Abstract
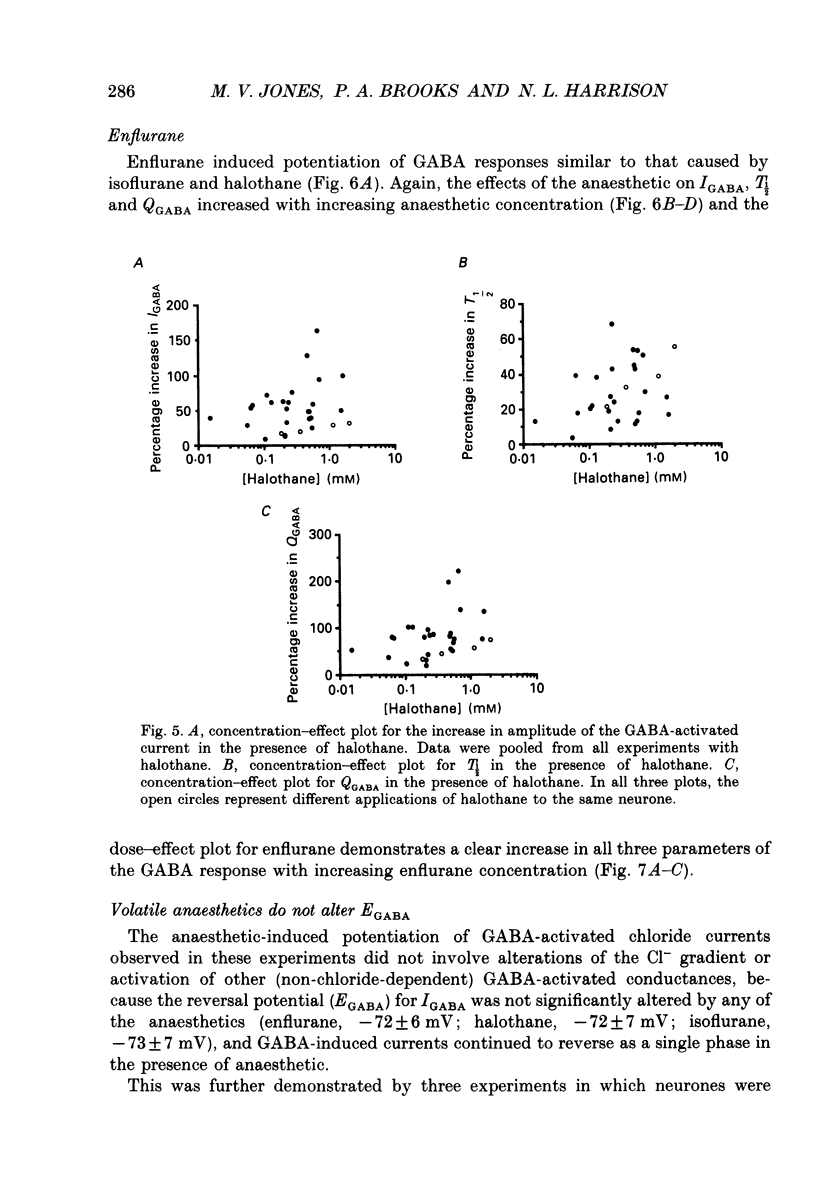
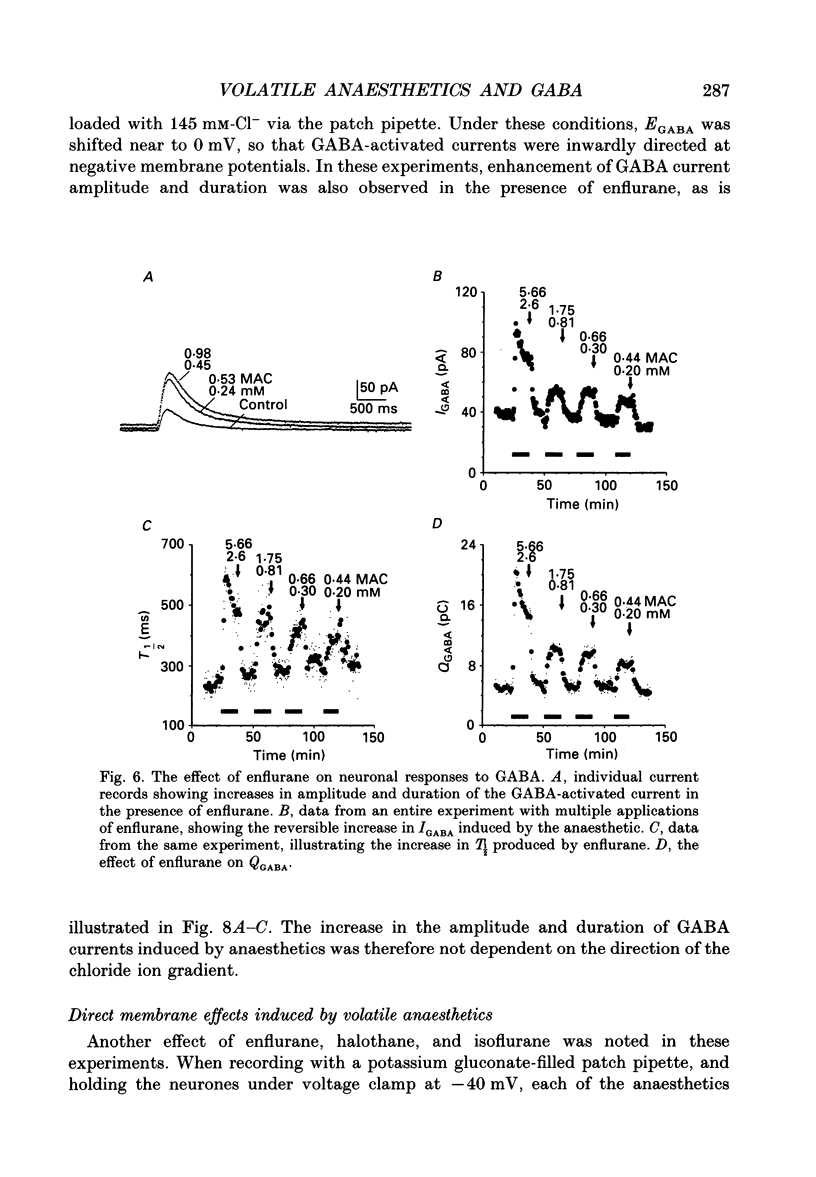
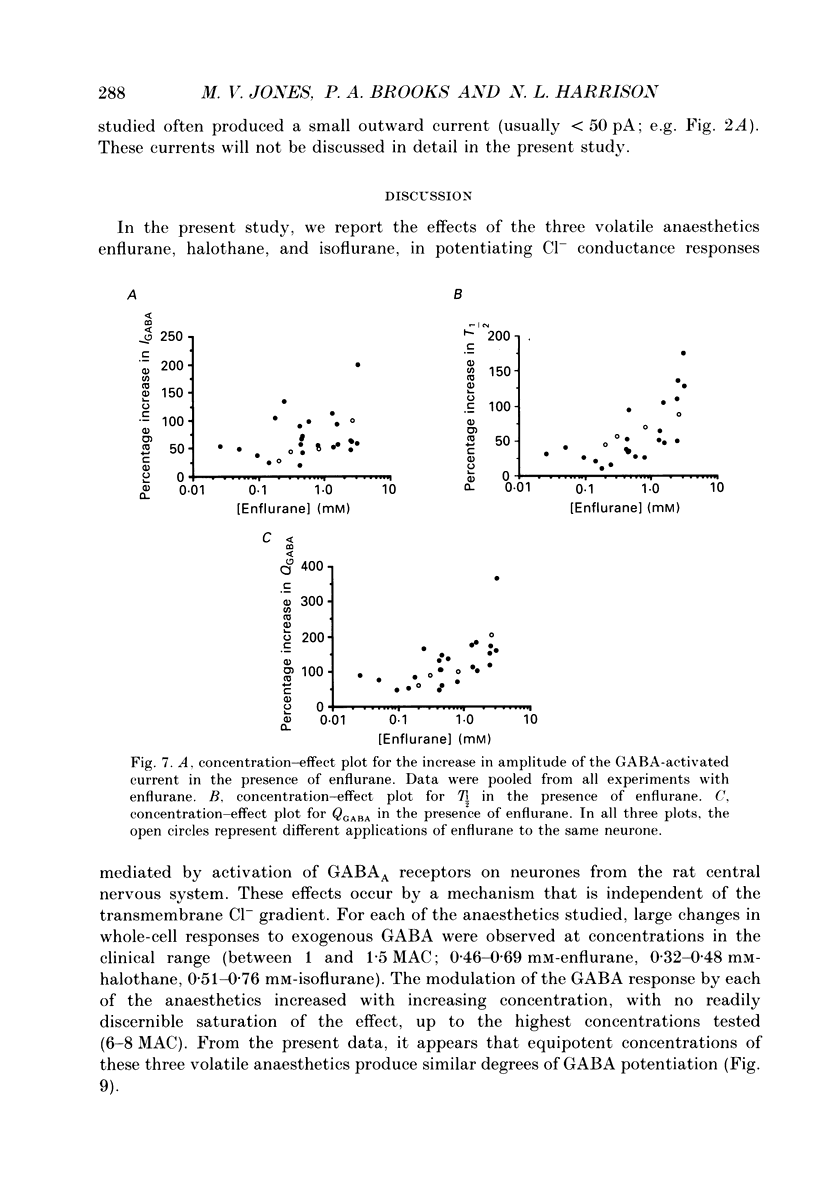
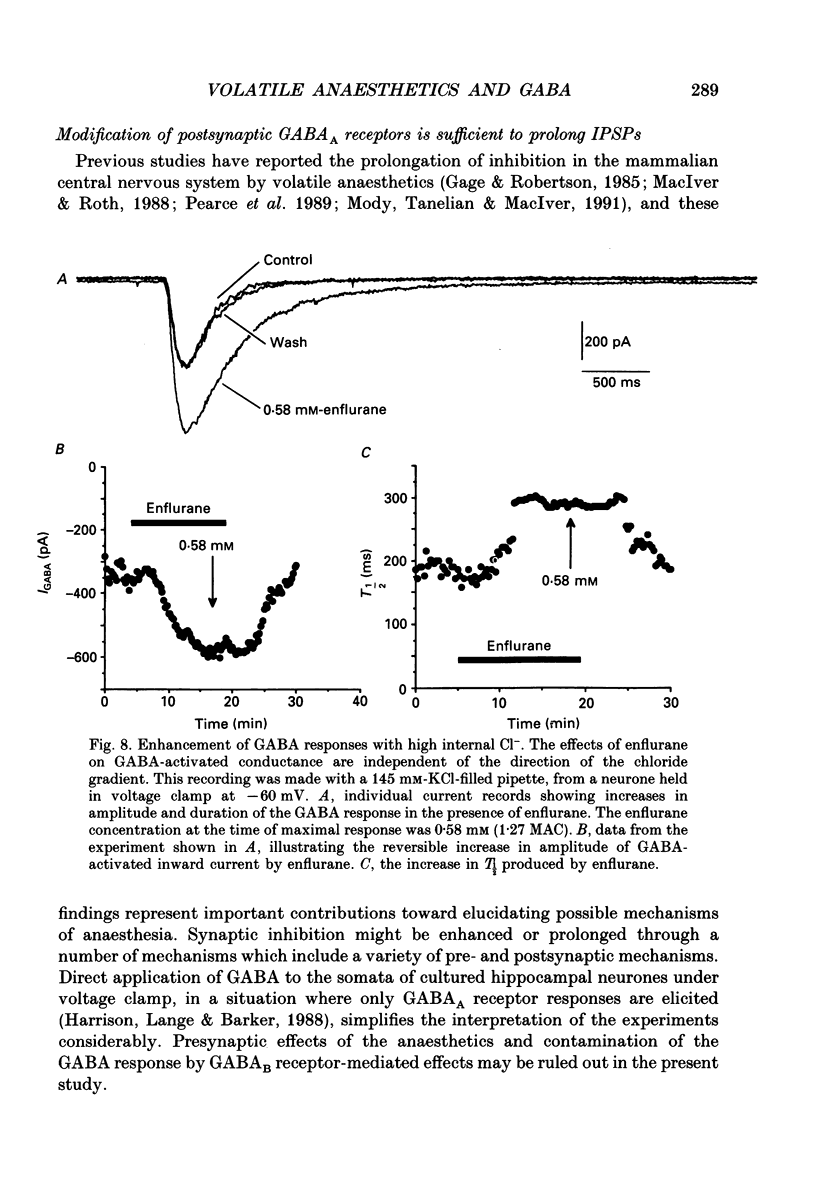
1. The effects of the volatile anaesthetics enflurane, halothane and isoflurane on gamma-aminobutyric acid (GABA)A receptor-mediated chloride currents were studied in cultured rat hippocampal neurones. Transient current responses were obtained by brief pressure application of GABA to the cell body of neurones under voltage clamp. 2. All three anaesthetics increased the peak amplitude and duration of current 2. All three anaesthetics increased the peak amplitude and duration of current responses to brief applications of GABA. These effects were fully reversible, and did not involve alterations in the reversal potential for GABA responses. 3. The experimental concentrations of anaesthetics were measured directly using gas chromatography. The enhancement of GABA currents increased with increasing anaesthetic concentration. Clinically effective concentrations of anaesthetics (between 1 and 1.5 times MAC (minimum alveolar concentration) produced significant enhancement of GABA currents. 4. These results demonstrate that the changes in the time course of synaptic inhibition reported in the presence of the volatile anaesthetics are likely to result from modification of the function of postsynaptic GABAA receptor-channel complexes. These findings also support the hypothesis that GABAA receptor complexes serve as common molecular target sites for a variety of structurally diverse anaesthetic molecules.
