Abstract
1. Bovine chromaffin cells were inflated by pressure applied through a pipette or swollen during intracellular perfusion with hypertonic solutions. Effects of such procedures on electrical properties of the membrane were studied by a combination of the tight-seal whole-cell patch-clamp technique and Fura-2 fluorescence measurements of free intracellular calcium concentration ([Ca2+]i). 2. Application of air pressure (about +5 cmH2O or 490 Pa) through the patch pipette caused an increase in the cell volume and concomitant development of an inwardly directed transient current at the holding potential of -60 mV. The current gradually increased to a peak value and subsequently decayed almost to its initial level within 5-10 min. A short pulse of pressure (5-10 s) was sufficient to elicit the whole sequence of events. 3. Intracellular free Ca2+ ion concentration, [Ca2+]i, steeply increased at the beginning of the pressure pulse to about 0.2 microM and either stayed at this level or decayed back to the more usual value of approximately 0.1 microM. 4. Similar changes in the transmembrane current and [Ca2+]i were observed during intracellular perfusion of cells with hypertonic solutions (30-50 mosM difference relative to the bath solution) or during extracellular application of hypotonic solution. 5. Swelling of non-perfused cells by extracellular application of hyposmotic solution caused the appearance of inward currents in cell-attached membrane patches held at a fixed potential -30 mV relative to the cell's resting potential. The kinetics of the current resembled those of the whole-cell current. 6. Intracellular introduction of guanosine triphosphate (GTP, 300 microM) significantly prolonged the duration (from 62 +/- 10 s, n = 5, to 98 +/- 8 s, n = 4, when measured at the level of half-amplitude), while introduction of the non-hydrolysable analogue of guanosine diphosphate (GDP), guanosine 5'-O-(2-thiodiphosphate) (GDP beta S, 300 microM), decreased the maximal rate of increase (from 11.4 +/- 2.6 pA/s, n = 6, to 3.2 +/- 2.1 pA/s, n = 10) of the current activated by pressure. 7. Lowering of the intracellular free Ca2+ ion concentration by introduction of 10 mM-EGTA did not significantly affect the current amplitude or time course. However, a rapid increase in the [Ca2+]i to micromolar levels (by activation of the voltage-operated calcium channels during membrane depolarization) could terminate development of the current activated by pressure and cause its fast decay to zero-current level.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF















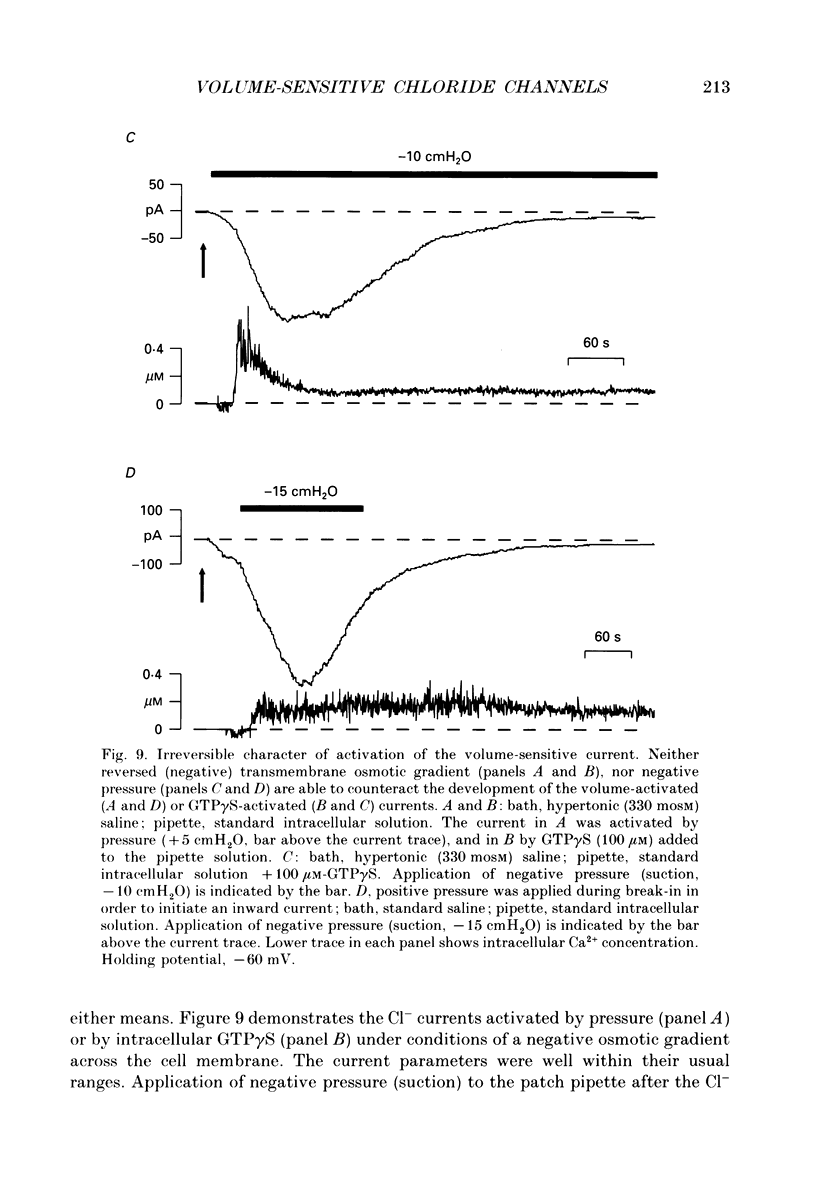





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cahalan M. D., Lewis R. S. Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc Gen Physiol Ser. 1988;43:281–301. [PubMed] [Google Scholar]
- Cala P. M., Mandel L. J., Murphy E. Volume regulation by Amphiuma red blood cells: cytosolic free Ca and alkali metal-H exchange. Am J Physiol. 1986 Mar;250(3 Pt 1):C423–C429. doi: 10.1152/ajpcell.1986.250.3.C423. [DOI] [PubMed] [Google Scholar]
- Christensen O. Mediation of cell volume regulation by Ca2+ influx through stretch-activated channels. Nature. 1987 Nov 5;330(6143):66–68. doi: 10.1038/330066a0. [DOI] [PubMed] [Google Scholar]
