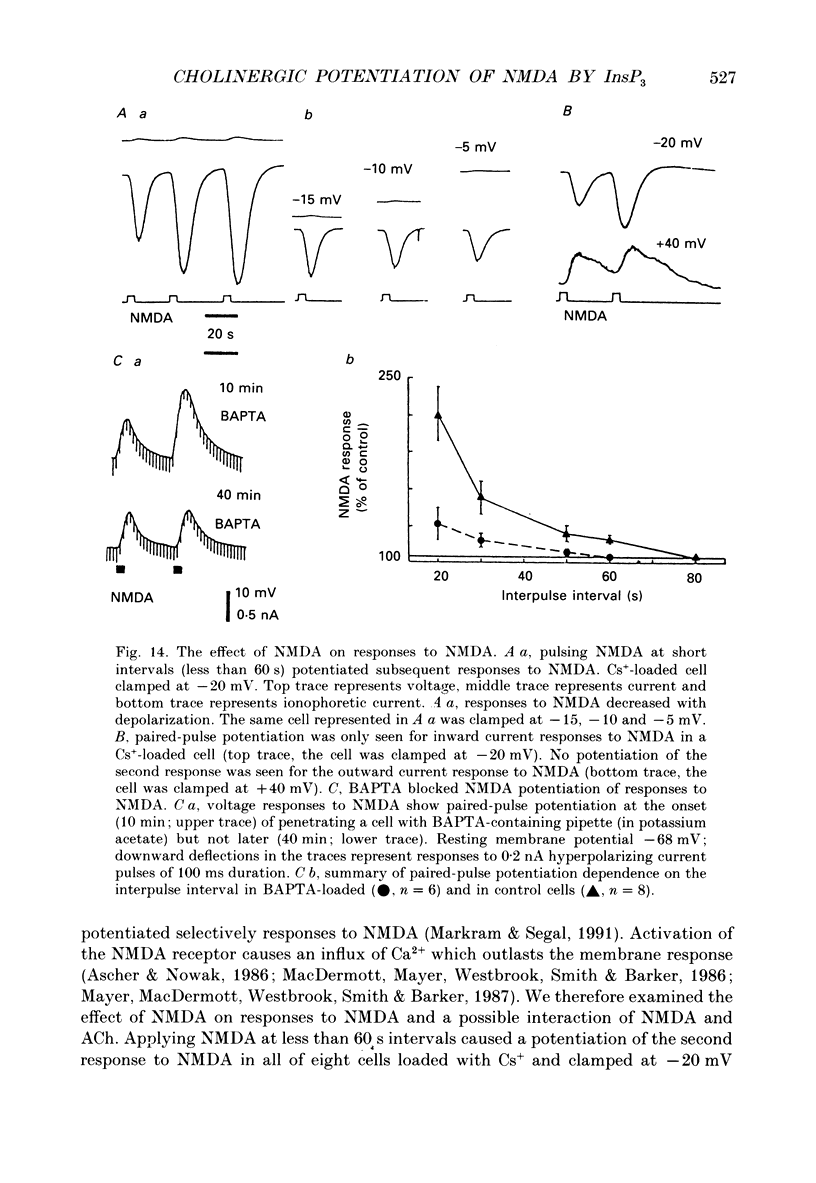
1. The cellular mechanism by which acetylcholine (ACh) potentiates neuronal responses to N-methyl-D-aspartate (NMDA) was investigated in CA1 neurones of hippocampal slices using current- and voltage-clamp techniques. 2. Loading cells with 5'-guanylylimidodiphosphate (GppNHp) caused a gradual increase in response to NMDA. Pulses of ACh accelerated this increase. Guanosine 5'-O-(2-thiodiphosphate) (GDP beta S) blocked the potentiating effect of ACh on responses to NMDA. 3. Acute LiCl caused a gradual decrease in the potentiating effect of ACh, while the potentiation was completely prevented by 3 day chronic 6 mequiv/kg (I.P.) LiCl treatment and restored by acute treatment with 10 mM-inositol. 4. Loading cells with a general protein kinase inhibitor, H-7, enhanced the potentiating effect of ACh on responses to NMDA and blocked the effect of ACh on the after-hyperpolarization (AHP). 5. Ultraviolet irradiation of cells loaded with a photolabile inositol 1,4,5-trisphosphate (InsP3) caused a transient increase in responses to NMDA, while penetrating cells with active InsP3-containing pipettes caused a gradual BAPTA-sensitive increase in responses to NMDA. 6. Reducing the rate of InsP3 metabolism, with 2,3-diphosphoglyceric acid (DPG), caused an increase and prolongation of the potentiating effect of ACh, while blocking the InsP3 receptor with heparin prevented the cholinergic potentiation. 7. NMDA, by itself, potentiated subsequent responses to NMDA, an effect that was blocked when [Ca2+]i was chelated with BAPTA. NMDA and ACh were also found to compete in potentiating responses to NMDA. Finally, the cholinergic potentiation was blocked when cells were loaded with BAPTA. 8. We propose that activation of the InsP3 branch of the phosphoinositide pathway potentiated responses to NMDA and that InsP3 exerted this effect by elevating [Ca2+]i.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURESOVA O., BURES J., BOHDANECKY Z., WEISS T. EFFECT OF ATROPINE ON LEARNING, EXTINCTION, RETENTION AND RETRIEVAL IN RATS. Psychopharmacologia. 1964 Mar 11;5:255–263. doi: 10.1007/BF02341258. [DOI] [PubMed] [Google Scholar]
- Bashir Z. I., Alford S., Davies S. N., Randall A. D., Collingridge G. L. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991 Jan 10;349(6305):156–158. doi: 10.1038/349156a0. [DOI] [PubMed] [Google Scholar]
- Benardo L. S., Prince D. A. Ionic mechanisms of cholinergic excitation in mammalian hippocampal pyramidal cells. Brain Res. 1982 Oct 14;249(2):333–344. doi: 10.1016/0006-8993(82)90067-1. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989 Nov 3;59(3):411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch J. A. The cholinergic synapse and the site of memory. Science. 1971 Nov 19;174(4011):788–794. doi: 10.1126/science.174.4011.788. [DOI] [PubMed] [Google Scholar]