Full text
PDF


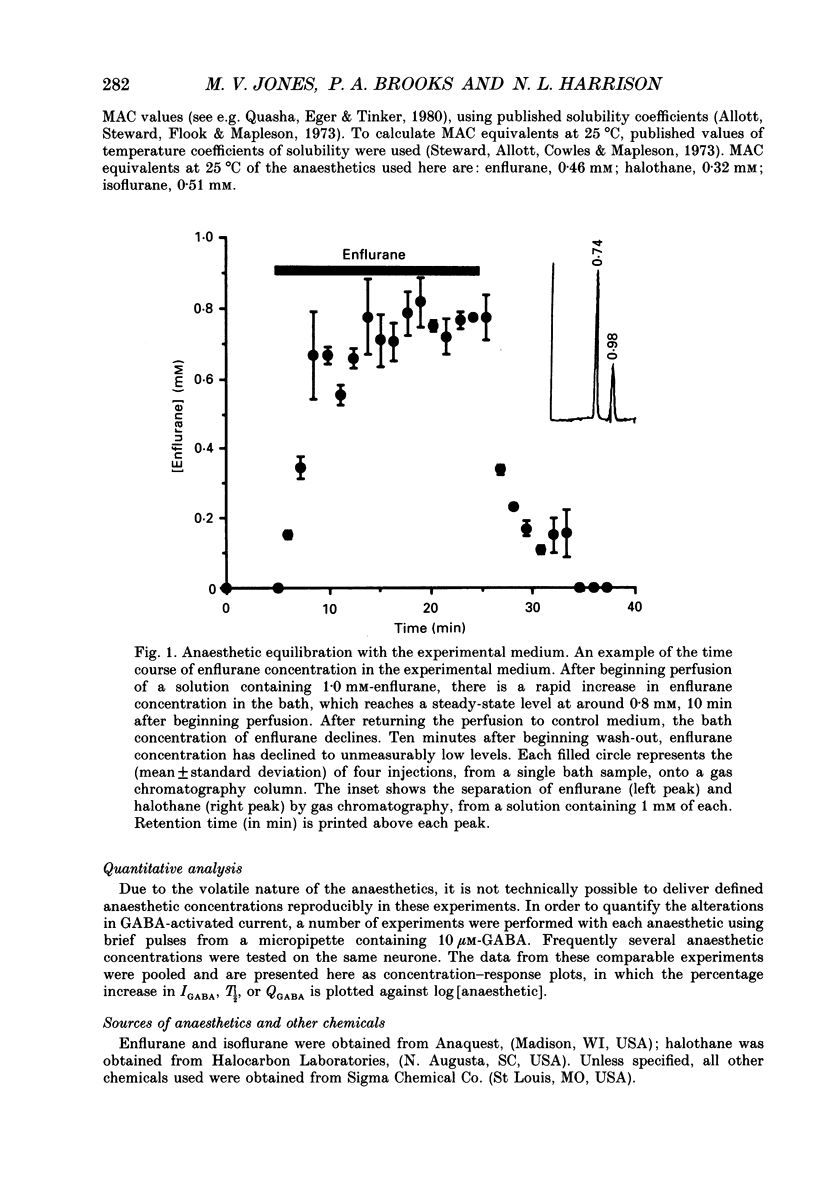
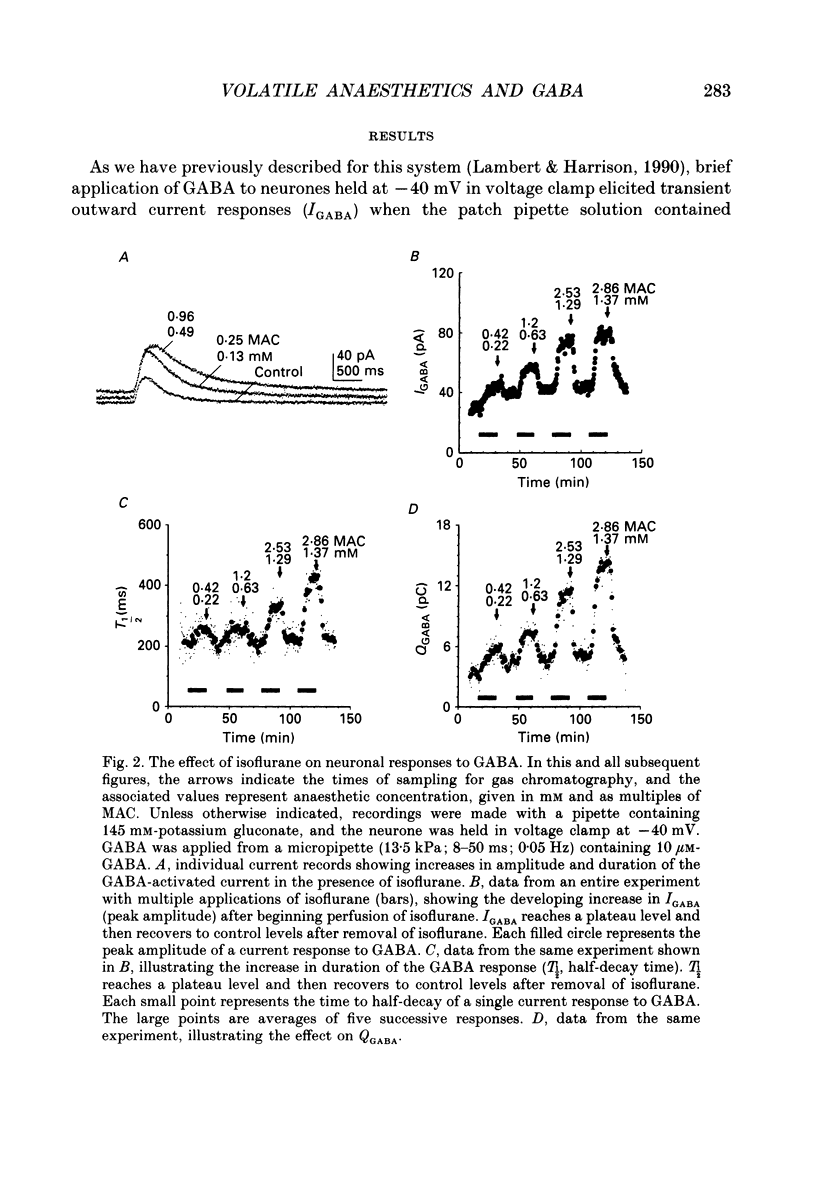
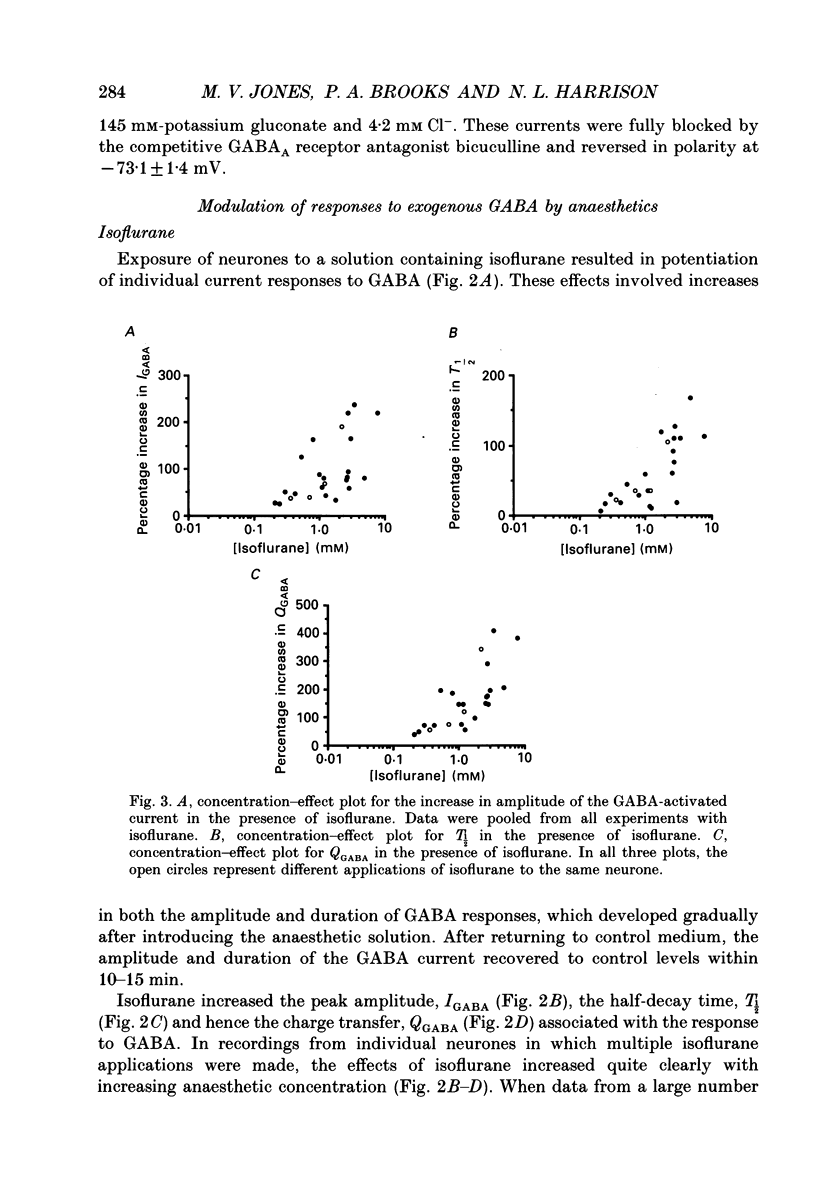
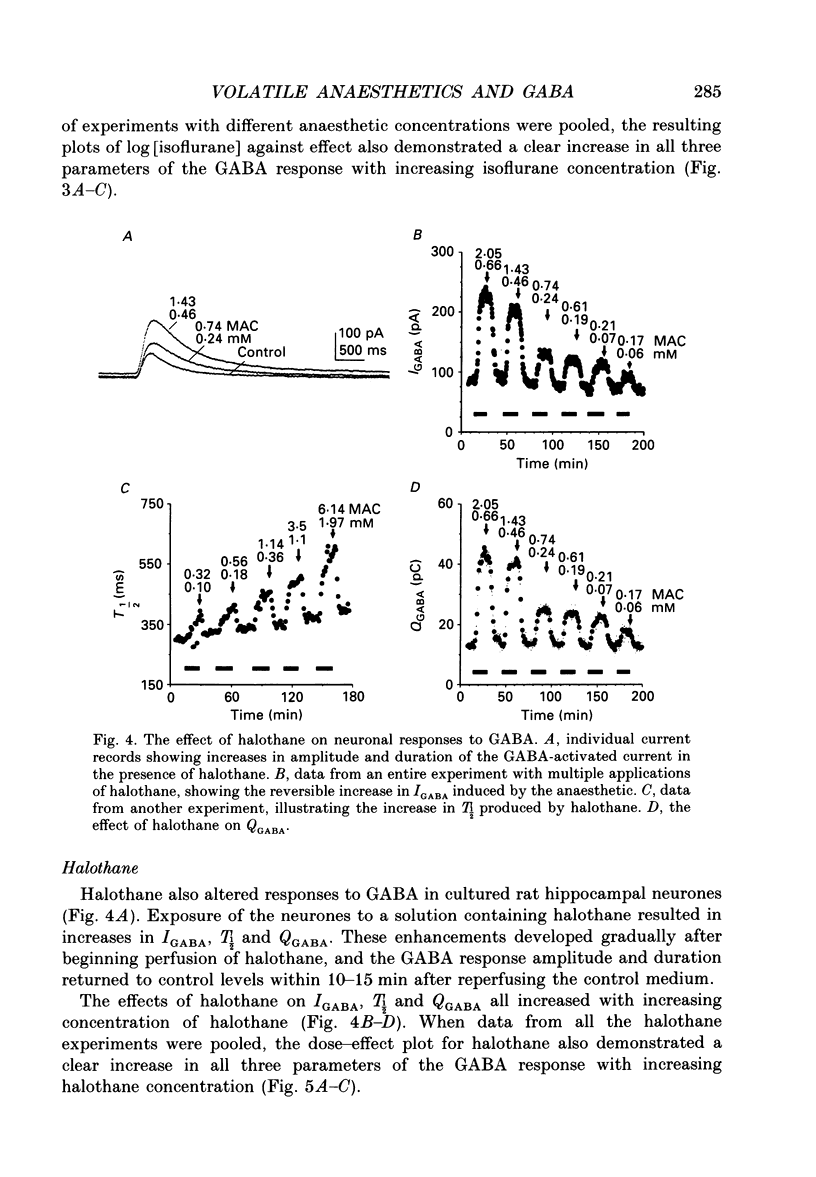



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Hattori K., Inomata N., Oomura Y. gamma-Aminobutyric-acid- and pentobarbitone-gated chloride currents in internally perfused frog sensory neurones. J Physiol. 1985 Mar;360:367–386. doi: 10.1113/jphysiol.1985.sp015622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Inomata N., Tokutomi N. Contribution of chloride shifts to the fade of gamma-aminobutyric acid-gated currents in frog dorsal root ganglion cells. J Physiol. 1987 Oct;391:219–234. doi: 10.1113/jphysiol.1987.sp016735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allott P. R., Steward A., Flook V., Mapleson W. W. Variation with temperature of the solubilities of inhaled anaesthetics in water, oil and biological media. Br J Anaesth. 1973 Mar;45(3):294–300. doi: 10.1093/bja/45.3.294. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Ransom B. R. Pentobarbitone pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:355–372. doi: 10.1113/jphysiol.1978.sp012388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. A physiological role for GABAB receptors in the central nervous system. Nature. 1988 Mar 10;332(6160):156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., SCHMIDT R., WILLIS W. D. PHARMACOLOGICAL STUDIES ON PRESYNAPTIC INHIBITION. J Physiol. 1963 Oct;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger E. I., 2nd, Saidman L. J., Brandstater B. Minimum alveolar anesthetic concentration: a standard of anesthetic potency. Anesthesiology. 