Abstract
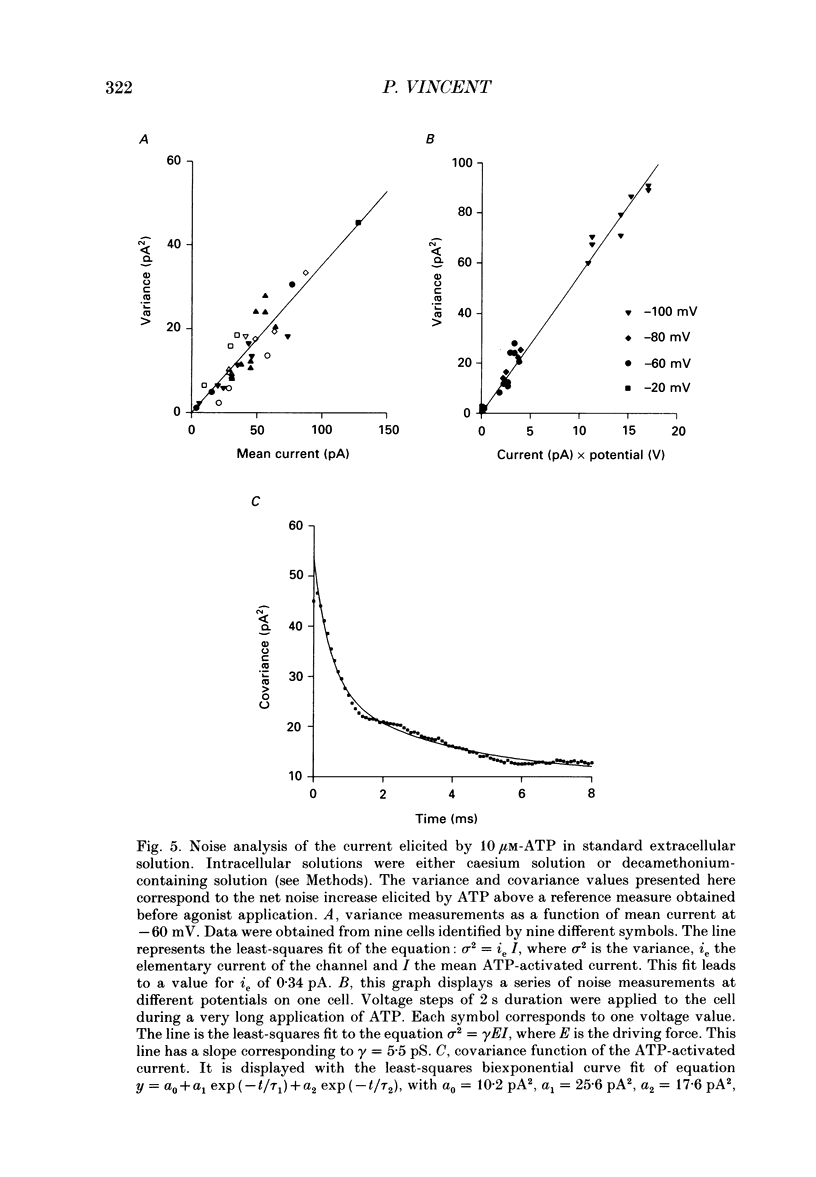
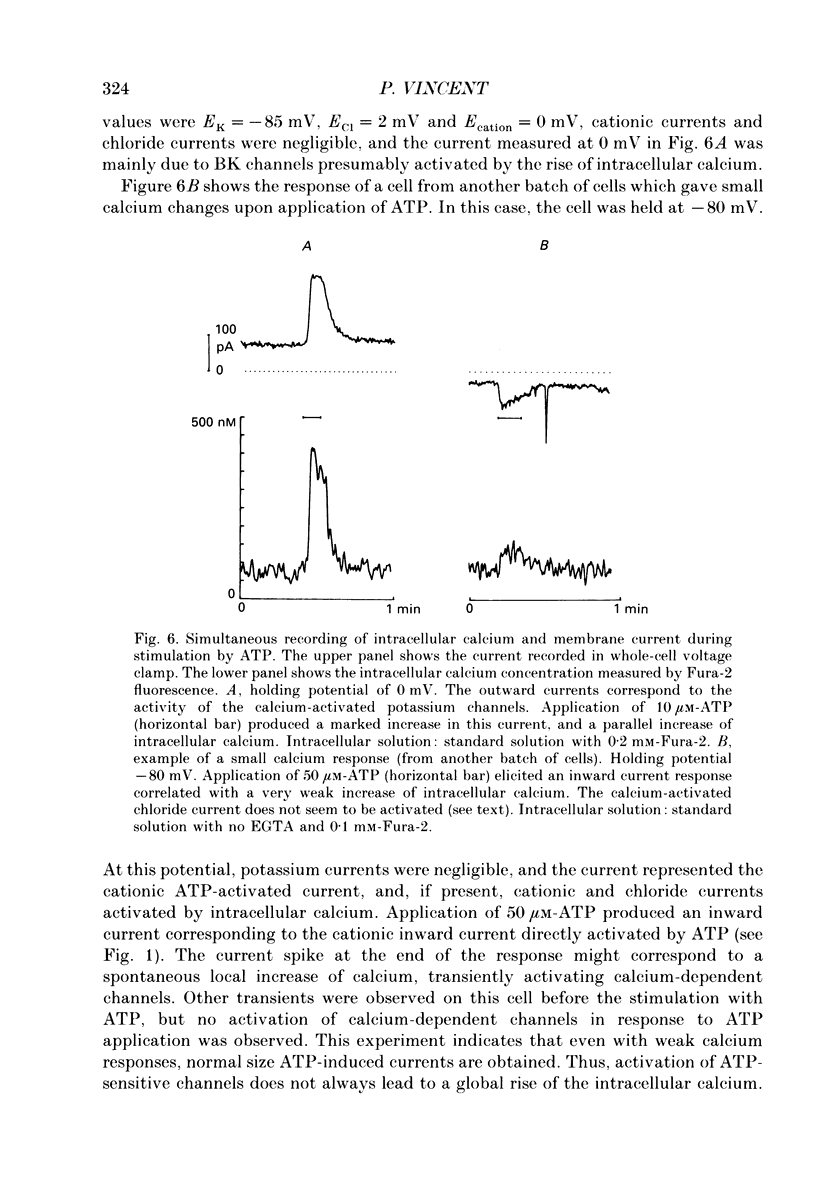
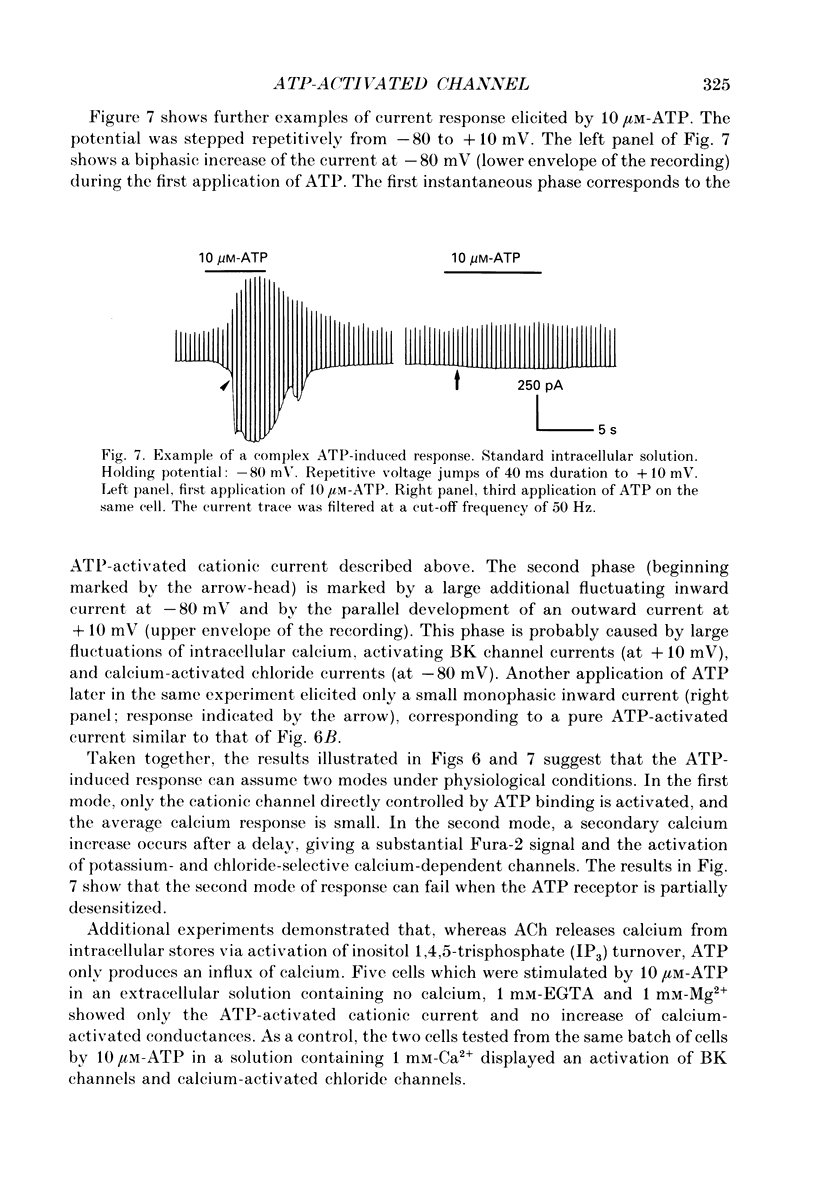
1. Responses of isolated rat lacrimal cells to local applications of ATP were studied using tight-seal whole-cell recording and/or Fura-2-derived calcium concentration measurements. 2. In cells where variations in Ca2+ concentration were prevented by use of a strong Ca2+ buffer, ATP was found to induce an inward current response at negative holding potentials. With 10 microM-ATP, the current amplitude ranged between 20 and 200 pA. The reversal potential of this ATP-induced current was close to 0 mV with normal external solution and shifted to -19 +/- 3 mV (mean +/- S.D.) when the concentration of external monovalent cations was halved. These results indicate that the channels have a cationic selectivity. The response amplitude decreased markedly from trial to trial, indicating a desensitization process which was irreversible on the time scale of the recordings. 