Abstract
Backgroud
In the diagnosis of bloodstream infections (BSI) in children, compared to the gold standard of blood culture, markers in the blood offer advantages such as rapid results and cost-effectiveness. Therefore, we investigated the clinical value of procalcitonin (PCT), C-reactive protein (CRP), white blood cell count (WBC), and neutrophil-to-lymphocyte ratio (NLR) in the early diagnosis of BSI in children.
Methods
This study included a retrospective analysis of 309 suspected BSI cases and patients were categorized into 2 groups based on blood culture results: blood culture-positive group, and blood culture-negative group. The blood culture-positive group was further partitioned into 3 sub-groups based on the type of pathogen: Gram-positive (G +) bacteria, Gram-negative (G-) bacteria, and fungi. Changes in PCT, CRP, WBC, and NLR were evaluated, and pathogen infections among these aforementioned groups were further determined. Moreover, the study employed the receiver operating characteristic (ROC) curve to evaluate the diagnostic value of these indicators in identifying BSI in pediatric patients at an early stage.
Results
Among the 98 strains of pathogens detected in blood culture, 58 (58.2%) strains were G- bacteria, 33 (33.7%) strains were G + bacteria, and 7 (7.1%) strains were fungi. The levels of PCT, CRP, WBC, and NLR were found to be significantly higher in the blood culture-positive group than the blood culture-negative group (p < 0.01). Upon comparing the levels of PCT and CRP in the three pathogen infections, it was found that the fungi group exhibited higher levels than the G- and G + bacteria groups (p < 0.01). The G- bacteria group exhibited higher levels of PCT, CRP, and WBC than the blood culture-negative group (p < 0.05). Similarly, the G + bacteria group exhibited higher levels of PCT, WBC, and NLR than the blood culture-negative group (p < 0.01). Besides, PCT presented the highest diagnostic efficiency among the single-item detections, with an AUC of 0.862 (95% CI: 0.819–0.906). The simultaneous detection of multiple parameters does not necessarily improve diagnostic performance but can enhance detection sensitivity.
Conclusions
PCT and CRP can provide important complementary information for the etiological diagnosis of BSI in children. Elevated levels of PCT and CRP were often associated with fungal or G- bacterial infections, with PCT showing particularly significant effects. Combined use of serum PCT, CRP, WBC, and NLR testing can improve the diagnostic sensitivity of pediatric BSI, reducing the risk of missed diagnoses, thereby enhancing the early diagnostic value of pediatric BSI.
Keywords: Pediatric Bloodstream Infections, Procalcitonin, C-Reactive Protein, White Blood Cell Count, Neutrophil-to-Lymphocyte Ratio
Background
Bloodstream infection (BSI) is a systemic disease caused by microorganisms entering the bloodstream and releasing toxins and metabolites [1]. This disease can easily lead to sepsis and multiple organ dysfunction syndrome, with a high mortality rate, making it a significant global public health issue [2]. In children, the immature immune system renders them more susceptible to invasive microorganisms, leading to rapid disease progression in cases of BSI. Therefore, early diagnosis and precise identification of the causative pathogens are crucial for the treatment of pediatric BSI [3].
Blood culture, considered the "gold standard" for diagnosing BSI, is vital in clinical practice. However, its accuracy can be compromised by various factors, such as detection time, bacterial growth characteristics, contamination by non-pathogenic organisms, and improper sampling techniques. These issues can result in false-negative or false-positive results, delaying treatment and wasting medical resources [4, 5].
Procalcitonin (PCT) is the precursor protein of human calcitonin. In the presence of an infectious disease, PCT levels peak within 6 h and plateau within 24 h. The levels of PCT can vary depending on the type of pathogen causing the infection [6]. C-reactive protein (CRP) is a protein released by the liver in response to injury or infection. It is commonly used as an auxiliary indicator to assess the activity of inflammation and monitor the effectiveness of treatment [7]. White blood cells (WBC), neutrophils, and lymphocytes are key cellular components in the body's defense against infections. During the course of BSI, changes in WBC count are closely related to the stage of infection, the patient's immune status, and the cause of the infection. An increase in neutrophils and a decrease in lymphocytes often indicate the presence of an infection. Therefore, the neutrophil-to-lymphocyte ratio (NLR), an easily obtainable inflammatory biomarker, holds significant value in assessing infectious diseases [8].