- Drachman D. A., Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974 Feb;30(2):113–121. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Classification of muscarinic responses in hippocampus in terms of receptor subtypes and second-messenger systems: electrophysiological studies in vitro. J Neurosci. 1988 Nov;8(11):4214–4224. doi: 10.1523/JNEUROSCI.08-11-04214.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. K., Bartus R. T. Regional differences in the coupling of muscarinic receptors to inositol phospholipid hydrolysis in guinea pig brain. J Neurochem. 1985 Oct;45(4):1085–1095. doi: 10.1111/j.1471-4159.1985.tb05527.x. [DOI] [PubMed] [Google Scholar]
- Guillemette G., Balla T., Baukal A. J., Catt K. J. Metabolism of inositol 1,4,5-trisphosphate to higher inositol phosphates in bovine adrenal cytosol. Am J Hypertens. 1989 May;2(5 Pt 1):387–394. doi: 10.1093/ajh/2.5.387. [DOI] [PubMed] [Google Scholar]
- Gurwitz D., Sokolovsky M. Dual pathways in muscarinic receptor stimulation of phosphoinositide hydrolysis. Biochemistry. 1987 Jan 27;26(2):633–638. doi: 10.1021/bi00376a039. [DOI] [PubMed] [Google Scholar]
- Harris E. W., Ganong A. H., Cotman C. W. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984 Dec 3;323(1):132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hirotsu I., Hori N., Katsuda N., Ishihara T. Effect of anticholinergic drug on long-term potentiation in rat hippocampal slices. Brain Res. 1989 Mar 13;482(1):194–197. doi: 10.1016/0006-8993(89)90561-1. [DOI] [PubMed] [Google Scholar]
- Ito T., Miura Y., Kadokawa T. Effects of physostigmine and scopolamine on long-term potentiation of hippocampal population spikes in rats. Can J Physiol Pharmacol. 1988 Aug;66(8):1010–1016. doi: 10.1139/y88-165. [DOI] [PubMed] [Google Scholar]
- Knöpfel T., Vranesic I., Gähwiler B. H., Brown D. A. Muscarinic and beta-adrenergic depression of the slow Ca2(+)-activated potassium conductance in hippocampal CA3 pyramidal cells is not mediated by a reduction of depolarization-induced cytosolic Ca2+ transients. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4083–4087. doi: 10.1073/pnas.87.11.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Zucker R. S., Nicoll R. A. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988 Oct 7;242(4875):81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- Malinow R., Schulman H., Tsien R. W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989 Aug 25;245(4920):862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Markram H., Segal M. Calcimycin potentiates responses of rat hippocampal neurons to N-methyl-D-aspartate. Brain Res. 1991 Feb 1;540(1-2):322–324. doi: 10.1016/0006-8993(91)90529-5. [DOI] [PubMed] [Google Scholar]