3. Steady state I-V curves for the ATP-induced current in normal saline showed a marked inward rectification. This rectification appeared to be linked to a time-dependent activation of the channels, as hyperpolarizing voltage jumps elicited a time-dependent current increase. This relaxation could be fitted by a double-exponential function, with time constants (at -120 mV) of 0.9 +/- 0.3 ms and 110 +/- 6.4 ms. 4. Variance analysis of the ATP-induced current gave a single-channel current value of 0.34 pA at -60 mV. The single-channel current amplitude varied linearly with potential, with a slope close to 6 pS. The relation between noise covariance and time could be fitted by a double-exponential function, with time constants (at -60 mV) of 0.8 +/- 0.4 ms and 6.8 +/- 3.4 ms (mean +/- S.D.). 5. In an isotonic Ca2+ solution, 10 microM-ATP induced an inward current at -60 mV with a calculated single-channel current amplitude obtained from noise analysis close to 0.2 pA. In an external solution containing 10 mM-calcium and no sodium, 50 microM-ATP elicited a current with a reversal potential of -19 mV. 6. Fura-2 measurements were performed in intact cells or in cells dialysed with a low concentration of Ca2+ buffer (e.g. 0.5 mM-EGTA). Under such conditions ATP induced increases of the internal Ca2+ concentration with very variable amplitudes. In some cells Ca2+ rises of 50 nM or lower were found. Minimal activation of Ca(2+)-dependent channels was then observed. In other cells large Ca2+ rises (up to 500 nM) were observed and were then correlated with marked activation of Ca(2+)-dependent channels.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



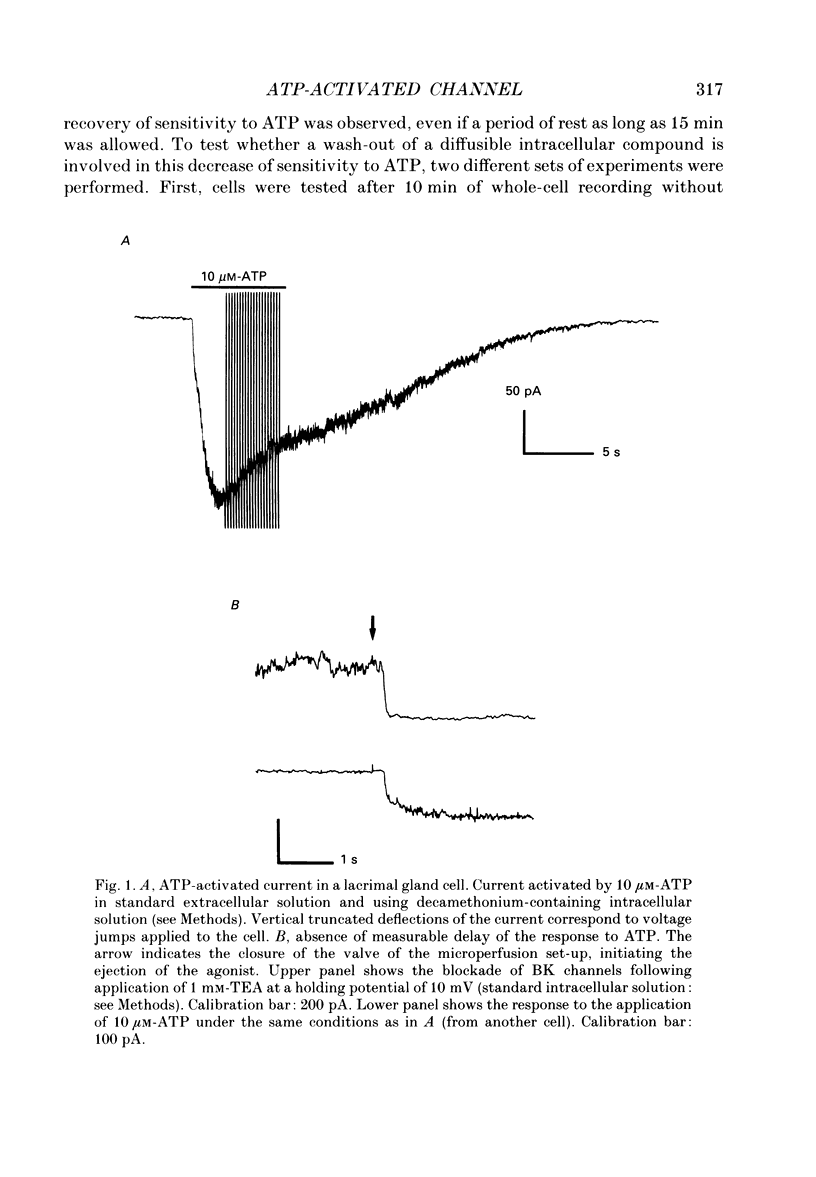
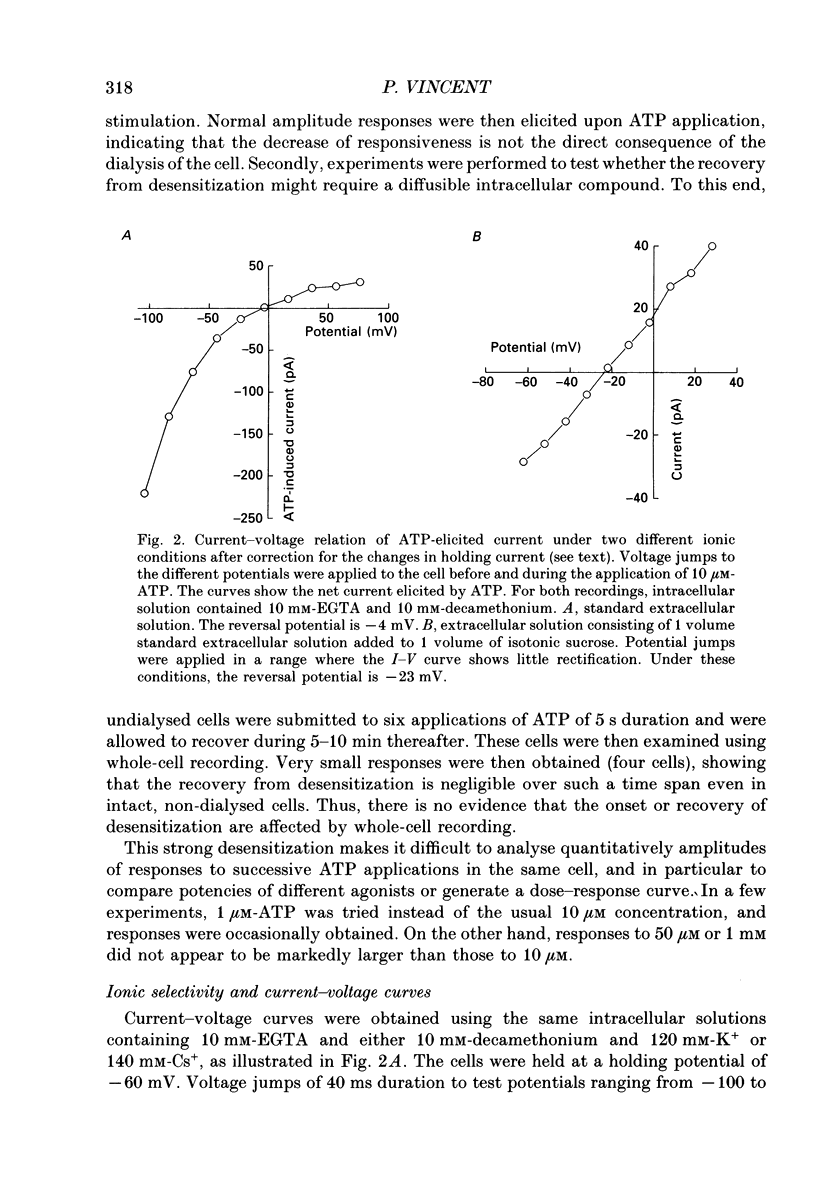
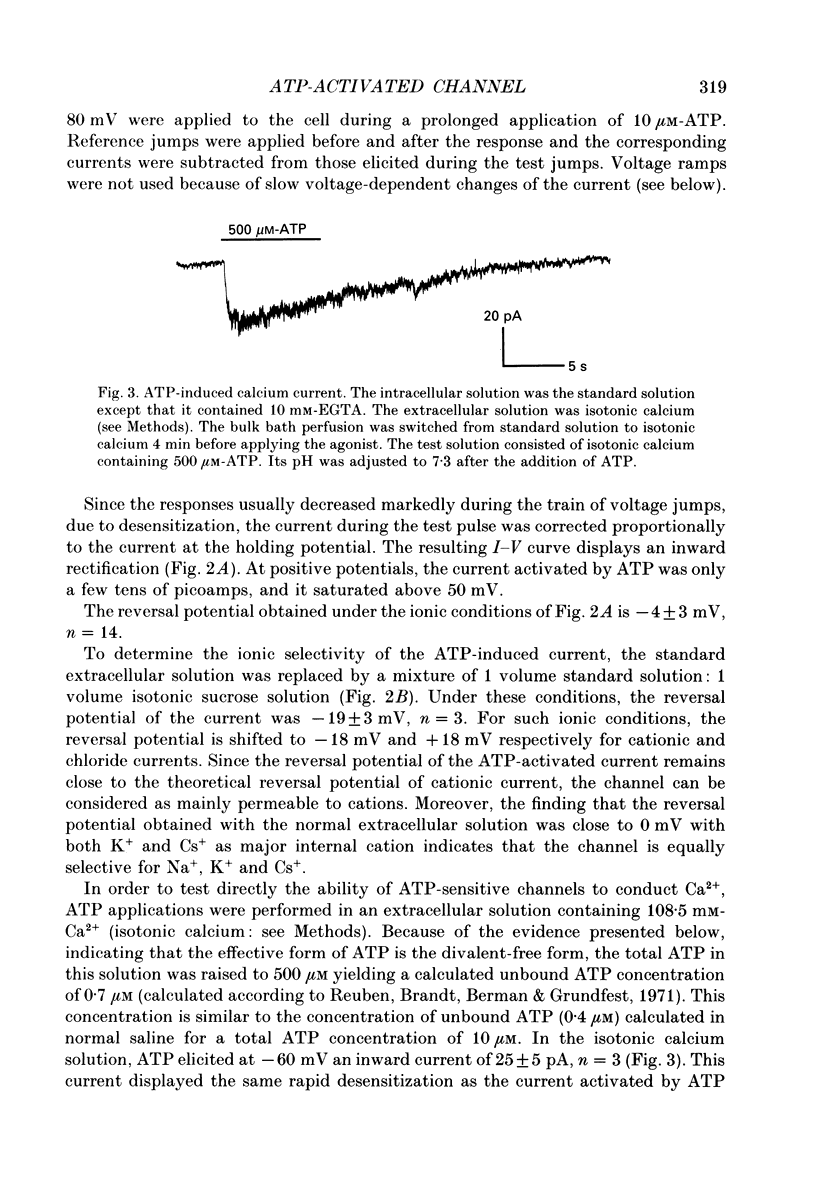
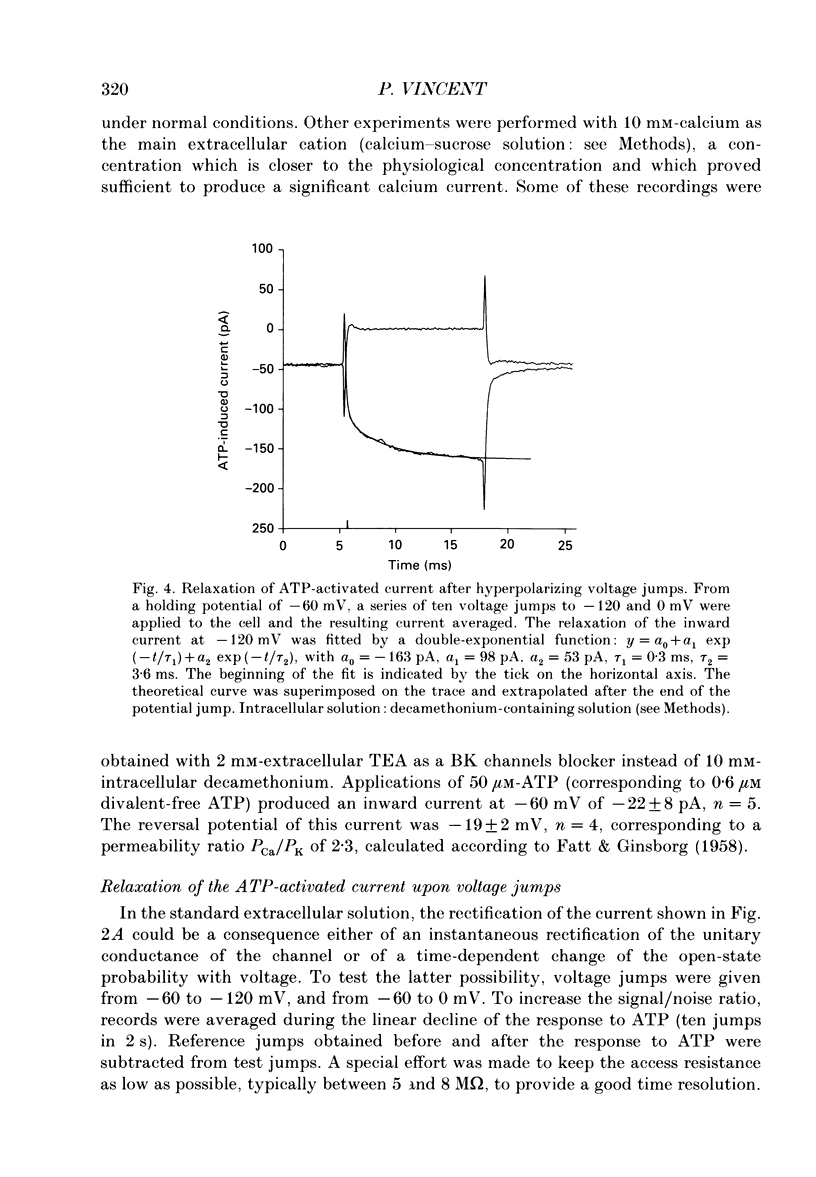








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Williams C. A., Ceelen P. W. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci. 1990 Jan;10(1):11–19. doi: 10.1523/JNEUROSCI.10-01-00011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Buisman H. P., Steinberg T. H., Fischbarg J., Silverstein S. C., Vogelzang S. A., Ince C., Ypey D. L., Leijh P. C. Extracellular ATP induces a large nonselective conductance in macrophage plasma membranes. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7988–7992. doi: 10.1073/pnas.85.21.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol. 1986 Sep;378:437–460. doi: 10.1113/jphysiol.1986.sp016229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I. A patch-clamp study of potassium channels and whole-cell currents in acinar cells of the mouse lacrimal gland. J Physiol. 1984 May;350:179–195. doi: 10.1113/jphysiol.1984.sp015195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D. An ATP-sensitive conductance in single smooth muscle cells from the rat vas deferens. J Physiol. 1988 Jul;401:361–380. doi: 10.1113/jphysiol.1988.sp017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V. Are there purinergic receptors on parotid acinar cells? Nature. 1982 Mar 4;296(5852):83–86. doi: 10.1038/296083a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Obukhov A. G. Cationic channels activated by extracellular ATP in rat sensory neurons. Neuroscience. 1988 Dec;27(3):995–1000. doi: 10.1016/0306-4522(88)90203-5. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Tanguy J. Dependence of intracellular effects of GTP gamma S and inositoltrisphosphate on cell membrane potential and on external Ca ions. Pflugers Arch. 1987 Aug;409(4-5):499–506. doi: 10.1007/BF00583807. [DOI] [PubMed] [Google Scholar]
- Marty A., Tan Y. P. The initiation of calcium release following muscarinic stimulation in rat lacrimal glands. J Physiol. 1989 Dec;419:665–687. doi: 10.1113/jphysiol.1989.sp017892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y., Gallacher D. V., Petersen O. H. Voltage and Ca2+-activated K+ channel in baso-lateral acinar cell membranes of mammalian salivary glands. Nature. 1983 Apr 28;302(5911):827–829. doi: 10.1038/302827a0. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Peterson O. H. Single-channel currents in isolated patches of plasma membrane from basal surface of pancreatic acini. Nature. 1982 Sep 9;299(5879):159–161. doi: 10.1038/299159a0. [DOI] [PubMed] [Google Scholar]
- McMillian M. K., Soltoff S. P., Lechleiter J. D., Cantley L. C., Talamo B. R. Extracellular ATP increases free cytosolic calcium in rat parotid acinar cells. Differences from phospholipase C-linked receptor