Abstract
Protein quality control is an essential function of the endoplasmic reticulum. Misfolded proteins unable to acquire their native conformation are retained in the endoplasmic reticulum, retro-translocated back into the cytosol, and degraded via the ubiquitin-proteasome system. We show that efficient degradation of soluble malfolded proteins in yeast requires a fully competent early secretory pathway. Mutations in proteins essential for ER-Golgi protein traffic severely inhibit ER degradation of the model substrate CPY*. We found ER localization of CPY* in WT cells, but no other specific organelle for ER degradation could be identified by electron microscopy studies. Because CPY* is degraded in COPI coat mutants, only a minor fraction of CPY* or of a proteinaceous factor required for degradation seems to enter the recycling pathway between ER and Golgi. Therefore, we propose that the disorganized structure of the ER and/or the mislocalization of Kar2p, observed in early secretory mutants, is responsible for the reduction in CPY* degradation. Further, we observed that mutations in proteins directly involved in degradation of malfolded proteins (Der1p, Der3/Hrd1p, and Hrd3p) lead to morphological changes of the endoplasmic reticulum and the Golgi, escape of CPY* into the secretory pathway and a slower maturation rate of wild-type CPY.
INTRODUCTION
Protein quality control with subsequent elimination of malfolded proteins or unassembled subunits is essential for cellular function. Disturbed quality control leads to disease and eventually to cell death (Plemper and Wolf, 1999; Kopito and Sitia, 2000). The endoplasmic reticulum (ER) is the folding compartment for proteins destined to function within the ER itself and for secretory proteins of the Golgi, endosomes, vacuoles, and plasma membrane as well as for proteins secreted extracellularly. It contains a multitude of folding enzymes and chaperones to perform this function (Ellgaard et al., 1999; Zapun et al., 1999). Failure in folding or in the assembly of multimeric complexes leads to recognition by the quality control machinery in the ER. The proteins are then transported back into the cytoplasm, most likely via the Sec61 translocon, and degraded by the ubiquitin–proteasome system (Kopito, 1997; Sommer and Wolf, 1997; Brodsky and McCracken, 1999; Plemper and Wolf, 1999). Polyubiquitination is mediated through the action of the E2 enzymes, Ubc1p and Ubc7p, and the membrane bound RING-H2 ubiquitin–protein ligase (E3) Der3/Hrd1p, which is complexed to another membrane protein, Hrd3p (Hiller et al., 1996; Plemper et al., 1999; Friedländer et al., 2000; Gardner et al., 2000; Bays et al., 2001a; Deak and Wolf, 2001). The final degradation is carried out by the proteasome, a multicatalytic and multifunctional proteinase machinery (Hilt and Wolf, 2000). Depending on the nature of the malfolded substrate protein, additional components of the ubiquitination machinery (i.e., the ubiquitin conjugating enzyme [E2 Ubc6p; Biederer et al., 1996; Hiller et al., 1996) and of the lumenal ER folding machinery (the Hsp70 chaperone Kar2p, protein-disulfide isomerase [PDI], α-1,2 mannosidase, the lectin-like protein Mnl1/Htm1 (Knop et al., 1996b; Plemper et al., 1997; Brodsky et al., 1999; Gillece et al., 1999; Jakob et al., 2001; Nakatsukasa et al., 2001) or an ER membrane protein of unknown function (Der1p; Knop et al., 1996a) are required for the degradation event.
We had previously shown that a defect in ERD1, involved in the retrieval of HDEL-containing proteins from the Golgi to the ER (Hardwick et al., 1990), leads to the escape of CPY* from the ER, despite the fact that CPY* does not contain the HDEL retrieval sequence (Knop et al., 1996a). We were, therefore, interested in the question whether the secretory competence of the ER in general is necessary for the degradation of malfolded proteins. Intracellular transport of proteins is mediated by coated vesicles: proteins are packed into COPII-coated vesicles at ER exit sites and transported to the Golgi apparatus, where they fuse with the target membrane (Rothman and Wieland, 1996; Schekman and Orci, 1996; Kuehn et al., 1998). On arrival at the Golgi complex proteins are sorted to the peripheral compartments of the cell such as vacuoles, plasma membrane, and secretory vesicles. Proteins can also be retrieved to the ER by retrograde transport from either the ER-Golgi intermediate compartment or the Golgi complex itself, by COPI-coated vesicles (Letourneur et al., 1994; Allan and Balch, 1999).
It has been previously shown that extensive protein misfolding and accumulation in the ER activates the unfolded protein response (UPR; Knop et al., 1996a; Chapman et al., 1998; Casagrande et al., 2000; Friedländer et al., 2000; Travers et al., 2000). Signaling of malfolded proteins in the ER occurs via Ire1p, a kinase/nuclease of the ER and the nuclear envelope, which activates the transcription factor Hac1p. This activation leads to transcriptional upregulation of genes necessary to relieve the cell from ER stress (Chapman et al., 1998). As one might expect, genes involved in ER degradation directly, such as DER1, DER3/HRD1, HRD3, and UBC7 are targets of Hac1p (Travers et al., 2000). Furthermore, transcription of genes involved in ER-to-Golgi trafficking, protein targeting to the vacuole and the cell surface, lipid metabolism, and glycosylation are also upregulated upon ER stress (Travers et al., 2000). In this study, we investigated the requirement of a fully operational early secretory pathway and a competent UPR for efficient ER degradation. As a soluble model substrate of ER degradation in yeast we examined the disappearance of malfolded carboxypeptidase yscY (CPY*; Finger et al., 1993; Hiller et al., 1996; Knop et al., 1996a). In addition, we tested the influence of defective ER degradation on the secretory system.
MATERIALS AND METHODS
Construction and Growth Conditions of Strains
Previously described standard methods were used in media preparation and for genetic and molecular biological techniques (Guthrie and Fink, 1991; Ausubel, 1992). The Saccharomyces cerevisiae strains used in this study are summarized in Table 1. Yeast cells were grown at 25°C (temperature-sensitive strains) or 30°C. For generation of the ufe1-1 integration plasmid, pUT1 (Lewis et al., 1997), containing the ufe1-1 allele, was digested with SalI and SpeI, and the 1450 base-pair ufe1-1 fragment was ligated into pRS306 (Sikorski and Hieter, 1989) to obtain pCT27. SnaBI linearized pCT27 was used to replace the chromosomal UFE1 allele by two-step gene replacement (Scherer and Davis, 1979). RSY281 (sec23-1; Kaiser and Schekman, 1990), CBY263 (sed5-1; Cao et al., 1998), and RSY277 (sec21-1; Letourneur et al., 1994) were backcrossed multiple times through W303-1C or YCT458 to obtain the isogenic strains YCT441, YCT480, and YCT611, respectively. The IRE1 gene was deleted using plasmid pJU341, containing the IRE1 knock out fragment (Friedländer et al., 2000). Crossing of the respective single mutants to each other produced the double-mutant strains YCT541 and YCT542. To obtain strains YCT458, YCT462, YCT460, YCT437, and YCT438, BglII linearized plasmid pRS306prc1-1 (Knop et al., 1996a) was used to introduce the prc1-1 allele into strains YR1070 (wild-type), YR1068 (sec12-1), YR1069 (sec18-1; H. Rudolph), RH448 (wild-type), and RH2688 (sec27-1; Schröder-Köhne et al., 1998) using two-step gene replacement (Scherer and Davis, 1979). YCT614 was generated from strain YCT438 by deleting the PEP4 gene with PvuII-digested pWO139. Integration was confirmed by Southern blotting. Plasmid pWO139 was generated through ligation of the 1.1-kb URA3 containing an EcoRI/SmaI fragment from Yep24 (Botstein et al., 1979) into EcoRI/MscI digested plasmid pWO261. For generation of plasmid pWO261 a 1.9-kb SacI/XhoI fragment from pTZ18 (Rupp and Wolf, 1995), containing PEP4, was ligated into pBluescript KS+ (Stratagene, La Jolla, CA).
Table 1.
