Abstract
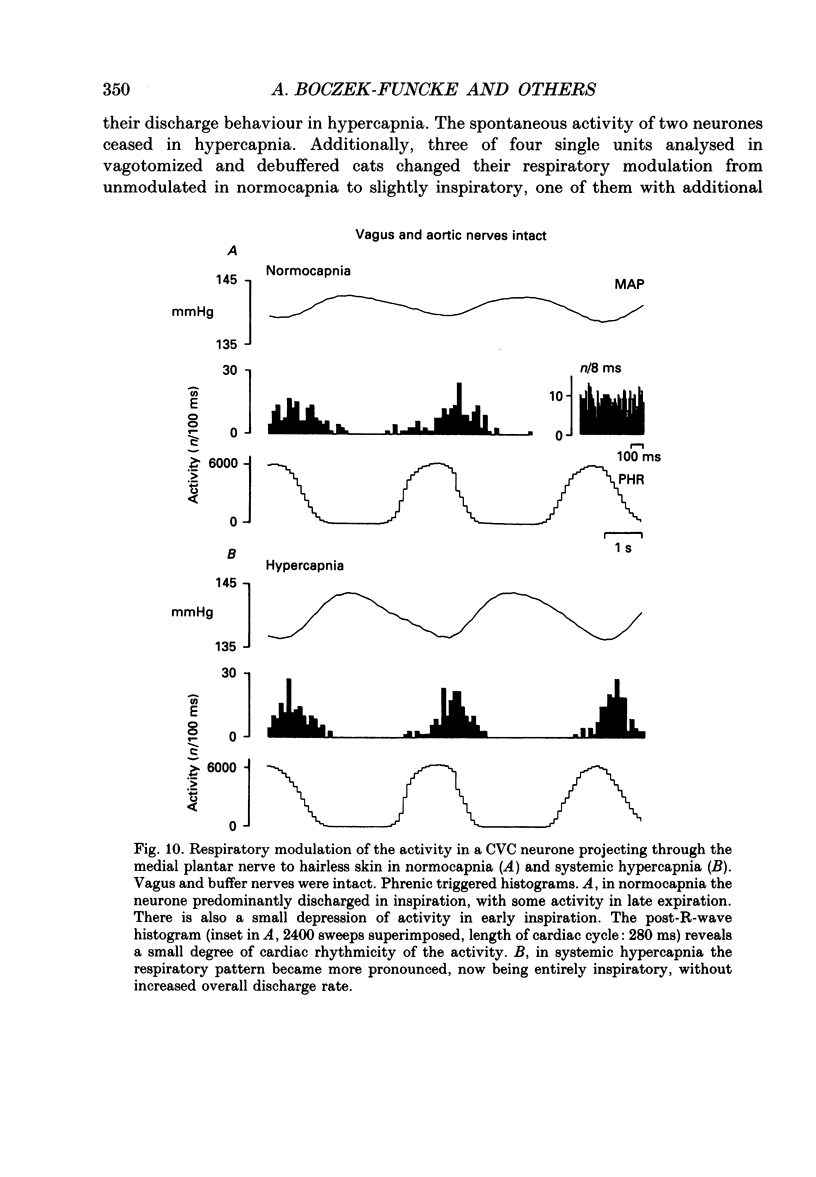