- Markram H., Segal M. Long-lasting facilitation of excitatory postsynaptic potentials in the rat hippocampus by acetylcholine. J Physiol. 1990 Aug;427:381–393. doi: 10.1113/jphysiol.1990.sp018177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., MacDermott A. B., Westbrook G. L., Smith S. J., Barker J. L. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987 Oct;7(10):3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry D. K., Hablitz J. J. Nystatin-perforated patch recordings disclose NMDA-induced outward currents in cultured neocortical neurons. Brain Res. 1990 Dec 10;535(2):318–322. doi: 10.1016/0006-8993(90)91616-o. [DOI] [PubMed] [Google Scholar]
- Monnet F. P., Debonnel G., de Montigny C. Neuropeptide Y selectively potentiates N-methyl-D-aspartate-induced neuronal activation. Eur J Pharmacol. 1990 Jun 21;182(1):207–208. doi: 10.1016/0014-2999(90)90516-9. [DOI] [PubMed] [Google Scholar]
- Muller D., Joly M., Lynch G. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science. 1988 Dec 23;242(4886):1694–1697. doi: 10.1126/science.2904701. [DOI] [PubMed] [Google Scholar]
- Rana R. S., Hokin L. E. Role of phosphoinositides in transmembrane signaling. Physiol Rev. 1990 Jan;70(1):115–164. doi: 10.1152/physrev.1990.70.1.115. [DOI] [PubMed] [Google Scholar]
- Reynolds J. N., Baskys A., Carlen P. L. The effects of serotonin on N-methyl-D-aspartate and synaptically evoked depolarizations in rat neocortical neurons. Brain Res. 1988 Jul 26;456(2):286–292. doi: 10.1016/0006-8993(88)90230-2. [DOI] [PubMed] [Google Scholar]
- Segal M. Multiple action of acetylcholine at a muscarinic receptor studied in the rat hippocampal slice. Brain Res. 1982 Aug 19;246(1):77–87. doi: 10.1016/0006-8993(82)90144-5. [DOI] [PubMed] [Google Scholar]
- Segal M. Synaptic activation of a cholinergic receptor in rat hippocampus. Brain Res. 1988 Jun 14;452(1-2):79–86. doi: 10.1016/0006-8993(88)90011-x. [DOI] [PubMed] [Google Scholar]
- Smith S. S. Estrogen administration increases neuronal responses to excitatory amino acids as a long-term effect. Brain Res. 1989 Dec 4;503(2):354–357. doi: 10.1016/0006-8993(89)91691-0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Sakurai M., Hayashi S. Effect of scopolamine and HP 029, a cholinesterase inhibitor, on long-term potentiation in hippocampal slices of the guinea pig. Neurosci Lett. 1989 Mar 27;98(2):179–183. doi: 10.1016/0304-3940(89)90506-5. [DOI] [PubMed] [Google Scholar]
- Teyler T. J., Discenna P. Long-term potentiation as a candidate mnemonic device. Brain Res. 1984 Mar;319(1):15–28. doi: 10.1016/0165-0173(84)90027-4. [DOI] [PubMed] [Google Scholar]
- Walden J., Speckmann E. J., Bingmann D. Augmentation of glutamate responses by GABA in the rat's motorcortex in vivo. Neurosci Lett. 1989 Jun 19;101(2):209–213. doi: 10.1016/0304-3940(89)90532-6. [DOI] [PubMed] [Google Scholar]
- Wenk G., Hughey D., Boundy V., Kim A., Walker L., Olton D. Neurotransmitters and memory: role of cholinergic, serotonergic, and noradrenergic systems. Behav Neurosci. 1987 Jun;101(3):325–332. doi: 10.1037//0735-7044.101.3.325. [DOI] [PubMed] [Google Scholar]