Yeast strains used in this study
| Name | Genotype | Source |
|---|---|---|
| W303-1B | Mat α ade2-1 leu2-3,112 trp1-1 ura3-1 his3-11,15 can1-100 | Chiang and Schekman (1991) |
| W303-1C | W303-1B prc1-1 | Knop et al. (1996a) |
| W303-CD | W303-1C Δder1∷URA3 | Knop et al. (1996a) |
| W303-CF | W303-1C Δpep4∷HIS3 | Knop et al. (1996a) |
| W303-CDF | W303-1C Δder1∷URA3 Δpep4∷HIS3 | Knop et al. (1996a) |
| W303-BD | W303-1B Δder1∷URA3 | Knop et al. (1996a) |
| YJB009 | W303-1C Δder3/hrd1∷HIS3 | Bordallo et al. (1998) |
| W303 ΔC | W303-1B Δprc1∷LEU2 | Plemper et al. (1999) |
| YCT431 | W303-1C ufe1-1 | This work |
| YCT441 | W303-1C sec23-1 | This work |
| YCT480 | W303-1C sed5-1 | This work |
| YRH68 | W303-1C Δire1∷LEU2 | This work |
| YCT542 | W303-1C sec23-1 Δire1∷LEU2 | This work |
| YCT540 | W303-1C ufe1-1 Δire1∷LEU2 | This work |
| YCT458 | Mat a ura3-52 prc1-1 | This work |
| YCT462 | YCT458 sec12-1 | This work |
| YCT460 | YCT458 sec18-1 | This work |
| YCT611 | YCT458 sec21-1 | This work |
| YCT437 | Mat a prc1-1 his4 lys2 ura3 leu2 bar1 | This work |
| YCT438 | YCT437 sec27-1 | This work |
| YCT614 | YCT437 sec27-1 Δpep4∷URA3 | This work |
To express CPY*-HA, we fused prc1-1HA behind the TDH3 (pCT43) or CUP1 (pCT52) promoter. Similarly, PRC1 was fused behind the TDH3 promoter (pCT70) for expression of CPY. The cloning strategy to obtain plasmids pCT41, pCT43, pCT52, and pCT70 is available on request. The yeast strain bearing the prc1-1HA allele was obtained according to M. Longtine (Longtine et al., 1998) using plasmid pFA6a-3HA-kanMX6. For generation of plasmid pWO152, a 3-kb BamHI/HindIII fragment, containing SEC18, was ligated into pRS426 (Christianson et al., 1992).
Pulse-chase Analysis
Yeast strains were grown logarithmically in CM medium and labeled for 20 min with 35S-methionine (pulse). Temperature-sensitive strains were shifted to 37°C for the times indicated in legends to figures. After addition of an excess of nonradioactive methionine, samples were taken at the indicated time points. Cell disruption, immunoprecipitation, and SDS-PAGE were performed as previously described (Plemper et al., 1999). Curves were generated plotting the mean values of two to four independent experiments. Maturation analysis of CPY and proteinase yscA (PrA) was performed similarly, except that the strains were labeled for only 5 min.
Electron Microscopy
For immuno-electron microscopy (EM), cells were fixed in a mixture of freshly prepared 4% formaldehyde and 0.5% glutaraldehyde in 0.1 M citrate buffer. Temperature and pH were chosen according to growth conditions, as described previously (Kärgel et al., 1996; Zimmer et al., 1997). Cells were washed with PBS, and then 1% sodium metaperiodate was added for 1 h to prepare the cell wall for the penetration of the cryoprotectant. The hydrated specimens were immersed in a cryoprotectant mixture of 25% polyvinyl-pyrrolidone (PVP, K15/MW 10000; Fluka, Buchs, Switzerland) and 1.6 M sucrose, as described in Tokuyasu (1989). To reach complete immersion of the cryoprotectant, incubation at 30°C for 2–3 h was strictly necessary. The cells were subsequently mounted on specimen holders, frozen in liquid nitrogen, and sectioned at −115°C with an ultracryotom Ultracut S attached with a FCS unit (Leica, Heerbrugg, Switzerland). Ultra-thin, thawed cryosections were prepared with glass knifes and placed on formvar/carbon-coated copper grids (200 mesh, hexagonal).
Labeling with primary antibodies and immunogold-complexes (12 nm) was performed according to Griffiths (1993). Finally, the frozen-thawed sections were stained and stabilized using a mixture of freshly prepared 3% tungstosilicic acid hydrate (STA; Fluka) and 2.5% polyvinylalcohol (Mr 10,000; Sigma, Deisenhofen, Germany).
CPY* Secretion
Cells were grown to late log phase and then sedimented by centrifugation at 500 × g for 5 min. Cells were washed once with ice-cold water and centrifuged again, and the supernatants from both centrifugation steps were combined. Proteins were precipitated with trichloroacetic acid (10%) for 30 min on ice and sedimented for 15 min at 12,000 × g. The pellet was washed twice with ice-cold acetone and dissolved in 100 μl urea buffer (5% SDS, 8 M urea, 200 mM Tris-HCl, pH 6.8, 0.1 mM EDTA 1.5% dithiothreithol, 0.03% bromophenol blue). To obtain the intracellular fraction of CPY*, an amount of cells corresponding to 2 OD600 were alkaline lysed (Hiller et al., 1996), and the samples were subjected to SDS-PAGE and immunodetection as described previously (Knop et al., 1996a).
Cycloheximide Chase
Cells were grown to log phase at 25°C and incubated for 1 h at 37°C. Cycloheximide was added (0.25 mg/ml), and 2 OD of cells were taken after 0 and 60 min of chase. Cell extracts were prepared by alkaline lysis method (Hiller et al., 1996), and the samples were subjected to SDS-PAGE followed by immunodetection (Knop et al., 1996a).
Antisera
Polyclonal anti-CPY and polyclonal anti-PrA sera were described previously (Finger et al., 1993); anti-Emp47p serum was a generous gift of H. Riezman (Schröder et al., 1995). Monoclonal anti-CPY and anti-PGK sera were purchased from Molecular Probes (Eugene, OR), HRPO-conjugated anti-mouse antibody from Sigma, monoclonal anti-HA antibody from Babco (HA.11; Berkley, CA), and anti-mouse immunogold-complexes (12 nm) from Dianova (Hamburg, Germany).
RESULTS
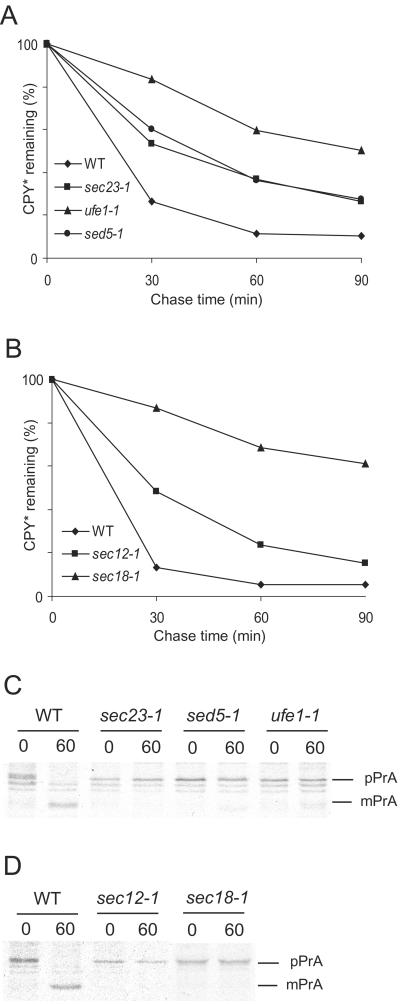
We analyzed the degradation of CPY* in mutants defective in anterograde transport between the ER and Golgi. We used the mutant alleles sec12-1, sec23-1, ufe1-1, sed5-1, and sec18-1, which block vesicular transport at restrictive conditions (Stevens et al., 1982; Hardwick and Pelham, 1992; Lewis and Pelham, 1996). Sec12p, the nucleotide-exchange factor, which recruits Sar1p to the ER membrane (Campbell and Schekman, 1997), and Sec23p (Kuehn et al., 1998; Springer and Schekman, 1998) are both necessary for COPII-coated vesicle formation. Ufe1p (Lewis et al., 1997; Patel et al., 1998) and Sed5p (Banfield et al., 1995; Wooding and Pelham, 1998; Tsui and Banfield, 2000) are t-SNAREs of the ER and Golgi, respectively. They are involved in the fusion of vesicles with the target membrane. Sec18p, the yeast homologue of the mammalian NSF, is required for vesicular transport in multiple stages of the secretory pathway (Graham and Emr, 1991; Mayer et al., 1996). A defect in any of these proteins leads to a considerably reduced degradation rate of CPY* (Figure 1, A and B). The half-life of CPY* increases ∼2- (sec12-1, sec23-1, and sed5-1) to 6- (ufe1-1) fold in mutant cells compared with wild type. We had previously analyzed the function of Sec18p in the degradation of CPY* and PrA*, a rapidly degraded mutant form of proteinase yscA (Finger et al., 1993). Because the data had not been quantified, we had concluded that both misfolded proteins were degraded under restrictive conditions in sec18-1 cells. Reinvestigation and quantification of CPY* degradation in these sec18-1 mutant cells, however, revealed a 6- to 7-fold increase in the half-life of CPY* (Figure 1B). The block of anterograde transport between ER and Golgi was confirmed by monitoring the maturation of proteinase yscA (PrA) in the mutant strains at restrictive conditions. In case of the ufe1-1 strain, a tiny fraction of matured PrA was visible after 60 min of chase; all the other mutants retained PrA in the proform (Figure 1, C and D). The degradation of CPY* observed in the sec12-1 mutant cells might be due to the action of a close homologue, Sed4p, which is also involved in the generation of COPII-coated vesicles at the ER membrane (Gimeno et al., 1995). We tested if a deletion of SED4 influences the degradation rate of CPY*, either as a single knockout or in conjunction with the sec12-1 mutation. In both cases there was no detectable change in the half-life of CPY* (our unpublished results).