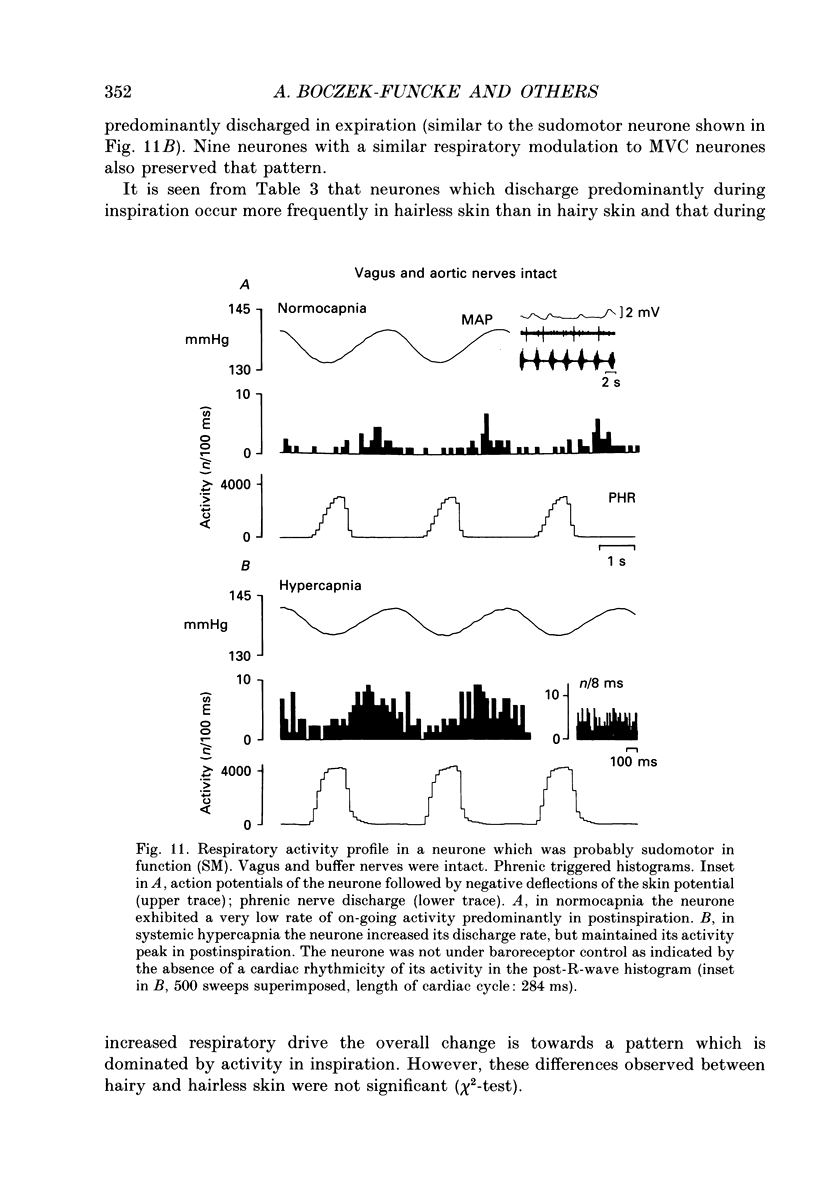