1965 Nov-Dec;26(6):756–763. doi: 10.1097/00000542-196511000-00010. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Do general anaesthetics act by competitive binding to specific receptors? Nature. 1984 Aug 16;310(5978):599–601. doi: 10.1038/310599a0. [DOI] [PubMed] [Google Scholar]
- Fujiwara N., Higashi H., Nishi S., Shimoji K., Sugita S., Yoshimura M. Changes in spontaneous firing patterns of rat hippocampal neurones induced by volatile anaesthetics. J Physiol. 1988 Aug;402:155–175. doi: 10.1113/jphysiol.1988.sp017198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Robertson B. Prolongation of inhibitory postsynaptic currents by pentobarbitone, halothane and ketamine in CA1 pyramidal cells in rat hippocampus. Br J Pharmacol. 1985 Jul;85(3):675–681. doi: 10.1111/j.1476-5381.1985.tb10563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Lange G. D., Barker J. L. (-)-Baclofen activates presynaptic GABAB receptors on GABAergic inhibitory neurons from embryonic rat hippocampus. Neurosci Lett. 1988 Feb 15;85(1):105–109. doi: 10.1016/0304-3940(88)90437-5. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Majewska M. D., Harrington J. W., Barker J. L. Structure-activity relationships for steroid interaction with the gamma-aminobutyric acidA receptor complex. J Pharmacol Exp Ther. 1987 Apr;241(1):346–353. [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Baughman R. W. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci. 1986 Oct;6(10):3044–3060. doi: 10.1523/JNEUROSCI.06-10-03044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huidobro-Toro J. P., Bleck V., Allan A. M., Harris R. A. Neurochemical actions of anesthetic drugs on the gamma-aminobutyric acid receptor-chloride channel complex. J Pharmacol Exp Ther. 1987 Sep;242(3):963–969. [PubMed] [Google Scholar]
- Lambert N. A., Harrison N. L. Analogs of cyclic AMP decrease gamma-aminobutyric acidA receptor-mediated chloride current in cultured rat hippocampal neurons via an extracellular site. J Pharmacol Exp Ther. 1990 Oct;255(1):90–94. [PubMed] [Google Scholar]
- MacDonald R. L., Rogers C. J., Twyman R. E. Barbiturate regulation of kinetic properties of the GABAA receptor channel of mouse spinal neurones in culture. J Physiol. 1989 Oct;417:483–500. doi: 10.1113/jphysiol.1989.sp017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver M. B., Roth S. H. Inhalation anaesthetics exhibit pathway-specific and differential actions on hippocampal synaptic responses in vitro. Br J Anaesth. 1988 May;60(6):680–691. doi: 10.1093/bja/60.6.680. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Rogers C. J., Twyman R. E. Kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol. 1989 Mar;410:479–499. doi: 10.1113/jphysiol.1989.sp017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A. GABA as an inhibitory neurotransmitter in human cerebral cortex. J Neurophysiol. 1989 Nov;62(5):1018–1027. doi: 10.1152/jn.1989.62.5.1018. [DOI] [PubMed] [Google Scholar]
- Mody I., Tanelian D. L., MacIver M. B. Halothane enhances tonic neuronal inhibition by elevating intracellular calcium. Brain Res. 1991 Jan 11;538(2):319–323. doi: 10.1016/0006-8993(91)90447-4. [DOI] [PubMed] [Google Scholar]