While numerous studies [9, 10] have focused on marker monitoring of bloodstream infections in adults, there remains a dearth of research regarding marker monitoring of bloodstream infections in children. In the diagnosis of BSI among pediatric patients, blood markers such as PCT, CRP, WBC, and NLR assume particular significance due to their ability to provide rapid results and cost-effectiveness, advantages that are lacking in traditional blood culture methods. Consequently, this study aims to investigate the clinical value of these markers for early diagnosis of BSI in children, providing a theoretical basis for the early diagnosis and timely treatment of BSI in children.
Materials and methods
General information
This study was conducted at Fujian Children’s Hospital, Selecting 309 hospitalized pediatric patients suspected of having BSI from January 2022 to December 2023. Based on blood culture results, the patients were divided into two groups: the blood culture-positive group with 98 cases (65 males and 33 females) and the blood culture-negative group with 211 cases (130 males and 81 females).
Inclusion criteria
Age from 1 month to less than 18 years, excluding those who were exactly 18 years old (under the age of 18 is considered a minor under Chinese law).
Presence of significant infection symptoms, such as fever (≥ 38 °C) or hypothermia (≤ 36 °C), with or without chills.
PCT, CRP, WBC, and NLR tests were conducted within 24 h of the blood culture.
Isolation of a single strain from two consecutive blood cultures or a single positive blood culture with clinical symptoms consistent with BSI [11].
Exclusion criteria
Patients with hematological diseases, autoimmune diseases, extensive burns, multiple traumas, abdominal surgery or malignant tumors.
Isolation of different strains from two or more blood cultures taken from the same site.
Clinical symptoms not consistent with BSI, with positive blood culture considered as contamination.
Use of antibiotics prior to blood sampling.
This study has been approved by the hospital’s medical ethics committee (No: 2024ETKLRK04009).
PCT/CRP/WBC/NLR determination
In this study, all patients were required to have their blood collected without the use of any antibiotic medications. The collection, transportation, and preservation of specimens for subsequent culture were performed in accordance with the Clinical and Laboratory Standards Institute guidelines [12]. Each subject provided a 2 mL blood sample, which was centrifuged at 3500 rpm for 5 min to obtain serum for testing. PCT levels were measured using a fully automated chemiluminescence immunoassay analyzer (Roche cobas e 801 and cobas e 601) with electrochemiluminescence as the detection method. PCT ≥ 0.05 ng/mL was considered positive. CRP levels were assessed using the Neph-EZ400 specific protein analyzer and reagents from Suzhou DeWo Biotechnology Co., Ltd., with the immunoturbidimetric method. CRP ≥ 5 mg/L was considered abnormally elevated. WBC were determined using the Mindray BC7500 fully automated hematology analyzer, and the NLR was calculated based on the neutrophil and lymphocyte counts obtained from this analyzer. All procedures were strictly conducted according to laboratory standards and reagent kit instructions to minimize errors.
Blood culture and pathogen identification
Blood samples from pediatric patients with BSI were collected for blood culture. The procedure included a three-step disinfection method and the cleaning and disinfection of the blood culture bottle caps. Within 24 h, 1–5 mL of venous blood was collected from each child using the BD BACTEC™ FX blood culture system with appropriate culture bottles. Analysis was performed using the microflex LT/SH mass spectrometer. Pathogen identification and antimicrobial susceptibility testing were conducted using the Vitek-2 automated analysis system. Based on the type of pathogen, infections were categorized into G- bacterial, G + bacterial, and fungal infections. Coagulase-negative staphylococci, corynebacteria, and other skin commensals isolated from only one set of blood cultures, lacking clinical and/or laboratory data supporting their pathogenicity, were considered potential contaminants [13]. Throughout the blood culture process, strict aseptic techniques were adhered to in order to prevent specimen contamination.