Figure 1.
CPY* degradation is impaired in mutants defective in ER-to-Golgi transport. Pulse-chase analysis was performed to measure CPY* degradation and maturation of PrA in wild-type and isogenic mutant strains. Cells were shifted to restrictive temperature 5 min before the chase and lysed at the indicated time points. CPY* or PrA were immunoprecipitated and separated by SDS-PAGE. Quantification was done using a PhosphorImager. (A) Formation of COPII-coated vesicles (Sec23p) and ER and Golgi t-SNARES (Ufe1p and Sed 5p) are necessary for efficient degradation of CPY*. (B) CPY* degradation requires a functional Sar1p activating factor (Sec12p) and yeast NSF (Sec18p). (C and D) Transport is blocked in the mutant strains as evidenced by the maturation defect of PrA (pPrA, p1 PrA precursor of the ER; mPrA, mature PrA of the vacuole).
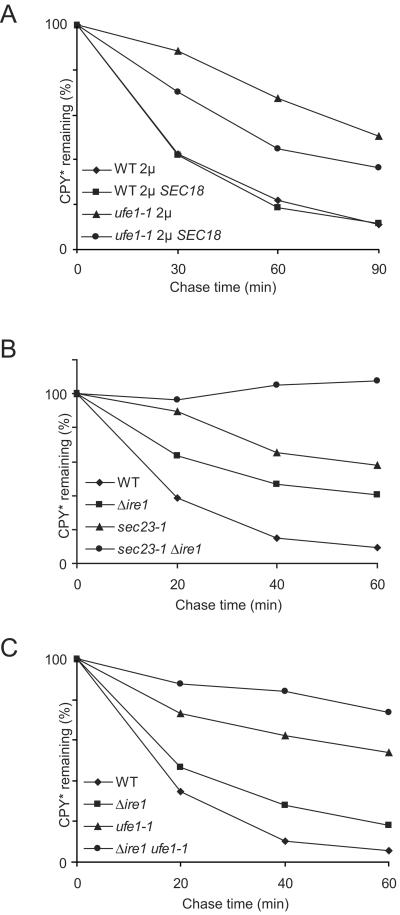
Ufe1p is known to function in two different membrane fusion events: it is involved in the homotypic fusion of ER membranes and in the heterotypic fusion of COPI-coated vesicles with the ER membrane. To distinguish between the two different fusion events, we overexpressed the AAA-ATPases Cdc48p and Sec18p in temperature-sensitive ufe1-1 mutant cells. CDC48p is involved in homotypic membrane fusion, whereas Sec18p is the homologue used in heterotypic fusion of COPI vesicles with the ER membrane (Lewis et al., 1997; Patel et al., 1998). Overexpression of Cdc48p rescued the temperature sensitivity of the ufe1-1 mutant (Patel et al., 1998) but was unable to enhance the degradation of CPY* (our unpublished results). Overexpression of Sec18p, on the other hand, did not rescue the temperature sensitivity of the ufe1-1 mutant (our unpublished results) but, interestingly, was able to partially overcome the ufe1-1–mediated degradation defect of CPY* in cells under restrictive conditions (Figure 2A).
Figure 2.
(A) Over expression of Sec18p partially suppresses the CPY* degradation defect of ufe1-1 mutant cells. Vector (pRS426) or SEC18 on a high copy plasmid (pWO152) were transformed into wild-type (WT) and ufe1-1 mutant cells. Pulse-chase analysis was done as described in Figure 1. (B and C) Synergistic effect of ER-Golgi transport mutants and the unfolded protein response on degradation of CPY*. Pulse-chase analysis was done as described in Figure 1.
As a control, we tested if Sec1p, a protein involved in docking of secretory transport vesicles to the plasma membrane (Novick and Schekman, 1979; Aalto et al., 1997), is involved in ER degradation. As expected, a temperature-sensitive mutant, sec1-1, did not alter the degradation of CPY* under restrictive conditions compared with wild type (our unpublished results). This rules out any unspecific influence of defects connected to various sec mutant alleles on ER degradation (Mizuta and Warner, 1994).
Using the DNA microarray technique, Travers and coworkers could show that transcription of many genes involved in ER-Golgi transport is upregulated upon stress in the ER. Transcription of Sec23p is enhanced by induction of the UPR, whereas transcription of Ufe1p is not (Travers et al., 2000). Previous studies did not find an alteration in CPY* degradation in Δire1 cells under nonstress conditions at 30°C (Friedländer et al., 2000). However, we observed that the degradation rate of CPY* is prolonged in Δire1 cells at 37°C. To examine whether there is a synergistic effect between UPR and ER-Golgi transport, we combined the Δire1 mutation with mutations in Sec23p and Ufe1p. Indeed, double mutants of Δire1 with either ufe1-1 or sec23-1 resulted in nearly complete arrest of CPY* degradation under restrictive conditions (Figure 2, B and C).
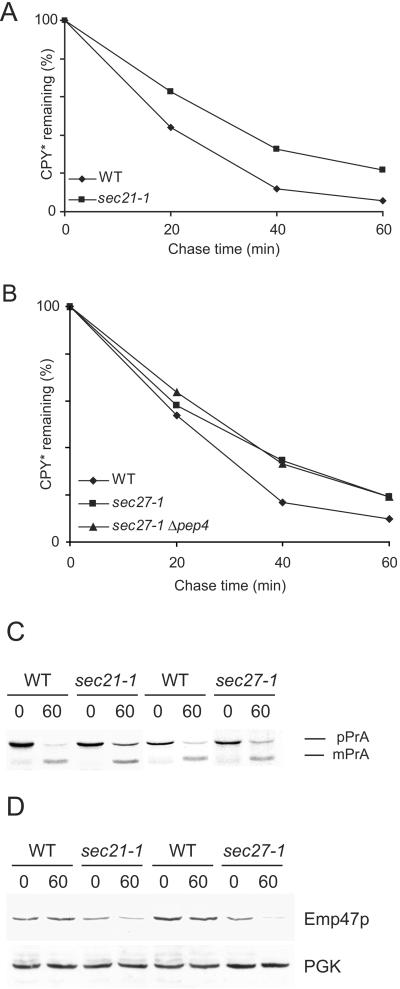
Consequently, we tested if a block in retrograde transport from the Golgi to the ER would influence the degradation rate of CPY* as much as a block in anterograde transport does. We measured the degradation of CPY* in yeast cells defective in Sec21p or Sec27p, two components of the COPI complex (Hosobuchi et al., 1992; Duden et al., 1994; Letourneur et al., 1994). As shown in Figure 3, A and B, degradation of CPY* is only moderately affected in sec21-1 or sec27-1 cells at restrictive conditions. It is known that proteins, which cannot be retrograde transported from the Golgi to the ER, may travel to the vacuole for degradation. Therefore, we combined a mutation in Sec27p with a deletion of the PEP4 gene, leading to a proteolysis defective vacuole (Knop et al., 1993; Van Den Hazel et al., 1996), to check if vacuolar degradation contributes to the decay of CPY* in this COPI mutant. We found that degradation of CPY* was not altered in the double mutant (Figure 3B), whereas a pep4 single mutant degrades CPY* like a wild-type strain (our unpublished results and Figure 5B). Anterograde transport is partially affected in sec21-1 and to a lesser degree in sec27-1 cells (Letourneur et al., 1994). We measured the maturation process of PrA, applying the conditions for the CPY* degradation experiment, and found that in fact PrA is not fully matured in the mutant cells after 60 min of chase (Figure 3C). In wild-type cells 80% of PrA is matured after 60 min of chase, whereas in sec21-1 cells only 64% and in sec27-1 cells 74% is in the mature form. Retrograde transport is defective in these two mutants at 37°C. It is known that Emp47p is degraded in the vacuole, when its recycling via COPI-coated vesicles is inhibited (Lewis and Pelham, 1996; Schröder-Köhne et al., 1998). We, therefore, tested the stability of Emp47p in sec21-1 and sec27-1 cells and found that Emp47p is degraded rapidly in the mutants, but it is stable in the respective wild-type cells at restrictive conditions (Figure 3D). This shows that even although retrograde transport is defective in these cells, CPY* is degraded efficiently.