- Moody E. J., Suzdak P. D., Paul S. M., Skolnick P. Modulation of the benzodiazepine/gamma-aminobutyric acid receptor chloride channel complex by inhalation anesthetics. J Neurochem. 1988 Nov;51(5):1386–1393. doi: 10.1111/j.1471-4159.1988.tb01102.x. [DOI] [PubMed] [Google Scholar]
- Nakahiro M., Yeh J. Z., Brunner E., Narahashi T. General anesthetics modulate GABA receptor channel complex in rat dorsal root ganglion neurons. FASEB J. 1989 May;3(7):1850–1854. doi: 10.1096/fasebj.3.7.2541038. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Eccles J. C., Oshima T., Rubia F. Prolongation of hippocampal inhibitory postsynaptic potentials by barbiturates. Nature. 1975 Dec 18;258(5536):625–627. doi: 10.1038/258625a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Madison D. V. General anesthetics hyperpolarize neurons in the vertebrate central nervous system. Science. 1982 Sep 10;217(4564):1055–1057. doi: 10.1126/science.7112112. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The effects of anaesthetics on synaptic excitation and inhibition in the olfactory bulb. J Physiol. 1972 Jun;223(3):803–814. doi: 10.1113/jphysiol.1972.sp009875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numann R. E., Wong R. K. Voltage-clamp study on GABA response desensitization in single pyramidal cells dissociated from the hippocampus of adult guinea pigs. Neurosci Lett. 1984 Jun 29;47(3):289–294. doi: 10.1016/0304-3940(84)90528-7. [DOI] [PubMed] [Google Scholar]