Statistical analysis
Statistical analysis was conducted using SPSS version 29.0. Measurement data were expressed as percentages and compared using the χ2 test. Quantitative data were presented as mean ± standard deviation (x̄ ± s) and analyzed using F and t tests. ROC curves were plotted using the report ROC package in R language (version 4.1.) to calculate diagnostic sensitivity, specificity, positive predictive value, negative predictive value, and the area under the ROC curve (AUC). An AUC value greater than 0.9 indicated very high diagnostic performance, 0.7–0.9 indicated good diagnostic performance, and 0.5–0.7 indicated poor diagnostic performance. A P-value of less than 0.05 was considered statistically significant.
Results
Composition of pathogens in positive blood cultures
Among the 98 strains of pathogens identified in positive blood cultures, 33 strains (33.7%) were G + bacteria, predominantly Staphylococcus aureus (9 strains) and Staphylococcus epidermidis (8 strains). G- bacteria accounted for 58 strains (58.2%), with Escherichia coli (13 strains) and Salmonella spp. (10 strains) being the most common. Additionally, 7 strains (7.1%) were fungi, primarily Candida parapsilosis (3 strains).
Comparison of PCT, CRP, WBC, and NLR levels between blood culture positive and negative groups
The levels of PCT, CRP, WBC, and NLR in the blood culture-positive group were significantly higher than those in the blood culture-negative group, with all differences being statistically significant (p < 0.01), as shown in Table 1.
Table 1.
Comparison of PCT, CRP, WBC, and NLR Levels Between Blood Culture Positive Group and Blood Culture Negative Group
| Group | n | PCT(ng/mL) | CRP(mg/L) | WBC(× 109/L) | NLR |
|---|---|---|---|---|---|
| Blood Culture Positive | 98 | 16.8 ± 36.4 | 52.1 ± 48.2 | 13.1 ± 8.5 | 4.1 ± 4.9 |
| Blood Culture Negative | 211 | 0.3 ± 0.6 | 21.5 ± 26.2 | 9.9 ± 4.6 | 2.7 ± 2.9 |
| t | 6.571 | 7.196 | 4.242 | 3.430 | |
| P | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
Comparison of PCT, CRP, WBC, and NLR levels between blood culture negative group and the other three group
Compared with the blood culture-negative group, the G- bacteria group had significantly higher levels of PCT, CRP, and WBC (p < 0.05), while NLR showed no significant difference (p > 0.05). In the G + bacteria group, levels of PCT, WBC, and NLR were significantly higher than those in the blood culture-negative group (p < 0.01), with no significant difference in CRP levels (p > 0.05). The fungi group exhibited significantly higher levels of PCT and CRP compared to the blood culture-negative group (p < 0.01), but there were no significant differences in WBC and NLR levels (p > 0.05), as shown in Table 2.
Table 2.