- Doroshenko P., Penner R., Neher E. Novel chloride conductance in the membrane of bovine chromaffin cells activated by intracellular GTP gamma S. J Physiol. 1991 May;436:711–724. doi: 10.1113/jphysiol.1991.sp018575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshenko P. Second messengers mediating activation of chloride current by intracellular GTP gamma S in bovine chromaffin cells. J Physiol. 1991 May;436:725–738. doi: 10.1113/jphysiol.1991.sp018576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke L. C., Misler S. Activity of ion channels during volume regulation by clonal N1E115 neuroblastoma cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3919–3923. doi: 10.1073/pnas.86.10.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett J. K., Spring K. R. Involvement of calcium and cytoskeleton in gallbladder epithelial cell volume regulation. Am J Physiol. 1985 Jan;248(1 Pt 1):C27–C36. doi: 10.1152/ajpcell.1985.248.1.C27. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A., Sarkadi B., Gelfand E. W. Responses of lymphocytes to anisotonic media: volume-regulating behavior. Am J Physiol. 1984 Mar;246(3 Pt 1):C204–C215. doi: 10.1152/ajpcell.1984.246.3.C204. [DOI] [PubMed] [Google Scholar]
- Hampton R. Y., Holz R. W. Effects of changes in osmolality on the stability and function of cultured chromaffin cells and the possible role of osmotic forces in exocytosis. J Cell Biol. 1983 Apr;96(4):1082–1088. doi: 10.1083/jcb.96.4.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazama A., Okada Y. Ca2+ sensitivity of volume-regulatory K+ and Cl- channels in cultured human epithelial cells. J Physiol. 1988 Aug;402:687–702. doi: 10.1113/jphysiol.1988.sp017229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Hudson R. L., Schultz S. G. Sodium-coupled glycine uptake by Ehrlich ascites tumor cells results in an increase in cell volume and plasma membrane channel activities. Proc Natl Acad Sci U S A. 1988 Jan;85(1):279–283. doi: 10.1073/pnas.85.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert I. H., Hoffmann E. K., Christensen P. Role of prostaglandins and leukotrienes in volume regulation by Ehrlich ascites tumor cells. J Membr Biol. 1987;98(3):247–256. doi: 10.1007/BF01871187. [DOI] [PubMed] [Google Scholar]
- Morris C. E. Mechanosensitive ion channels. J Membr Biol. 1990 Feb;113(2):93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmichl M., Friedrich F., Maly K., Lang F. The effect of hypoosmolarity on the electrical properties of Madin Darby canine kidney cells. Pflugers Arch. 1989 Mar;413(5):456–462. doi: 10.1007/BF00594173. [DOI] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Rothstein A., Mack E. Volume-activated K+ and Cl- pathways of dissociated epithelial cells (MDCK): role of Ca2+. Am J Physiol. 1990 May;258(5 Pt 1):C827–C834. doi: 10.1152/ajpcell.1990.258.5.C827. [DOI] [PubMed] [Google Scholar]
- Sackin H. A stretch-activated K+ channel sensitive to cell volume. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1731–1735. doi: 10.1073/pnas.86.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Kawahara K., Ogawa A., Morita T., Kawaguchi Y., Kurihara S., Sakai O. [Ca2+]i rises via G protein during regulatory volume decrease in rabbit proximal tubule cells. Am J Physiol. 1990 Mar;258(3 Pt 2):F690–F696. doi: 10.1152/ajprenal.1990.258.3.F690. [DOI] [PubMed] [Google Scholar]
- Ubl J., Murer H., Kolb H. A. Ion channels activated by osmotic and mechanical stress in membranes of opossum kidney cells. J Membr Biol. 1988 Sep;104(3):223–232. doi: 10.1007/BF01872324. [DOI] [PubMed] [Google Scholar]
- Ubl J., Murer H., Kolb H. A. Simultaneous recording of cell volume, membrane current and membrane potential: effect of hypotonic shock. Pflugers Arch. 1989 Dec;415(3):381–383. doi: 10.1007/BF00370891. [DOI] [PubMed] [Google Scholar]
- Wakade A. R., Malhotra R. K., Sharma T. R., Wakade T. D. Changes in tonicity of perfusion medium cause prolonged opening of calcium channels of the rat chromaffin cells to evoke explosive secretion of catecholamines. J Neurosci. 1986 Sep;6(9):2625–2634. doi: 10.1523/JNEUROSCI.06-09-02625.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson E. H., DiBona D. R., Schafer J. A. Analysis of structural changes during hypotonic swelling in Ehrlich ascites tumor cells. Am J Physiol. 1986 Jul;251(1 Pt 1):C104–C114. doi: 10.1152/ajpcell.1986.251.1.C104. [DOI] [PubMed] [Google Scholar]