- Olsen R. W. Drug interactions at the GABA receptor-ionophore complex. Annu Rev Pharmacol Toxicol. 1982;22:245–277. doi: 10.1146/annurev.pa.22.040182.001333. [DOI] [PubMed] [Google Scholar]
- Pearce R. A., Stringer J. L., Lothman E. W. Effect of volatile anesthetics on synaptic transmission in the rat hippocampus. Anesthesiology. 1989 Oct;71(4):591–598. doi: 10.1097/00000542-198910000-00019. [DOI] [PubMed] [Google Scholar]
- Quasha A. L., Eger E. I., 2nd, Tinker J. H. Determination and applications of MAC. Anesthesiology. 1980 Oct;53(4):315–334. doi: 10.1097/00000542-198010000-00008. [DOI] [PubMed] [Google Scholar]
- Roth S. H. Physical mechanisms of anesthesia. Annu Rev Pharmacol Toxicol. 1979;19:159–178. doi: 10.1146/annurev.pa.19.040179.001111. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Scholfield C. N. Potentiation of inhibition by general anaesthetics in neurones of the olfactory cortex in vitro. Pflugers Arch. 1980 Feb;383(3):249–255. doi: 10.1007/BF00587527. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: voltage-clamp analysis of inhibitory synaptic connections. J Neurophysiol. 1984 Sep;52(3):469–487. doi: 10.1152/jn.1984.52.3.469. [DOI] [PubMed] [Google Scholar]
- Shivers B. D., Killisch I., Sprengel R., Sontheimer H., Köhler M., Schofield P. R., Seeburg P. H. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989 Sep;3(3):327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Steward A., Allott P. R., Cowles A. L., Mapleson W. W. Solubility coefficients for inhaled anaesthetics for water, oil and biological media. Br J Anaesth. 1973 Mar;45(3):282–293. doi: 10.1093/bja/45.3.282. [DOI] [PubMed] [Google Scholar]