Comparison of PCT, CRP, WBC, and NLR Levels Among G- Bacteria Group, G + Bacteria Group, Fungi Group, and Blood Culture Negative Group
| Group | n | PCT(ng/mL) | CRP(mg/L) | WBC(× 109/L) | NLR |
|---|---|---|---|---|---|
| G- Bacteria | 58 | 17.6 ± 40.2★ | 60.6 ± 51.9★ | 12.3 ± 5.7☆ | 3.5 ± 4.7 |
| G + Bacteria | 33 | 11.6 ± 25.3★ | 33.1 ± 29.4 | 15.3 ± 12.2★ | 4.6 ± 4.1★ |
| Fungi | 7 | 37.7 ± 45.9★ | 70.4 ± 65.5★ | 10.0 ± 3.7 | 7.3 ± 3.3 |
| Blood Culture Negative | 211 | 0.3 ± 0.6 | 21.5 ± 26.3 | 10.0 ± 4.6 | 2.7 ± 2.9 |
| t | 12.674 | 4.524 | 0.001 | 4.132 | |
| P | < 0.01 | < 0.01 | 0.382 | 0.291 |
Notes:★ indicates P < 0.01 compared with the blood culture negative group; ☆ indicates P < 0.05 compared with the blood culture negative group
Clinical value of single and combined detection of PCT, CRP, WBC, and NLR in the diagnosis of pediatric BSI
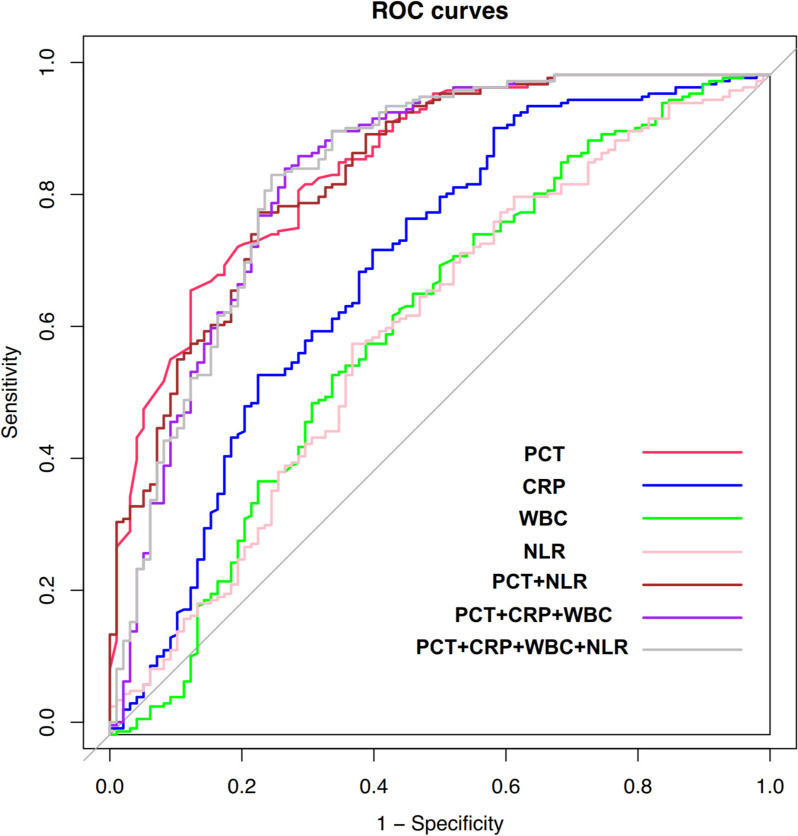
ROC curve analysis demonstrated that among single-item tests, PCT had the highest diagnostic efficiency. When the cutoff value was 0.2 ng/mL, the AUC was 0.862 (95% CI: 0.819–0.906). Among two-item combined tests, the combination of PCT and NLR had the highest diagnostic efficiency, with an AUC of 0.852 (95% CI: 0.805–0.898). For three-item combined tests, the combination of PCT, CRP, and WBC showed the highest diagnostic efficiency, with an AUC of 0.845 (95% CI: 0.793–0.897). The AUC for the four-item combined test was 0.847 (95% CI: 0.795–0.828), as shown in Table 3 and Fig. 1.
Table 3.
Analysis of the Diagnostic Value of PCT, CRP, WBC, and NLR, Individually and in Combination, for Early Diagnosis of Pediatric BSI
| Cut-off value | AUC(95%CI) |
Sensitivity (%) |
P |
Specificity (%) |
Positive predictive value (%) | Negative predictive value (%) | |
|---|---|---|---|---|---|---|---|
| PCT | 0.2 | 0.862(0.819–0.906) | 67.3 | 0.000 | 87.8 | 92.2 | 55.5 |
| CRP | 46.9 | 0.708(0.640–0.773) | 91.9 | 0.000 | 42.9 | 77.6 | 71.2 |
| WBC | 12.4 | 0.618(0.547–0.689) | 71.1 | 0.000 | 50 | 75.4 | 44.5 |
| NLR | 2.1 | 0.613(0.544–0.683) | 59.2 | 0.001 | 63.3 | 77.6 | 41.9 |
| PCT + NLR | — | 0.852(0.805–0.898) | 79.1 | 0.000 | 77.6 | 88.4 | 63.3 |
|
PCT + CRP + WBC |
— | 0.845(0.793–0.897) | 85.8 | 0.000 | 73.5 | 87.4 | 70.6 |
|
PCT + CRP + WBC + NLR |
— | 0.847(0.795–0.828) | 84.8 | 0.000 | 75.5 | 88.2 | 69.8 |
Fig. 1.