Figure 3.
CPY* degradation is affected only moderately in cells defective in COPI-coated vesicle formation at restrictive temperature. Pulse-chase analysis was performed as described in Figure 1, except that the cells were shifted to restrictive temperature 60 min before the chase. (A) CPY* degradation in isogenic wild-type and sec21-1 mutant strain. (B) CPY* degradation in isogenic wild-type, sec27-1, and sec27-1 Δpep4 mutant strains. (C) Maturation of PrA was measured in a pulse-chase experiment in COPI mutant and isogenic wild-type strains. The experiment was done as described for Figure 3A, except that the cells were labeled with radioactive methionine for 10 min. (D) COPI-coated vesicle transport is affected in the mutant strains under the conditions used. Emp47p degradation was followed in a cycloheximide chase experiment after a 60-min preincubation at 37°C. Equal amounts of cells were taken at the indicated time points. Pgk1p served as a loading control.
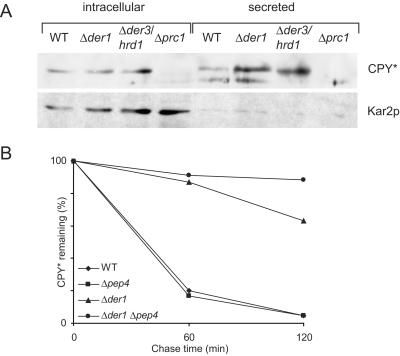
Figure 5.
(A) CPY* is secreted into the medium. Cellular and secreted CPY* was analyzed by SDS-PAGE and immunodetection. The same blot was reprobed against Kar2p. Approximately five times more material was loaded in the extracellular fraction. (B) Vacuolar hydrolysis contributes to degradation of CPY* in Δder1 cells. Degradation of CPY* was measured by pulse-chase analysis in yeast strains defective in vacuolar hydrolysis (Δpep4), ER degradation (Δder1), or double mutants (Δpep4 Δder1).
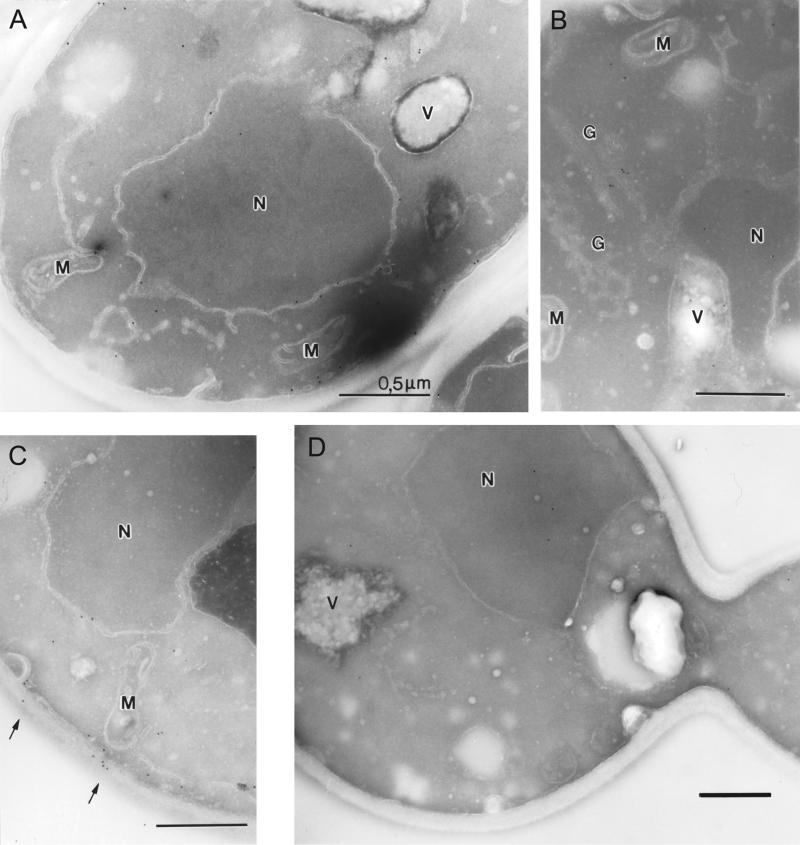
One of the possible models that would explain these results is the existence of a special ER-derived compartment for degradation, as proposed recently for mammalian cells by Kamhi-Nesher et al. (2001). To test this model, we analyzed the localization of CPY* by electron microscopy and immuno-gold visualization of an HA-tagged version of CPY*. CPY*-HA is degraded in the same way and with similar kinetics as nontagged CPY* (our unpublished results). CPY*-HA is localized to the ER/nuclear envelope and to the peripheral ER, whereas the later compartments of the secretory pathway are almost free of label in wild-type cells (Figure 4A). A yeast strain deleted in Der1p, which is defective in the degradation of CPY* (Knop et al., 1996a), was used to analyze whether CPY*-HA is accumulating in a novel compartment. In Δder1 cells, as in wild-type, most of the CPY*-HA was found at the ER/nuclear envelope and the peripheral ER. Additionally, CPY*-HA was found in the Golgi apparatus and the vacuole, and a fraction was secreted outside the cell (Figure 4, B and C). This finding complements the results of Knop et al., 1996a, who showed Golgi glycosylation of CPY* in Der1p mutant cells. In control cells, not expressing CPY*-HA, few gold particles can typically be found inside the nucleus and in the cytosol but not in any membraneous structures (Figure 4D).
Figure 4.
Localization of CPY*-HA by immuno-EM. Ultra-thin cryosections of wild-type (A) and Der1p-deleted cells (B and C) expressing HA-tagged CPY* or wild-type cells without tag (D) were labeled with antibodies against HA and anti-mouse-gold complexes (12 nm). Yeast strains in A, B, and C were transformed with plasmid pCT52, carrying CPY*-HA under the control of the CUP1 promoter. The expression of CPY*-HA was induced with addition of copper sulfate (100 μM final concentration) for 3 h. (A) CPY*-HA is localized to the ER in wild-type cells. (B) CPY*-HA is distributed in structures of the secretory system in Δder1 cells. (C) Δder1 cells secrete CPY*-HA to the outside of the cell. (D) Unspecific staining of the used antibodies in wild-type cells. N, nucleus; M, mitochondrion; V, vacuole; G, Golgi elements. Bars, 500 nm.
The localization of CPY* was also analyzed by Western blotting, which confirmed the EM data: in wild-type cells a minor fraction of CPY* was secreted into the medium, whereas in Δder1 and Δder3/hrd1 cells more CPY* appeared in the medium (Figure 5A). Additionally, the amount of secreted CPY* was higher than that of Kar2p in these cells, as evidenced by a very faint Kar2p signal in the extracellular fraction (Figure 5A). It is known that Kar2p is secreted when the HDEL retrieval pathway is saturated (Belden and Barlowe, 2001). To analyze the fate of CPY* in the vacuole, we performed pulse-chase analysis in cells with impaired vacuolar degradation due to deletion of the PEP4 gene (Knop et al., 1993; Van Den Hazel et al., 1996). In Δpep4 cells CPY* was degraded as in wild-type cells; however, in Δder1 Δpep4 double mutants the degradation was slower than in Δder1 single mutants (Figure 5B).
