Abstract
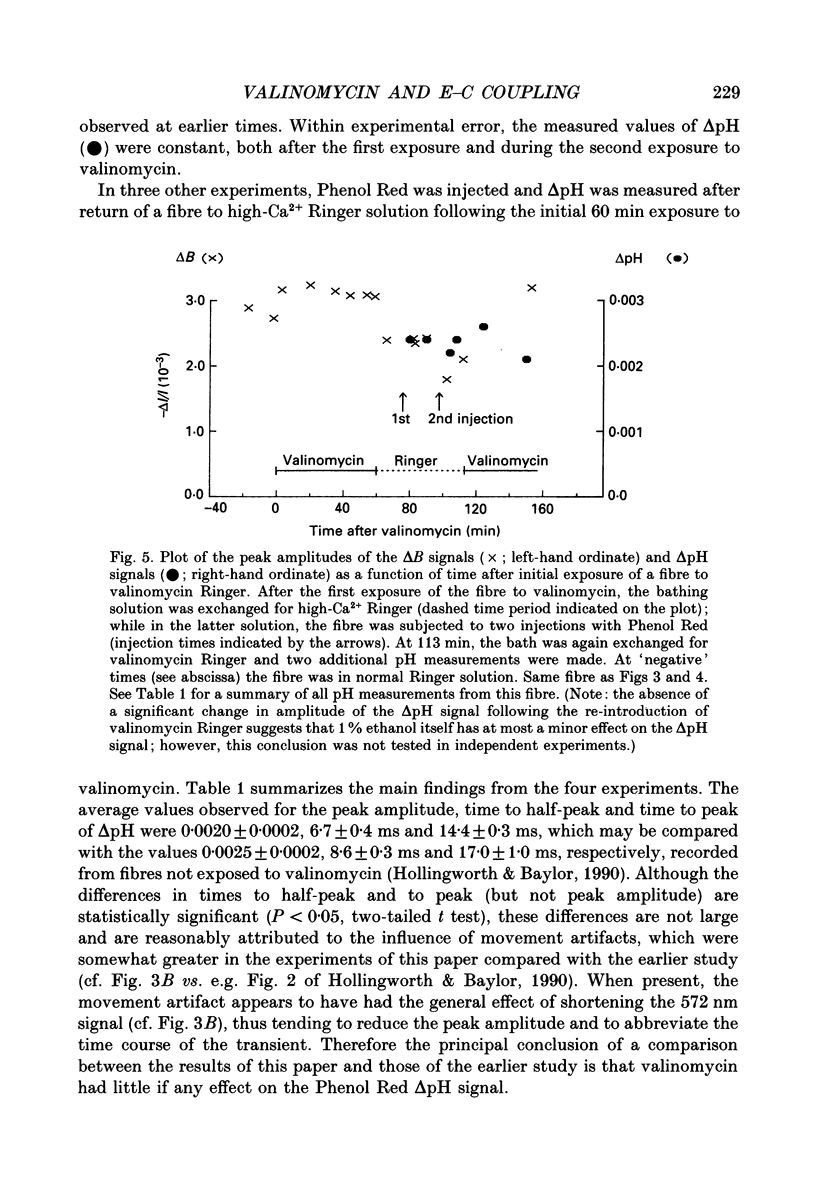
1. Experiments were carried out on intact frog skeletal muscle fibres to study the role of H+ and K+ as counter-ions during the release of Ca2+ from the sarcoplasmic reticulum (SR). A specific focus was to test whether valinomycin, a potassium ionophore, markedly reduces or abolishes H+ counter-ions fluxes across the SR membrane in response to electrical stimulation. 2. Single twitch fibres, mounted on an optical bench apparatus and stretch to long sarcomere length (3.6-4.0 microns), were activated by single action potentials (16 degrees C). Two optical signals related to excitation-contraction coupling were measured: (i) the 'second component' of the intrinsic birefringence signal, which is closely related to the myoplasmic free [Ca2+] transient, and (ii) the transient myoplasmic alkalization (delta pH) detectable from the pH indicator Phenol Red, a signal thought to reflect the movement of protons from the myoplasm into the SR in partial electrical exchange for released Ca2+. 3. Exposure of a fibre to 5 microM-valinomycin produced a slight, progressive decrease in the amplitude of the birefringence signal, approximately 5-6% per hour. This result suggests that, if anything, the peak rate at which Ca2+ is released from the sarcoplasmic reticulum is slightly decreased by valinomycin. 4. The amplitude of the Phenol Red delta pH signal, measured after exposure of a fibre to valinomycin for a period of at least 60 min, averaged 0.0020 +/- 0.0002 (+/- S.E.M.); this value is slightly smaller than, but not significantly different from (P greater than 0.05; two-tailed t test) that measured in fibres not exposed to valinomycin (0.0025 +/- 0.0002). This result does not support the idea that valinomycin, but virtue of increasing the flux of K+ into the SR, markedly reduces the flux of protons during Ca2+ release. 5. Our findings of minimal changes in the birefringence and delta pH signals are consistent with the idea that, at the time of Ca2+ release, the potassium conductance of the SR membrane is large and not substantially increased by the addition of valinomycin to Ringer solution.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli T. E., Tieffenberg M., Tosteson D. C. The effect of valinomycin on the ionic permeability of thin lipid membranes. J Gen Physiol. 1967 Dec;50(11):2527–2545. doi: 10.1085/jgp.50.11.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Calcium release and sarcoplasmic reticulum membrane potential in frog skeletal muscle fibres. J Physiol. 1984 Mar;348:209–238. doi: 10.1113/jphysiol.1984.sp015106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Optical measurements of intracellular pH and magnesium in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:105–137. doi: 10.1113/jphysiol.1982.sp014367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients. J Physiol. 