ROC curves for early diagnosis of pediatric BSI using individual and combined detection of PCT, CRP, WBC, and NLR
Discussion
Early diagnosis, differentiation of diverse pathogens, and guidance on the rational use of antibiotics are crucial for improving the prognosis of pediatric BSI and reducing mortality rates [14, 15]. In this study, the blood culture results of 309 pediatric patients suspected of having BSI were analyzed. The results showed that the hematological markers PCT, CRP, WBC and NLR were significantly elevated (P < 0.01) in the 98 positive blood culture cases. Among these, PCT had the highest sensitivity, with an area under the ROC curve of 0.862, indicating high diagnostic value in predicting bloodstream infections, consistent with the findings of Cha JK's study [16].
PCT, the precursor protein of calcitonin, is found in very low levels in healthy individuals. It is commonly used as a biomarker for monitoring bacterial infections and assessing the prognosis of critically ill patients [17]. Due to the unique composition of lipopolysaccharide (LPS) in the cell wall of G- bacteria, as compared to G + bacteria, LPS can independently or synergistically with various inflammatory factors such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) induce the production and release of PCT. Therefore, elevated levels of PCT can aid in distinguishing BSI caused by G- bacteria from those caused by G + bacteria [18]. Wang ZH highlights that both Pcv-aCO2 and PCT possess similar and high diagnostic value for the early detection of BSI caused by G- bacteria [19]. Leli C proposes that PCT could potentially serve as a valuable tool for distinguishing between G- bacteria, G + bacteria, and fungal bloodstream infections [20]. The present study validates these findings, demonstrating the effective differentiation of bloodstream infections caused by diverse pathogens through the utilization of PCT. The highest PCT levels were recorded in the fungal infection group, followed by the G- bacteria infection group, and finally the G + bacteria infection group (p < 0.05).
CRP, as a non-specific acute-phase reactant protein, is widely used in clinical diagnostics due to its universal elevation in various inflammatory responses [21]. In a large cohort study of febrile infants, Norman-Bruce H found that CRP and PCT demonstrated similar diagnostic accuracy for identifying serious bacterial infections when using internationally recognized cutoff values [22]. Our study revealed that CRP levels were significantly elevated in the fungal blood culture group, indicating that fungal-induced pediatric BSI triggers the most severe systemic inflammatory response. However, there was no significant difference in CRP levels between the G- bacteria bacterial blood culture group and the blood culture-negative group (p > 0.05). This suggests that while CRP can differentiate between fungal or G- bacterial infections in the blood, its diagnostic value in identifying G + bacterial infections is limited. This limitation may be attributed to CRP's high sensitivity but relatively low specificity. Additionally, since the blood culture-negative group in this study primarily consisted of patients with localized infections, this might have further impacted the accuracy of CRP in diagnosing systemic infections caused by G + bacterial.
WBC serves as a classic clinical indicator of inflammation, whereas NLR is deemed to possess superior diagnostic value in identifying bacterial infections [23]. Colak observed a significantly higher NLR in patients with positive blood cultures compared to those with negative cultures [24]. This study revealed that while WBC and NLR levels exhibit efficacy in discriminating between pediatric BSI blood culture-positive and blood culture-negative cohorts, their discriminatory ability is limited when distinguishing between blood culture-positive fungal groups and blood culture-negative counterpart (p > 0.05). Additionally, there was no significant difference in NLR between the blood culture-positive group, G- bacterial group and the blood culture-negative group (p > 0.05). These findings suggest that WBC and NLR may not be optimal for pathogen identification in pediatric BSI. This observation could potentially be attributed to the immaturity of children's immune systems and the influence of various physiological factors on WBC and NLR.