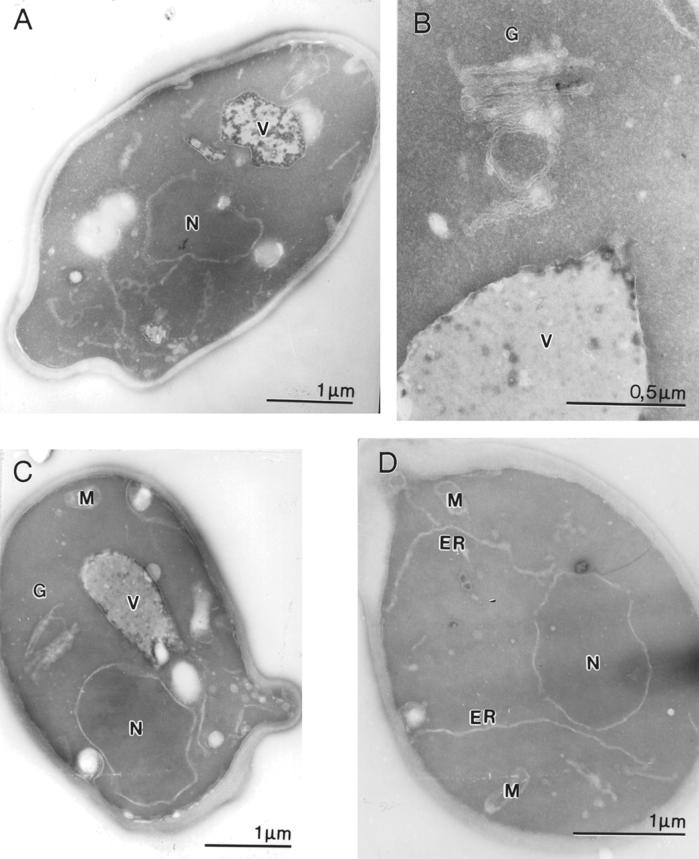
The EM images revealed that in cells deficient in ER degradation some interesting morphological changes are visible. In these cells (Δder1, Δder3/hrd1, and Δhrd3) the Golgi apparatus appears as stacked cisternae in many cells (Figure 6, B and C, and our unpublished results), whereas a typical wild-type cell contains disconnected Golgi structures (Figure 6A). Stacked Golgi structures were previously observed in yeast mutant cells defective in intra-Golgi transport, such as in sec7-1 cells at nonpermissive conditions (Rambourg et al., 1993) but rarely in wild-type cells (Rambourg et al., 1995). Mutants defective in ER degradation (Δder1, Δder3/hrd1, and Δhrd3) also exhibit a considerably proliferated ER (Figure 6D and our unpublished results). These morphological changes are absent in cells where ER degradation is abolished in conjunction with the unfolded protein response. In Δder1 Δire1 cells the ER does not proliferate and the Golgi apparatus has normal appearance (our unpublished results).
Figure 6.
Morphology of secretory organelles is changed in mutants defective in ER degradation. (A) Wild-type. (B) Golgi structure in Δder1 cells. (C) Golgi structures present in Der3/Hrd1p-deficient cells. (D) ER proliferates in Δder3/hrd1 cells. N, nucleus; M, mitochondrion; V, vacuole; ER, endoplasmic reticulum. Bars, 1000 and 500 nm, respectively.
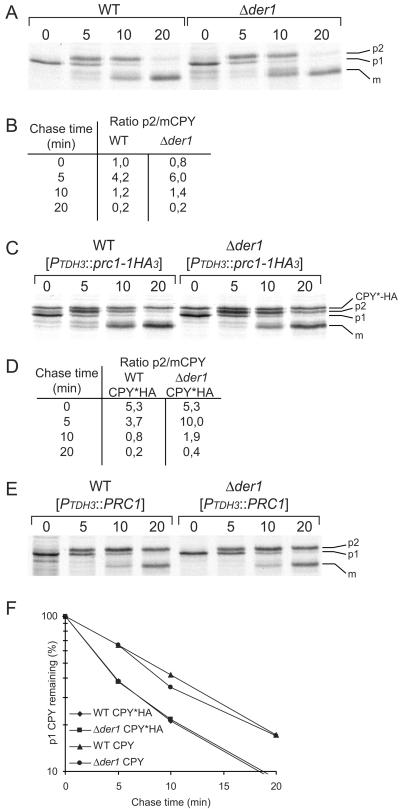
These observations raised the question of whether the morphological changes in the ER and Golgi influence the secretory function of these organelles. To address this question, we measured the maturation kinetics of wild-type CPY in wild-type and Δder1 mutant cells. Distinct forms of CPY reflect the transport of this enzyme from the ER via the Golgi to the vacuole. The core glycosylated pro-CPY precursor in the ER has a molecular mass of ∼67 kDa (p1CPY). In the Golgi, the carbohydrate of CPY is modified, resulting in the p2CPY form of 69 kDa. After transport to the vacuole, CPY is matured (mCPY) to a final form of 61 kDa (Rendueles and Wolf, 1988). Figure 7, A and B, shows that the transport of wild-type CPY is not delayed to a significant degree in cells lacking Der1p. The calculated ratio between Golgi p2CPY and vacuolar mCPY species is almost the same in wild-type and Δder1 mutant cells at the different time points (Figure 7B). However, when CPY*-HA is expressed simultaneously with wild-type CPY in Der1p-deficient cells, the maturation of CPY from the p2CPY Golgi form to the mature vacuolar form is considerably delayed (Figure 7C). The ratio between p2 and mCPY is higher in Δder1 than in wild-type cells when CPY*-HA is coexpressed, indicating a slower transport of the p2CPY form (Figure 7D). Surprisingly, the exit of pro-CPY from the ER seems to be undisturbed: there is no difference in the rate of disappearance of p1CPY between wild-type and Δder1 cells expressing CPY*-HA (Figure 7F), and it is also the same as that observed in wild-type cells not coexpressing CPY or CPY*-HA (our unpublished results). As a control, we expressed CPY exogenously in wild-type and Δder1 cells and followed the maturation of CPY. Under these conditions, every step of the process was delayed in both strains, starting with a slower exit from the ER (Figure 7, E and F). Taken together, the EM data and the CPY maturation experiments suggest that escape of unfolded proteins from the ER disturbs the secretory competence of the later stages of the secretory pathway.
Figure 7.
Maturation of CPY is prolonged, upon presence of unfolded proteins in the secretory pathway. Cells expressing CPY were labeled for 5 min with radioactive methionine. Cells were lysed at the indicated time points and treated as described in Figure 1. The positions of p1 (ER), p2 (Golgi), m (vacuolar) CPY and CPY*-HA are shown. (A) Maturation of CPY is not dependent on the presence of Der1p. (B) Calculated ratio between p2 and mCPY in wild-type and Δder1 cells. (C) Expression of CPY*-HA leads to delay in maturation of CPY in Δder1 cells. Plasmid pCT43, carrying CPY*-HA under the control of the TDH3 promoter, was introduced into wild-type and Δder1 cells. (D) Calculated ratio between p2 and mCPY in wild-type and Δder1 cells expressing additional CPY*-HA. (E) Expression of additional CPY from the TDH3 promoter leads to delayed maturation of CPY in wild-type and Δder1 cells. (F) Conversion of the p1-form to the p2 Golgi form of CPY in wild-type and Δder1 cells expressing additional CPY or CPY*-HA, respectively.
DISCUSSION
Using mutants defective in ER-to-Golgi traffic, we discovered a connection between the secretory competence of the ER itself and the degradation of the ERAD substrate CPY*. Mutants defective in Ufe1p, Sec12p, Sec23p, Sed5p, and Sec18p exhibited an extended half-life of CPY* (Figure 1). After completion of our studies, reports appeared that also communicate disturbed degradation of soluble ER degradation substrates in mutants defective in ER-Golgi transport (Caldwell et al., 2001; Vashist et al., 2001). The authors give two explanations for the involvement of ER-to-Golgi transport in ER degradation: (i) Soluble ER substrates may travel to the Golgi and back to the ER, either to receive a modification that enhances degradation (Caldwell et al., 2001) or as the default route to degradation (Vashist et al., 2001). (ii) Alternatively, an yet unidentified factor, which is required for the degradation of soluble substrates, cycles between ER and Golgi. In this model, the substrate itself remains in the ER (Caldwell et al., 2001).