1983 Nov;344:625–666. doi: 10.1113/jphysiol.1983.sp014959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Use of metallochromic dyes to measure changes in myoplasmic calcium during activity in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:139–177. doi: 10.1113/jphysiol.1982.sp014368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Absorbance signals from resting frog skeletal muscle fibers injected with the pH indicator dye, phenol red. J Gen Physiol. 1990 Sep;96(3):449–471. doi: 10.1085/jgp.96.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. A large birefringence signal preceding contraction in single twitch fibres of the frog. J Physiol. 1977 Jan;264(1):141–162. doi: 10.1113/jphysiol.1977.sp011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. Birefringence experiments on isolated skeletal muscle fibres suggest a possible signal from the sarcoplasmic reticulum. Nature. 1975 Jan 10;253(5487):97–101. doi: 10.1038/253097a0. [DOI] [PubMed] [Google Scholar]
- Best P. M., Abramcheck C. W. Potassium efflux from single skinned skeletal muscle fibers. Biophys J. 1985 Dec;48(6):907–913. doi: 10.1016/S0006-3495(85)83853-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Horowicz P. Fluorescence intensity changes associated with contractile activation in frog muscle stained with Nile Blue A. J Physiol. 1975 Apr;246(3):709–735. doi: 10.1113/jphysiol.1975.sp010912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Evans R. H., Francis A. A., Martin M. R., Watkins J. C., Davies J., Dray A. D-alpha-Aminoadipate as a selective antagonist of amino acid-induced and synaptic excitation of mammalian spinal neurones. Nature. 1977 Dec 22;270(5639):743–745. doi: 10.1038/270743a0. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Allen D. G. Model of calcium movements during activation in the sarcomere of frog skeletal muscle. Biophys J. 1984 May;45(5):913–925. doi: 10.1016/S0006-3495(84)84238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello W. C. Membrane sealing in frog skeletal-muscle fibers. Proc Natl Acad Sci U S A. 1973 Apr;70(4):982–984. doi: 10.1073/pnas.70.4.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Ferguson D. G., Castellani L., Kenney L. The density and disposition of Ca-ATPase in in situ and isolated sarcoplasmic reticulum. Ann N Y Acad Sci. 1986;483:44–56. doi: 10.1111/j.1749-6632.1986.tb34495.x. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C. Membrane particles and transmission at the triad. Fed Proc. 1975 Apr;34(5):1382–1389. [PubMed] [Google Scholar]
- Garcia A. M., Miller C. Channel-mediated monovalent cation fluxes in isolated sarcoplasmic reticulum vesicles. J Gen Physiol. 1984 Jun;83(6):819–839. doi: 10.1085/jgp.83.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselbach W., Oetliker H. Energetics and electrogenicity of the sarcoplasmic reticulum calcium pump. Annu Rev Physiol. 1983;45:325–339. doi: 10.1146/annurev.ph.45.030183.001545. [DOI] [PubMed] [Google Scholar]
- Hinkle M., Van der Kloot W. The effects of valinomycin on striated muscles of the frog and the crayfish. Comp Biochem Physiol A Comp Physiol. 1973 Oct 1;46(2):269–278. doi: 10.1016/0300-9629(73)90417-9. [DOI] [PubMed] [Google Scholar]