In this study, we evaluated the application value of PCT, CRP, WBC, and NLR in pediatric BSI by establishing their ROC curves. The results showed that among the individual tests, PCT had a high diagnostic efficiency at a cutoff value of 0.2 ng/mL [AUC 0.862(95% CI: 0.819–0.906)], with a sensitivity and specificity of 67.3% and 87.8%, respectively. Considering the utilization of the precise and dependable electrochemiluminescence technique in this study, capable of detecting PCT at exceedingly low concentration levels, this method assumes paramount importance for the early diagnosis and timely treatment of pediatric BSI.
In clinical practice, we have observed a higher frequency of Complete Blood Count (CBC) testing compared to CRP or PCT. Despite PCT testing alone exhibiting the largest ROC area and highest specificity, its sensitivity was only 67.3%. In this study, combining PCT with CRP, WBC, and NLR either in pairs or all four together significantly enhanced the diagnostic sensitivity. However, the diagnostic accuracy of WBC and NLR for pediatric BSI is significantly inferior compared to CRP or PCT. Therefore, we recommend conducting both complete blood count (CBC) and CRP/PCT testing in pediatric patients suspected of BSI. This comprehensive approach can facilitate prompt treatment initiation and intervention for pediatric patients with suspected BSI.
Although our research has demonstrated that PCT levels in pediatric BSI can effectively differentiate between various bacterial species, as supported by the studies conducted by Brodská H et al. [25], our findings indicate that PCT levels were notably elevated in the fungal group. The findings of our study demonstrate a significant elevation in PCT levels within the fungal group, which contradicts previously reported research outcomes [13]. The relatively high levels of PCT and CRP observed in these 7 cases may be attributed to the small number of fungal cases included and potential sample bias, which could account for the discrepancies in our study findings compared to previous research. In order to address this limitation, we intend to expand our sample size and continue monitoring these results in future investigations.
The efficacy of diagnostic testing for BSI can be improved through the utilization of multi-parameter combined testing, as confirmed by recent reports [26]. The present study demonstrates that the combined utilization of PCT, CRP, WBC, and NLR for testing significantly enhances both sensitivity and negative predictive value compared to the utilization of a single parameter. However, in comparison to the utilization of PCT alone, it does not significantly augment the diagnostic value for pediatric bloodstream infections (BSI). This observation may be attributed to the prompt testing conducted by clinicians upon manifestation of suspected BSI symptoms. At present, PCT might have reached its zenith plateau while the other three parameters are yet to attain their maximum values, potentially leading to statistical bias. Additionally, the test outcomes can be influenced by factors such as the study's sample size and the specific infectious pathogens.
We acknowledge that our study is subject to certain limitations. Ideally, the prognostic evaluation of biomarkers should be conducted in larger-scale multicenter studies and prospective clinical trials. For small sample size: Our study is limited by a small sample size, which may affect the generalizability of our findings. This sample size was determined by the availability of eligible patients during the study period. Future studies with larger cohorts will be necessary to validate our results. Due to the limited number of cases in the fungal group (only 7 cases), higher levels of PCT and CRP were observed, leading to divergent findings compared to previous reports. Besides, the retrospective nature of this study introduces potential biases that might influence our findings. While we have taken steps to minimize these biases through careful data collection and analysis, prospective studies are needed to confirm our observations. The last, inclusion of multiple pathogen types has resulted in some subgroups being underrepresented (e.g., only 7 fungal cases). This limitation restricts our ability to draw definitive conclusions about the performance of our methods for less common pathogens. Further research focusing on individual pathogen types is warranted.