Our results lead to different conclusions: in the sec12-1, sec18-1, and sec23-1 mutants, ER-to-Golgi transport is blocked completely as evidenced by the lack of the Golgi localized p2CPY species (Stevens et al., 1982) or by the maturation defect of PrA (Figure 1, C and D). In contrast, degradation of CPY* is not completely blocked in these mutants (Figure 1, A and B). Instead, degradation of CPY* takes place at different rates in the various ER-Golgi trafficking mutants tested; the delay in degradation ranges between a factor of 2 (sec12-1, sec23-1, and sed5-1 mutant strains) and 6–7 (sec18-1 and ufe1-1 mutants). Deletion of Sed4p, a close homologue of Sec12p, in the presence of the sec12-1 mutation, did not enhance the half-life of CPY*. Because ER-to-Golgi traffic is blocked in these mutants, it is hard to envisage how some CPY* could still reach the Golgi to receive a specific modification or why the degradation route would be changed in sec12-1, sec23-1, or sed5-1 but not in sec18-1 or ufe1-1 cells. In COPI mutant cells (sec21-1 and sec27-1), only a moderate alteration in the degradation of CPY* compared with wild-type is visible (Figure 3, A and B), despite the fact that retrograde traffic from the Golgi to the ER is defective (Figure 3D and Letourneur et al., 1994). If CPY* would travel to the Golgi and back to the ER before degradation, one would expect a much larger influence of COPI-coat mutants on degradation of CPY*. Because the half-life of CPY* is not increased in sec27-1 Δpep4 double-mutant cells, we conclude that the vacuole does not contribute to the degradation seen in sec27-1 cells (Figure 3B). All together, the transport defects of the various mutants cannot be correlated with the degradation pattern of CPY*. These findings, therefore, argue against the idea of a relocation of CPY* to the Golgi and then back to the ER or of the recycling of a proteinaceous factor between these compartments for efficient degradation of CPY*. Only a yet-unknown Golgi-ER retrieval mechanism could possibly apply for a cycling-dependent degradation mechanism. Another recent publication reports that mutations in the early secretory pathway severely affect the structure of the ER (Prinz et al., 2000). They show that ufe1-1 or sec23-1 mutant cells, two mutants also used in our studies, exhibit a dramatically reduced amount of peripheral ER and a considerably disorganized organelle at nonpermissive temperature (Prinz et al., 2000). We find that under the same conditions degradation of CPY* is prolonged in these mutants (Figure 1A). Another possible explanation is that the mislocalization of Kar2p observed in various ER-Golgi transport mutants (Nishikawa et al., 1994) leads to defective degradation. Kar2p is necessary for degradation of CPY* (Plemper et al., 1997) but is not involved in the degradation of membrane proteins (Plemper et al., 1998; Kiser et al., 2001; Zhang et al., 2001). This model would explain why misfolded membrane proteins are degraded efficiently in the ER-Golgi transport mutants (Biederer et al., 1996; Katzmann et al., 1999; Vashist et al., 2001), whereas CPY* is not. Taken together, we conclude that the morphological disturbance of the ER, mislocalization of Kar2p, or both, are the cause of the changes in CPY* degradation. Analysis of the localization of HA-tagged CPY* via immuno-gold EM revealed that the protein resides at the ER/nuclear envelope and the peripheral ER. These EM images do not indicate the existence of a novel compartment, specialized in ER degradation (Figure 4, A–C), as recently proposed for mammalian cells (Kamhi-Nesher et al., 2001). In the light of the EM data, the degradation behavior of CPY* in the ER-Golgi transport mutants as well as the data of Prinz et al. (2000) and Nishikawa et al. (1994), we suggest that the decrease in degradation of CPY* is due to indirect or secondary effects caused by the mutations that lead to impaired ER-Golgi transport. The severe alteration in CPY* degradation observed in double mutants defective in Sec23p or Ufe1p and UPR signaling due to deletion of Ire1p (Figure 2, B and C), can be explained similarly: the amount of ER is diminished, chaperones like Kar2p are mislocalized, and the possibility to increase expression of proteins involved in ER-stress relieve is abolished. Recovery of CPY* degradation upon overexpression of Sec18p in ufe1-1 mutants indicates that the involvement of Ufe1p in vesicular transport is necessary to maintain proper CPY* degradation and that this is independent of its involvement in homotypic membrane fusion exerted together with Cdc48p (Figure 2A). Recent studies show that Cdc48p does indeed take part in CPY* degradation, being involved in retro-translocation of the malfolded protein into the cytosol (Ye et al., 2001; Bays et al., 2001b; Rabinovitch et al., 2002; Jarosch et al., 2002).
The ER is considerably enlarged in Δder1, Δder3/hrd1, and Δhrd3 cells (Figure 6D and our unpublished results). This alteration is most likely controlled by the UPR, because many genes involved in lipid metabolism are also upregulated upon ER stress (Travers et al., 2000). As expected, a Δder1 Δire1 double knockout strain has no proliferated ER (our unpublished results). The appearance of Golgi stacks could indicate a defect in intra-Golgi transport in the mutants deficient in ER degradation, which is less severe than the one seen in the sec7-1 mutant at restrictive conditions but significant enough to change the morphology of the organelle. On disruption of CPY* degradation by deletion of Der1p, we find some CPY*-HA in the Golgi apparatus, in the vacuole and in secreted form (Figure 4, B and C). This indicates a “leakage” of CPY*-HA out of the ER under these conditions. In addition, in a strain deleted for Der1p and Pep4p, CPY* has a longer half-life than in a strain lacking only Der1p, indicating transport of a fraction of CPY* into the vacuole. This finding complements the observation of Knop et al. (1996a), who reported some Golgi glycosylation of CPY* in Δder1 cells. Soluble, misfolded proteins can escape from the ER when their degradation is abolished. The reason is probably the saturation of the retrieval pathway from the Golgi back to the ER when too many unfolded proteins are present in the ER. It is known that the HDEL receptor takes part in the retention of unfolded proteins in the ER by recycling them back from the Golgi (Knop et al., 1996a; Yamamoto et al., 2001). Unfolded proteins that escape the retention machinery travel along the secretory pathway to the vacuole or are secreted (Figures 4, B and C, and 5). This seems to disturb the secretory pathway in a yet unknown way, as delivery of wild-type CPY from the Golgi to the vacuole is delayed in Δder1 cells simultaneously expressing malfolded CPY*-HA (Figure 7). This delay can be explained by a defect in transport through the Golgi due to the presence of misfolded proteins or, alternatively, by a competition between CPY and CPY*-HA for the CPY sorting receptor, Vps10p (Stack et al., 1995). Perturbations in vacuolar function may also explain this phenomenon. The maturation defect observed in Δder1 cells coexpressing CPY*-HA is not simply a consequence of overloading in the secretory pathway. Overloading through expression of additional CPY results in slower transport in every step of the secretory pathway, e.g., the half-life in exit from the ER is roughly doubled (Figure 7F). In Δder1 cells expressing CPY*-HA only the later transport or maturation steps are affected, which seems to be a consequence of unfolded proteins present in the secretory pathway (Figure 7, C and D). The data presented here indicate that efficient ER degradation requires an ER fully competent in secretion and, vice versa, that efficient secretion depends on an undisturbed quality control machinery in the ER.
ACKNOWLEDGMENTS
The authors thank M. Vogel for the preparation of the cryosections, R. Hitt, J. Strayle, H. Rudolph, H. Pelham, T. Sommer, H. Riezman, and C. Barlowe for antibodies, plasmids, and strains. We are grateful to Z. Kostova, R. Hitt, J. Strayle, S. Jäger, and the members of the Sommer lab for helpful discussions and Elisabeth Tosta for help with the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft, Bonn; the German-Israeli Project Cooperation (DIP) of the Bundesministerium für Bildung und Forschung (BMBF); and the Fonds der Chemischen Industrie, Frankfurt.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–08–0399. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–08–0399.