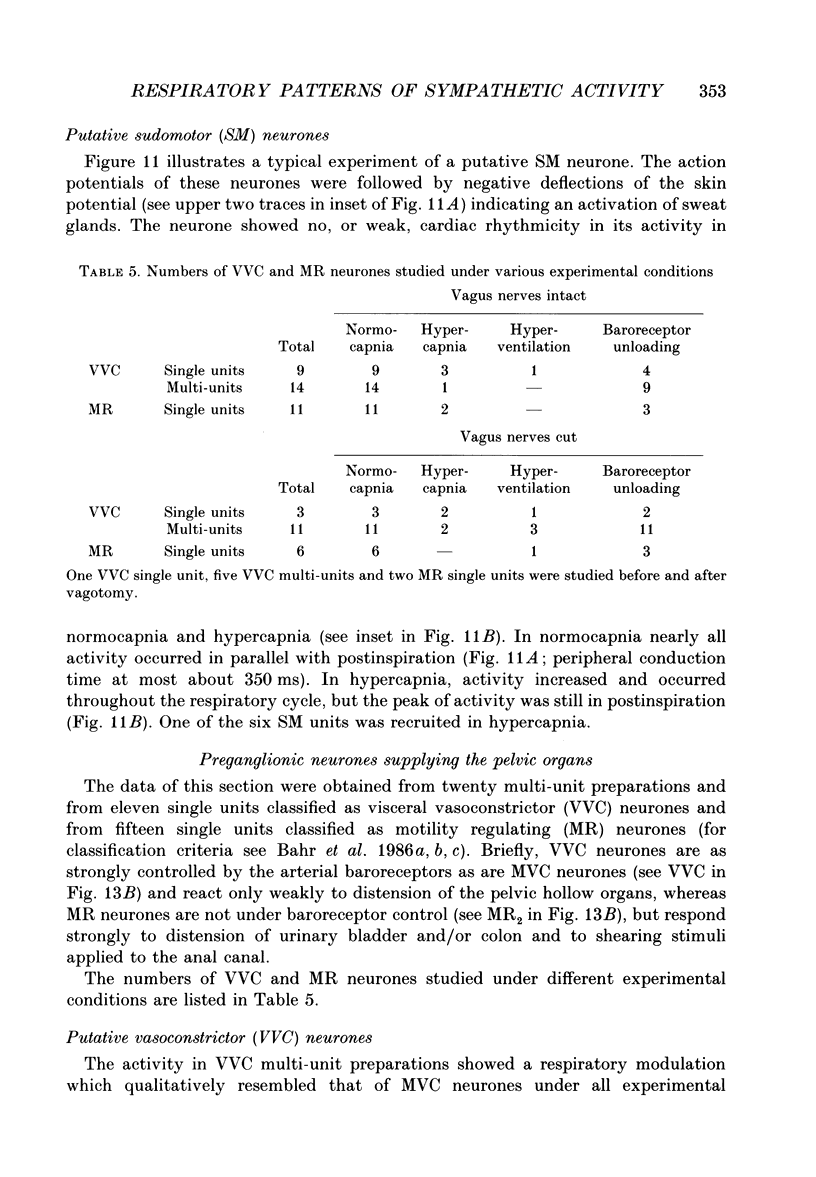
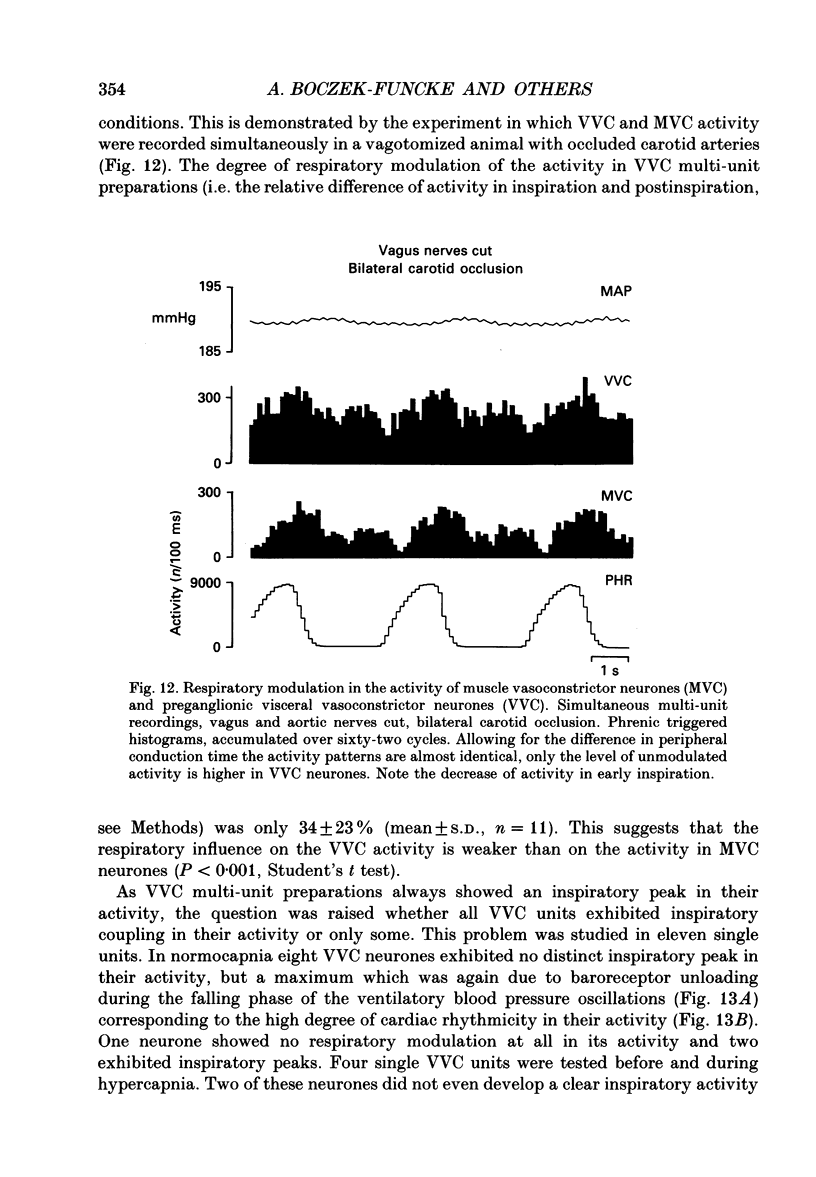