Conclusion
The combination of PCT and CRP can offer valuable complementary insights for the etiological diagnosis of pediatric BSI. Elevated levels of both PCT and CRP are frequently indicative of fungal or Gram-negative bacterial infections, with PCT demonstrating particularly noteworthy implications. However, the utility of WBC and NLR in differentiating the pathogen classification of pediatric BSI is currently limited. The combined utilization of serum PCT, CRP, WBC, and NLR testing can improve the diagnostic sensitivity of pediatric BSI, reducing the likelihood of missed diagnoses, thereby enhancing the early diagnostic value of pediatric BSI.
Acknowledgements
We are grateful to the laboratory clinicians in the Bacteria Room (Fujian Children’s Hospital) for their help in pathogen identification and helpful discussions. We thank all the researchers and participants who have made significant contributions to this study.
Abbreviations
- PCT
Procalcitonin
- CRP
C-reactive protein
- WBC
White blood cell count
- NLR
Neutrophil-to-lymphocyte ratio
- BSI
Bloodstream infections
- TNF-α
Tumor necrosis factor-alpha
- IL-6
Interleukin-6
- LPS
Lipopolysaccharide
- CBC
Complete Blood Count
Authors’ contributions
FC and QT contributed to the conception and supervision of the project. YL, ML, CL and WT contributed to the interpretation or analysis of the data. YL, ML and CL contributed to the preparation of the manuscript and to the revision of important intellectual content. All authors reviewed and approved the final manuscript.
Funding
This work was funded by Startup Fund for scientific research, Fujian Medical University (Grant number: 2021QH1176).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of Fujian Children’s Hospital (No: 2024ETKLRK04009) and adhered to the Declaration of Helsinki. Furthermore, informed consent to participate was obtained from the parents or legal guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yadong Li, Mingjie Li and Chenye Lin contributed equally to this work.
Contributor Information
Qiuyu Tang, Email: Tell.rain@163.com.
Feng Cheng, Email: 543323062@qq.com.
References
- 1.Kelly J, Tysall L, Dewar S. Daptomycin susceptibility testing and therapeutic use in enterococcal bloodstream infection (EBSI) in a setting with high rates of vancomycin-resistant Enterococcus faecium (VREfm) [J]. J Antimicrob Chemother. 2022;77(5):1432–5. [DOI] [PubMed] [Google Scholar]
- 2.Costa SP, Carvalho CM. Burden of bacterial bloodstream infections and recent advances for diagnosis [J]. Pathog Dis. 2022;80(1):ftac027. [DOI] [PubMed] [Google Scholar]
- 3.Wu H-G, Liu W-S, Zhu M, et al. Research and analysis of 74 bloodstream infection cases of Acinetobacter baumannii and drug resistance [J]. Eur Rev Med Pharmacol Sci. 2018;22(6):1782–6. [DOI] [PubMed] [Google Scholar]
- 4.Ombelet S, Barbé B, Affolabi D, et al. Best practices of blood cultures in low- and middle-income countries [J]. Front Med (Lausanne). 2019;6:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamy B, Dargère S, Arendrup MC, et al. How to optimize the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the-art [J]. Front Microbiol. 2016;7:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Memar MY, Varshochi M, Shokouhi B, et al. Procalcitonin: the marker of pediatric bacterial infection [J]. Biomed Pharmacother. 2017;96:936–43. [DOI] [PubMed] [Google Scholar]
- 7.Yadav R K, Kumar D, Gupta A, et al. C-reactive protein and procalcitonin: as predictor biomarkers of severity and outcome in children with community-acquired pneumonia [J]. Trop Doct. 2024;54:262–67. [DOI] [PubMed]