REFERENCES
- Aalto MK, Jantti J, Ostling J, Keranen S, Ronne H. Mso1p: a yeast protein that functions in secretion and interacts physically and genetically with Sec1p. Proc Natl Acad Sci USA. 1997;94:7331–7336. doi: 10.1073/pnas.94.14.7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan BB, Balch WE. Protein sorting by directed maturation of Golgi compartments. Science. 1999;285:63–66. doi: 10.1126/science.285.5424.63. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Kingston RE, Seidman FG, Struhl K, Moore DD, Brent R, Smith FA. Current protocols in molecular biology. New York: Greene; 1992. [Google Scholar]
- Banfield DK, Lewis MJ, Pelham HR. A SNARE-like protein required for traffic through the Golgi complex. Nature. 1995;375:806–809. doi: 10.1038/375806a0. [DOI] [PubMed] [Google Scholar]
- Bays NW, Gardner RG, Seelig LP, Joazeiro CA, Hampton RY. Hrd1p/Der3p is a membrane-anchored ubiquitin ligase required for ER- associated degradation. Nat Cell Biol. 2001a;3:24–29. doi: 10.1038/35050524. [DOI] [PubMed] [Google Scholar]
- Bays NW, Wilhovsky SK, Goradia A, Hodgkiss-Harlow K, Hampton RY. HRD4/NPL4 is required for the proteasomal processing of ubiquitinated ER proteins. Mol Biol Cell. 2001b;12:4114–4128. doi: 10.1091/mbc.12.12.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden WJ, Barlowe C. Deletion of yeast p24 genes activates the unfolded protein response. Mol Biol Cell. 2001;12:957–969. doi: 10.1091/mbc.12.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer T, Volkwein C, Sommer T. Degradation of subunits of the Sec61p complex, an integral component of the ER membrane, by the ubiquitin-proteasome pathway. EMBO J. 1996;15:2069–2076. [PMC free article] [PubMed] [Google Scholar]
- Botstein D, Falco SC, Stewart SE, Brennan M, Scherer S, Stinchcomb DT, Struhl K, Davis RW. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979;8:17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Brodsky JL, McCracken AA. ER protein quality control and proteasome-mediated protein degradation. Semin Cell Dev Biol. 1999;10:507–513. doi: 10.1006/scdb.1999.0321. [DOI] [PubMed] [Google Scholar]
- Brodsky JL, Werner ED, Dubas ME, Goeckeler JL, Kruse KB, McCracken AA. The requirement for molecular chaperones during endoplasmic reticulum-associated protein degradation demonstrates that protein export and import are mechanistically distinct. J Biol Chem. 1999;274:3453–3460. doi: 10.1074/jbc.274.6.3453. [DOI] [PubMed] [Google Scholar]
- Caldwell SR, Hill KJ, Cooper AA. Degradation of endoplasmic reticulum (ER) quality control substrates requires transport between the ER and Golgi. J Biol Chem. 2001;276:23296–23303. doi: 10.1074/jbc.M102962200. [DOI] [PubMed] [Google Scholar]
- Campbell JL, Schekman R. Selective packaging of cargo molecules into endoplasmic reticulum-derived COPII vesicles. Proc Natl Acad Sci USA. 1997;94:837–842. doi: 10.1073/pnas.94.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Ballew N, Barlowe C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 1998;17:2156–2165. doi: 10.1093/emboj/17.8.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande R, Stern P, Diehn M, Shamu C, Osario M, Zuniga M, Brown PO, Ploegh H. Degradation of proteins from the ER of S. cerevisiae requires an intact unfolded protein response pathway. Mol Cell. 2000;5:729–735. doi: 10.1016/s1097-2765(00)80251-8. [DOI] [PubMed] [Google Scholar]
- Chapman R, Sidrauski C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus. Annu Rev Cell Dev Biol. 1998;14:459–485. doi: 10.1146/annurev.cellbio.14.1.459. [DOI] [PubMed] [Google Scholar]
- Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Deak PM, Wolf DH. Membrane topology and function of Der3/Hrd1p as a ubiquitin-protein ligase (E3) involved in endoplasmic reticulum degradation. J Biol Chem. 2001;276:10663–10669. doi: 10.1074/jbc.M008608200. [DOI] [PubMed] [Google Scholar]
- Duden R, Hosobuchi M, Hamamoto S, Winey M, Byers B, Schekman R. Yeast beta- and beta′-coat proteins (COP). Two coatomer subunits essential for endoplasmic reticulum-to-Golgi protein traffic. J Biol Chem. 1994;269:24486–24495. [PubMed] [Google Scholar]
- Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999;286:1882–1888. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- Finger A, Knop M, Wolf DH. Analysis of two mutated vacuolar proteins reveals a degradation pathway in the endoplasmic reticulum or a related compartment of yeast. Eur J Biochem. 1993;218:565–574. doi: 10.1111/j.1432-1033.1993.tb18410.x. [DOI] [PubMed] [Google Scholar]
- Friedländer R, Jarosch E, Urban J, Volkwein C, Sommer T. A regulatory link between ER-associated protein degradation and the unfolded-protein response. Nat Cell Biol. 2000;2:379–384. doi: 10.1038/35017001. [DOI] [PubMed] [Google Scholar]
- Gardner RG, Swarbrick GM, Bays NW, Cronin SR, Wilhovsky S, Seelig L, Kim C, Hampton RY. Endoplasmic reticulum degradation requires lumen to cytosol signaling. Transmembrane control of Hrd1p by Hrd3p. J Cell Biol. 2000;151:69–82. doi: 10.1083/jcb.151.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillece P, Luz J M, Lennarz W J, de La Cruz F J, Römisch K. Export of a cysteine-free misfolded secretory protein from the endoplasmic reticulum for degradation requires interaction with protein disulfide isomerase. J Cell Biol. 1999;147:1443–1456. doi: 10.1083/jcb.147.7.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno RE, Espenshade P, Kaiser CA. SED4 encodes a yeast endoplasmic reticulum protein that binds Sec16p and participates in vesicle formation. J Cell Biol. 1995;131:325–338. doi: 10.1083/jcb.131.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TR, Emr SD. Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 (NSF) mutant. J Cell Biol. 1991;114:207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G. Labeling reactions for immunocytochemistry. In: Griffiths G, editor. Fine structure immunocytochemistry. Berlin, Germany: Springer; 1993. pp. 237–275. [Google Scholar]
- Guthrie C, Fink G R. Guide to yeast genetics and molecular biology. Vol. 194. New York: Academic Press; 1991. [PubMed] [Google Scholar]
- Hardwick KG, Lewis MJ, Semenza J, Dean N, Pelham HR. ERD1, a yeast gene required for the retention of luminal endoplasmic reticulum proteins, affects glycoprotein processing in the Golgi apparatus. EMBO J. 1990;9:623–630. doi: 10.1002/j.1460-2075.1990.tb08154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick KG, Pelham HR. SED5 encodes a 39-kD integral membrane protein required for vesicular transport between the ER and the Golgi complex. J Cell Biol. 1992;119:513–521. doi: 10.1083/jcb.119.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller MM, Finger A, Schweiger M, Wolf DH. ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science. 1996;273:1725–1728. doi: 10.1126/science.273.5282.1725. [DOI] [PubMed] [Google Scholar]
- Hilt W, Wolf DH. In: Proteasomes. The world of regulatory proteolysis. Hilt W, Wolf D H, editors. Austin: Landes Bioscience (Georgetown/Eurekah.com); 2000. pp. 1–387. [Google Scholar]
- Hosobuchi M, Kreis T, Schekman R. SEC21 is a gene required for ER-to-Golgi protein transport that encodes a subunit of a yeast coatomer. Nature. 1992;360:603–605. doi: 10.1038/360603a0. [DOI] [PubMed] [Google Scholar]
- Jakob CA, Bodmer D, Spirig U, Battig P, Marcil A, Dignard D, Bergeron JJ, Thomas DY, Aebi M. Htm1p, a mannosidase-like protein, is involved in glycoprotein degradation in yeast. EMBO Rep. 2001;2:423–430. doi: 10.1093/embo-reports/kve089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosch E, Taxis C, Volkwein C, Bordallo J, Finley D, Wolf D H, Sommer T. Protein dislocation from the ER requires polyubiquitination, and the AAA-ATPase Cdc48. Nat Cell Biol. 2002;4:134–139. doi: 10.1038/ncb746. [DOI] [PubMed] [Google Scholar]
- Kaiser CA, Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Kamhi-Nesher S, Shenkman M, Tolchinsky S, Fromm SV, Ehrlich R, Lederkremer GZ. A novel quality control compartment derived from the endoplasmic reticulum. Mol Biol Cell. 2001;12:1711–1723. doi: 10.1091/mbc.12.6.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärgel E, Menzel R, Honeck H, Vogel F, Bohmer A, Schunck WH. Candida maltosa NADPH-cytochrome P450 reductase: cloning of a full-length cDNA, heterologous expression in Saccharomyces cerevisiae and function of the N-terminal region for membrane anchoring and proliferation of the endoplasmic reticulum. Yeast. 1996;12:333–348. doi: 10.1002/(SICI)1097-0061(19960330)12:4%3C333::AID-YEA915%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Katzmann DJ, Epping EA, Moye-Rowley WS. Mutational disruption of plasma membrane trafficking of Saccharomyces cerevisiae Yor1p, a homologue of mammalian multidrug resistance protein. Mol Cell Biol. 1999;19:2998–3009. doi: 10.1128/mcb.19.4.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser GL, Gentzsch M, Kloser AK, Balzi E, Wolf DH, Goffeau A, Riordan JR. Expression and degradation of the cystic fibrosis transmembrane conductance regulator in Saccharomyces cerevisiae. Arch Biochem Biophys. 2001;390:195–205. doi: 10.1006/abbi.2001.2385. [DOI] [PubMed] [Google Scholar]