agonists. Biochem J. 1988 Oct 1;255(1):291–300. [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Fujimori K., Takanaka A., Inoue K. An ATP-activated conductance in pheochromocytoma cells and its suppression by extracellular calcium. J Physiol. 1990 Sep;428:257–272. doi: 10.1113/jphysiol.1990.sp018211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Inoue K., Fujimori K., Takanaka A. ATP-activated single-channel currents recorded from cell-free patches of pheochromocytoma PC12 cells. Neurosci Lett. 1990 Oct 30;119(1):5–8. doi: 10.1016/0304-3940(90)90741-q. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Matsuki N. Adenosine triphosphate-activated inward current in isolated smooth muscle cells from rat vas deferens. Pflugers Arch. 1987 Aug;409(6):644–646. doi: 10.1007/BF00584668. [DOI] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Gallacher D. V. Electrophysiology of pancreatic and salivary acinar cells. Annu Rev Physiol. 1988;50:65–80. doi: 10.1146/annurev.ph.50.030188.000433. [DOI] [PubMed] [Google Scholar]
- Reuben J. P., Brandt P. W., Berman M., Grundfest H. Regulation of tension in the skinned crayfish muscle fiber. I. Contraction and relaxation in the absence of Ca (pCa is greater than 9). J Gen Physiol. 1971 Apr;57(4):385–407. doi: 10.1085/jgp.57.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Gallacher D. V. Extracellular ATP activates receptor-operated cation channels in mouse lacrimal acinar cells to promote calcium influx in the absence of phosphoinositide metabolism. FEBS Lett. 1990 May 7;264(1):130–134. doi: 10.1016/0014-5793(90)80782-e. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., McMillian M. K., Cragoe E. J., Jr, Cantley L. C., Talamo B. R. Effects of extracellular ATP on ion transport systems and [Ca2+]i in rat parotid acinar cells. Comparison with the muscarinic agonist carbachol. J Gen Physiol. 1990 Feb;95(2):319–346. doi: 10.1085/jgp.95.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatham P. E., Lindau M. ATP-induced pore formation in the plasma membrane of rat peritoneal mast cells. J Gen Physiol. 1990 Mar;95(3):459–476. doi: 10.1085/jgp.95.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. A., Hume R. I. Irreversible desensitization of ATP responses in developing chick skeletal muscle. J Physiol. 1990 Nov;430:373–388. doi: 10.1113/jphysiol.1990.sp018296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. A., Hume R. I. Permeation of both cations and anions through a single class of ATP-activated ion channels in developing chick skeletal muscle. J Gen Physiol. 1990 Apr;95(4):569–590. doi: 10.1085/jgp.95.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann A., Marty A. Activation of Ca-dependent K channels by carbamoylcholine in rat lacrimal glands. Proc Natl Acad Sci U S A. 1984 Jan;81(2):611–615. doi: 10.1073/pnas.81.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth C. D., Johnson R. G. Acetylcholine and ATP are coreleased from the electromotor nerve terminals of Narcine brasiliensis by an exocytotic mechanism. Proc Natl Acad Sci U S A. 1990 Jan;87(2):553–557. doi: 10.1073/pnas.87.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H., Westhead E. The molecular organization of adrenal chromaffin granules. Neuroscience. 1980;5(11):1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]