- 8.Dursun A, Ozsoylu S, Akyildiz BN. Neutrophil-to-lymphocyte ratio and mean platelet volume can be useful markers to predict sepsis in children [J]. Pak J Med Sci. 2018;34(4):918–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Zeng J, Yu X, et al. PCT, IL-6, and IL-10 facilitate early diagnosis and pathogen classifications in bloodstream infection [J]. Ann Clin Microbiol Antimicrob. 2023;22(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamelytė E, Vaičekauskienė G, Dagys A, et al. Early blood biomarkers to improve sepsis/bacteremia diagnostics in pediatric emergency settings [J]. Medicina (Kaunas). 2019;55(4):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) [J]. JAMA. 2016;315(8):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Towns ML, Jarvis WR, Hsueh P-R. Guidelines on blood cultures [J]. J Microbiol Immunol Infect. 2010;43(4):347–9. [DOI] [PubMed] [Google Scholar]
- 13.Niu D, Huang Q, Yang F, et al. Serum biomarkers to differentiate gram-negative, gram-positive and fungal infection in febrile patients [J]. J Med Microbiol. 2021;70:1360. [DOI] [PubMed]
- 14.Brunkhorst FM, Weigand MA, Pletz M, et al. S3 guideline sepsis-prevention, diagnosis, therapy, and aftercare: long version] [J. Med Klin Intensivmed Notfmed. 2020;115(Suppl 2):37–109. [DOI] [PubMed] [Google Scholar]
- 15.Mboya EA, Sanga LA, Ngocho JS. Irrational use of antibiotics in the Moshi municipality northern Tanzania: a cross-sectional study [J]. Pan Afr Med J. 2018;31:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cha JK, Kwon KH, Byun SJ, et al. Clinical value of procalcitonin for suspected nosocomial bloodstream infection [J]. Korean J Intern Med. 2018;33(1):176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shim YE, Shin S, Ko Y, et al. Serum procalcitonin as a biomarker for differentiating between infectious and non-infectious fever after pancreas transplantation [J]. Clin Transplant. 2021;35(4):e14224. [DOI] [PubMed] [Google Scholar]
- 18.Park BS, Kim SE, Park SH, et al. Procalcitonin as a potential predicting factor for prognosis in bacterial meningitis [J]. J Clin Neurosci. 2017;36:129–33. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z-H, Wei X-B, Liao X-L, et al. PCV-ACO2 and procalcitonin levels for the early diagnosis of bloodstream infections caused by gram-negative bacteria [J]. Am J Med Sci. 2022;364(6):752–7. [DOI] [PubMed] [Google Scholar]
- 20.Leli C, Ferranti M, Moretti A, et al. Procalcitonin levels in gram-positive, gram-negative, and fungal bloodstream infections [J]. Dis Markers. 2015;2015:701480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mölkänen T, Ruotsalainen E, Rintala EM, et al. Predictive value of C-reactive protein (CRP) in identifying fatal outcome and deep infections in Staphylococcus aureus bacteremia [J]. PLoS ONE. 2016;11(5):e0155644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norman-Bruce H, Umana E, Mills C, et al. Diagnostic test accuracy of procalcitonin and C-reactive protein for predicting invasive and serious bacterial infections in young febrile infants: a systematic review and meta-analysis [J]. Lancet Child Adolesc Health. 2024;8(5):358–68. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Yuan X, Wang C. The clinical value of IL-3, IL-4, IL-12p70, IL-17A, IFN-γ, MIP-1β, NLR, P-selectin, and TNF-α in differentiating bloodstream infections caused by gram-negative, gram-positive bacteria and fungi in hospitalized patients: an observational study [J]. Medicine (Baltimore). 2019;98(38):e17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colak A, Aksit MZ, Toprak B, et al. Diagnostic values of neutrophil/lymphocyte ratio, platelet/lymphocyte ratio and procalcitonin in early diagnosis of bacteremia [J]. Turk J Biochem. 2020;45(1):57–64. [Google Scholar]
- 25.Brodská H, Malíčková K, Adámková V, et al. Significantly higher procalcitonin levels could differentiate gram-negative sepsis from gram-positive and fungal sepsis [J]. Clin Exp Med. 2013;13(3):165–70. [DOI] [PubMed] [Google Scholar]
- 26.Dambroso-Altafini D, Menegucci TC, Costa BB, et al. Routine laboratory biomarkers used to predict gram-positive or gram-negative bacteria involved in bloodstream infections [J]. Sci Rep. 2022;12(1):15466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript or supplementary information files.