- Knop M, Finger A, Braun T, Hellmuth K, Wolf DH. Der1, a novel protein specifically required for endoplasmic reticulum degradation in yeast. EMBO J. 1996a;15:753–763. [PMC free article] [PubMed] [Google Scholar]
- Knop M, Hauser N, Wolf DH. N-Glycosylation affects endoplasmic reticulum degradation of a mutated derivative of carboxypeptidase yscY in yeast. Yeast. 1996b;12:1229–1238. doi: 10.1002/(sici)1097-0061(19960930)12:12<1229::aid-yea15>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Knop M, Schiffer HH, Rupp S, Wolf DH. Vacuolar/lysosomal proteolysis: proteases, substrates, mechanisms. Curr Opin Cell Biol. 1993;5:990–996. doi: 10.1016/0955-0674(93)90082-2. [DOI] [PubMed] [Google Scholar]
- Kopito RR. ER quality control: the cytoplasmic connection. Cell. 1997;88:427–430. doi: 10.1016/s0092-8674(00)81881-4. [DOI] [PubMed] [Google Scholar]
- Kopito RR, Sitia R. Aggresomes, and Russell bodies Symptoms of cellular indigestion? EMBO Rep. 2000;1:225–231. doi: 10.1093/embo-reports/kvd052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn MJ, Herrmann JM, Schekman R. COPII-cargo interactions direct protein sorting into ER-derived transport vesicles. Nature. 1998;391:187–190. doi: 10.1038/34438. [DOI] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Pelham HR. SNARE-mediated retrograde traffic from the Golgi complex to the endoplasmic reticulum. Cell. 1996;85:205–215. doi: 10.1016/s0092-8674(00)81097-1. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Rayner JC, Pelham HR. A novel SNARE complex implicated in vesicle fusion with the endoplasmic reticulum. EMBO J. 1997;16:3017–3024. doi: 10.1093/emboj/16.11.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A, 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Mizuta K, Warner JR. Continued functioning of the secretory pathway is essential for ribosome synthesis. Mol Cell Biol. 1994;14:2493–2502. doi: 10.1128/mcb.14.4.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsukasa K, Nishikawa S, Hosokawa N, Nagata K, Endo T. Mnl1p, an alpha-mannosidase-like protein in yeast Saccharomyces cerevisiae, is required for endoplasmic reticulum-associated degradation of glycoproteins. J Biol Chem. 2001;276:8635–8638. doi: 10.1074/jbc.C100023200. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Hirata A, Nakano A. Inhibition of endoplasmic reticulum (ER)-to-Golgi transport induces relocalization of binding protein (BiP) within the ER to form the BiP bodies. Mol Biol Cell. 1994;5:1129–1143. doi: 10.1091/mbc.5.10.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1979;76:1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SK, Indig FE, Olivieri N, Levine ND, Latterich M. Organelle membrane fusion: a novel function for the syntaxin homolog Ufe1p in ER membrane fusion. Cell. 1998;92:611–620. doi: 10.1016/s0092-8674(00)81129-0. [DOI] [PubMed] [Google Scholar]
- Plemper RK, Bohmler S, Bordallo J, Sommer T, Wolf DH. Mutant analysis links the translocon and BiP to retrograde protein transport for ER degradation. Nature. 1997;388:891–895. doi: 10.1038/42276. [DOI] [PubMed] [Google Scholar]
- Plemper RK, Bordallo J, Deak PM, Taxis C, Hitt R, Wolf DH. Genetic interactions of Hrd3p and Der3p/Hrd1p with Sec61p suggest a retro-translocation complex mediating protein transport for ER degradation. J Cell Sci. 1999;112:4123–4134. doi: 10.1242/jcs.112.22.4123. [DOI] [PubMed] [Google Scholar]
- Plemper RK, Egner R, Kuchler K, Wolf DH. Endoplasmic reticulum degradation of a mutated ATP-binding cassette transporter Pdr5 proceeds in a concerted action of Sec61 and the proteasome. J Biol Chem. 1998;273:32848–32856. doi: 10.1074/jbc.273.49.32848. [DOI] [PubMed] [Google Scholar]
- Plemper RK, Wolf DH. Retrograde protein translocation: ERADication of secretory proteins in health and disease. Trends Biochem Sci. 1999;24:266–270. doi: 10.1016/s0968-0004(99)01420-6. [DOI] [PubMed] [Google Scholar]
- Prinz WA, Grzyb L, Veenhuis M, Kahana JA, Silver PA, Rapoport TA. Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Biol. 2000;150:461–474. doi: 10.1083/jcb.150.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich E, Kerem A, Fröhlich KU, Diamant N, Bar-Nun S. AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol Cell Biol. 2002;22:626–634. doi: 10.1128/MCB.22.2.626-634.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambourg A, Clermont Y, Kepes F. Modulation of the Golgi apparatus in Saccharomyces cerevisiae sec7 mutants as seen by three-dimensional electron microscopy. Anat Rec. 1993;237:441–452. doi: 10.1002/ar.1092370402. [DOI] [PubMed] [Google Scholar]
- Rambourg A, Clermont Y, Ovtracht L, Kepes F. Three-dimensional structure of tubular networks, presumably Golgi in nature, in various yeast strains: a comparative study. Anat Rec. 1995;243:283–293. doi: 10.1002/ar.1092430302. [DOI] [PubMed] [Google Scholar]
- Rendueles PS, Wolf DH. Proteinase function in yeast: biochemical and genetic approaches to a central mechanism of post-translational control in the eukaryote cell. FEMS Microbiol Rev. 1988;4:17–45. doi: 10.1111/j.1574-6968.1988.tb02706.x-i1. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Rupp S, Wolf DH. Biogenesis of the yeast vacuole (lysosome). The use of active-site mutants of proteinase yscA to determine the necessity of the enzyme for vacuolar proteinase maturation and proteinase yscB stability. Eur J Biochem. 1995;231:115–125. doi: 10.1111/j.1432-1033.1995.tb20677.x. [DOI] [PubMed] [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Scherer S, Davis RW. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci USA. 1979;76:4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder S, Schimmoller F, Singer-Krüger B, Riezman H. The Golgi-localization of yeast Emp47p depends on its di-lysine motif but is not affected by the ret1–1 mutation in alpha-COP. J Cell Biol. 1995;131:895–912. doi: 10.1083/jcb.131.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder-Köhne S, Letourneur F, Riezman H. Alpha-COP can discriminate between distinct, functional di-lysine signals in vitro and regulates access into retrograde transport. J Cell Sci. 1998;111:3459–3470. doi: 10.1242/jcs.111.23.3459. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer T, Wolf DH. Endoplasmic reticulum degradation: reverse protein flow of no return. FASEB J. 1997;11:1227–1233. doi: 10.1096/fasebj.11.14.9409541. [DOI] [PubMed] [Google Scholar]
- Springer S, Schekman R. Nucleation of COPII vesicular coat complex by endoplasmic reticulum to Golgi vesicle SNAREs. Science. 1998;281:698–700. doi: 10.1126/science.281.5377.698. [DOI] [PubMed] [Google Scholar]
- Stack JH, Horazdovsky B, Emr SD. Receptor-mediated protein sorting to the vacuole in yeast: roles for a protein kinase, a lipid kinase and GTP-binding proteins. Annu Rev Cell Dev Biol. 1995;11:1–33. doi: 10.1146/annurev.cb.11.110195.000245. [DOI] [PubMed] [Google Scholar]
- Stevens T, Esmon B, Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982;30:439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Tokuyasu KT. Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem J. 1989;21:163–171. doi: 10.1007/BF01007491. [DOI] [PubMed] [Google Scholar]
- Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- Tsui MM, Banfield DK. Yeast Golgi SNARE interactions are promiscuous. J Cell Sci. 2000;113:145–152. doi: 10.1242/jcs.113.1.145. [DOI] [PubMed] [Google Scholar]
- Van Den Hazel HB, Kielland-Brandt MC, Winther JR. Review: biosynthesis and function of yeast vacuolar proteases. Yeast. 1996;12:1–16. doi: 10.1002/(sici)1097-0061(199601)12:1<1::aid-yea902>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Vashist S, Kim W, Belden WJ, Spear ED, Barlowe C, Ng DT. Distinct retrieval and retention mechanisms are required for the quality control of endoplasmic reticulum protein folding. J Cell Biol. 2001;155:355–368. doi: 10.1083/jcb.200106123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooding S, Pelham HR. The dynamics of golgi protein traffic visualized in living yeast cells. Mol Biol Cell. 1998;9:2667–2680. doi: 10.1091/mbc.9.9.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Fujii R, Toyofuku Y, Saito T, Koseki H, Hsu VW, Aoe T. The KDEL receptor mediates a retrieval mechanism that contributes to quality control at the endoplasmic reticulum. EMBO J. 2001;20:3082–3091. doi: 10.1093/emboj/20.12.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Meyer HH, Rapoport TA. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature. 2001;414:652–656. doi: 10.1038/414652a. [DOI] [PubMed] [Google Scholar]
- Zapun A, Jakob CA, Thomas DY, Bergeron JJ. Protein folding in a specialized compartment: the endoplasmic reticulum. Struct Fold Des. 1999;7:R173–R82. doi: 10.1016/s0969-2126(99)80112-9. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Nijbroek G, Sullivan M L, McCracken A A, Watkins S C, Michaelis S, Brodsky J L. Hsp70 molecular chaperone facilitates endoplasmic reticulum-associated protein degradation of cystic fibrosis transmembrane conductance regulator in yeast. Mol Biol Cell. 2001;12:1303–1314. doi: 10.1091/mbc.12.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer T, Vogel F, Ohta A, Takagi M, Schunck WH. Protein quality—a determinant of the intracellular fate of membrane-bound cytochromes P450 in yeast. DNA Cell Biol. 1997;16:501–514. doi: 10.1089/dna.1997.16.501. [DOI] [PubMed] [Google Scholar]