- Hollingworth S., Baylor S. M. Changes in phenol red absorbance in response to electrical stimulation of frog skeletal muscle fibers. J Gen Physiol. 1990 Sep;96(3):473–491. doi: 10.1085/jgp.96.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T., Somlyo A. P., Somlyo A. V. The effects of valinomycin on ion movements across the sarcoplasmic reticulum in frog muscle. J Physiol. 1984 May;350:253–268. doi: 10.1113/jphysiol.1984.sp015199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács L., Schümperli R. A., Szücs G. Comparison of birefringence signals and calcium transients in voltage-clamped cut skeletal muscle fibres of the frog. J Physiol. 1983 Aug;341:579–593. doi: 10.1113/jphysiol.1983.sp014825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca P. P., Miller C. A K+-selective, three-state channel from fragmented sarcoplasmic reticulum of frog leg muscle. J Membr Biol. 1981;61(1):31–38. doi: 10.1007/BF01870750. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Maylie J., Irving M., Sizto N. L., Chandler W. K. Calcium signals recorded from cut frog twitch fibers containing antipyrylazo III. J Gen Physiol. 1987 Jan;89(1):83–143. doi: 10.1085/jgp.89.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Monovalent ion and calcium ion fluxes in sarcoplasmic reticulum. Mol Cell Biochem. 1983;55(1):65–82. doi: 10.1007/BF00229243. [DOI] [PubMed] [Google Scholar]
- Meissner G., Young R. C. Proton permeability of sarcoplasmic reticulum vesicles. J Biol Chem. 1980 Jul 25;255(14):6814–6819. [PubMed] [Google Scholar]
- Mobley B. A., Eisenberg B. R. Sizes of components in frog skeletal muscle measured by methods of stereology. J Gen Physiol. 1975 Jul;66(1):31–45. doi: 10.1085/jgp.66.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetliker H., Schümperli R. A. Influence of sarcomere length, tonicity, and external sodium concentration on conduction velocity in frog muscle fibres. J Physiol. 1982 Nov;332:203–221. doi: 10.1113/jphysiol.1982.sp014410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape P. C., Konishi M., Hollingworth S., Baylor S. M. Perturbation of sarcoplasmic reticulum calcium release and phenol red absorbance transients by large concentrations of fura-2 injected into frog skeletal muscle fibers. J Gen Physiol. 1990 Sep;96(3):493–516. doi: 10.1085/jgp.96.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Scales D., Giuseppeinesi Assembly of ATPase protein in sarcoplasmic reticulum membranes. Biophys J. 1976 Jul;16(7):735–751. doi: 10.1016/S0006-3495(76)85725-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Imagawa T., Ma J., Fill M., Campbell K. P., Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988 Jul;92(1):1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G., Ketterer B., Benz R., Läuger P. The rate constants of valinomycin-mediated ion transport through thin lipid membranes. Biophys J. 1971 Dec;11(12):981–994. doi: 10.1016/S0006-3495(71)86272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara J., Bezanilla F., Salzberg B. M. Nile blue fluorescence signals from cut single muscle fibers under voltage or current clamp conditions. J Gen Physiol. 1978 Dec;72(6):775–800. doi: 10.1085/jgp.72.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]