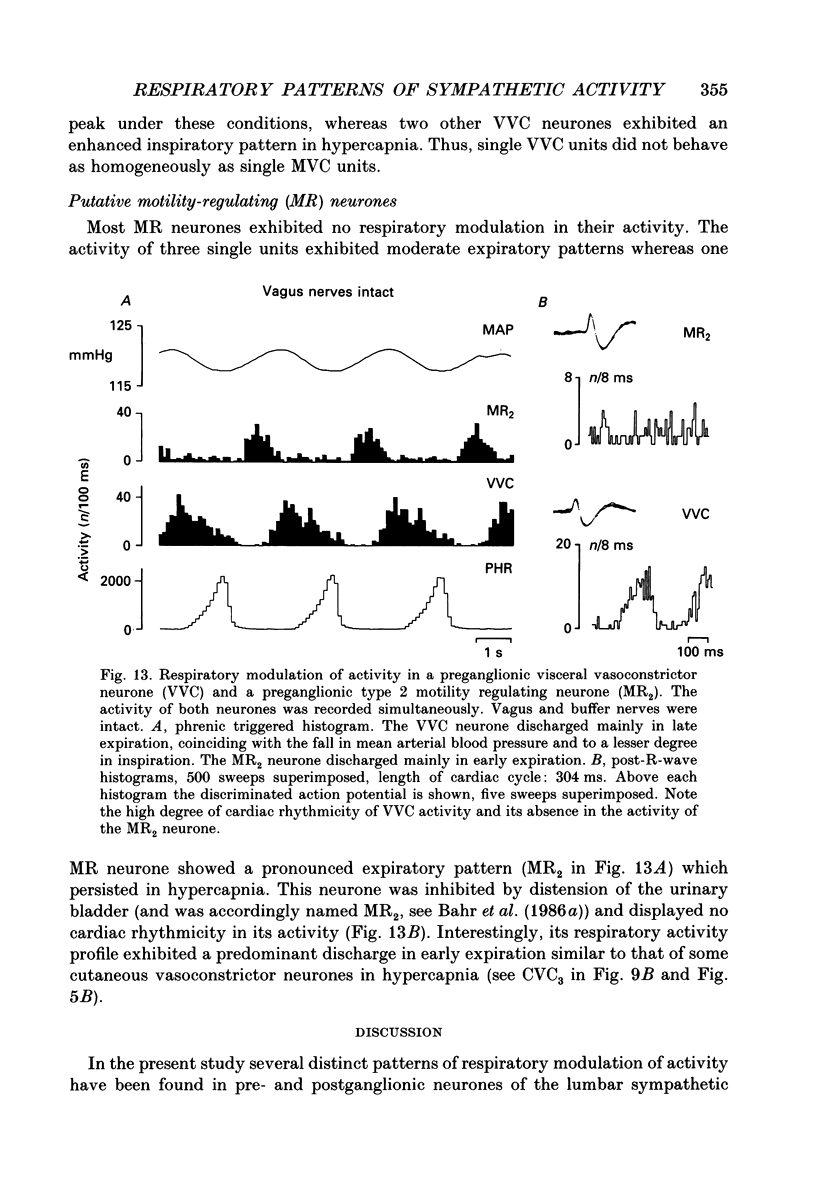
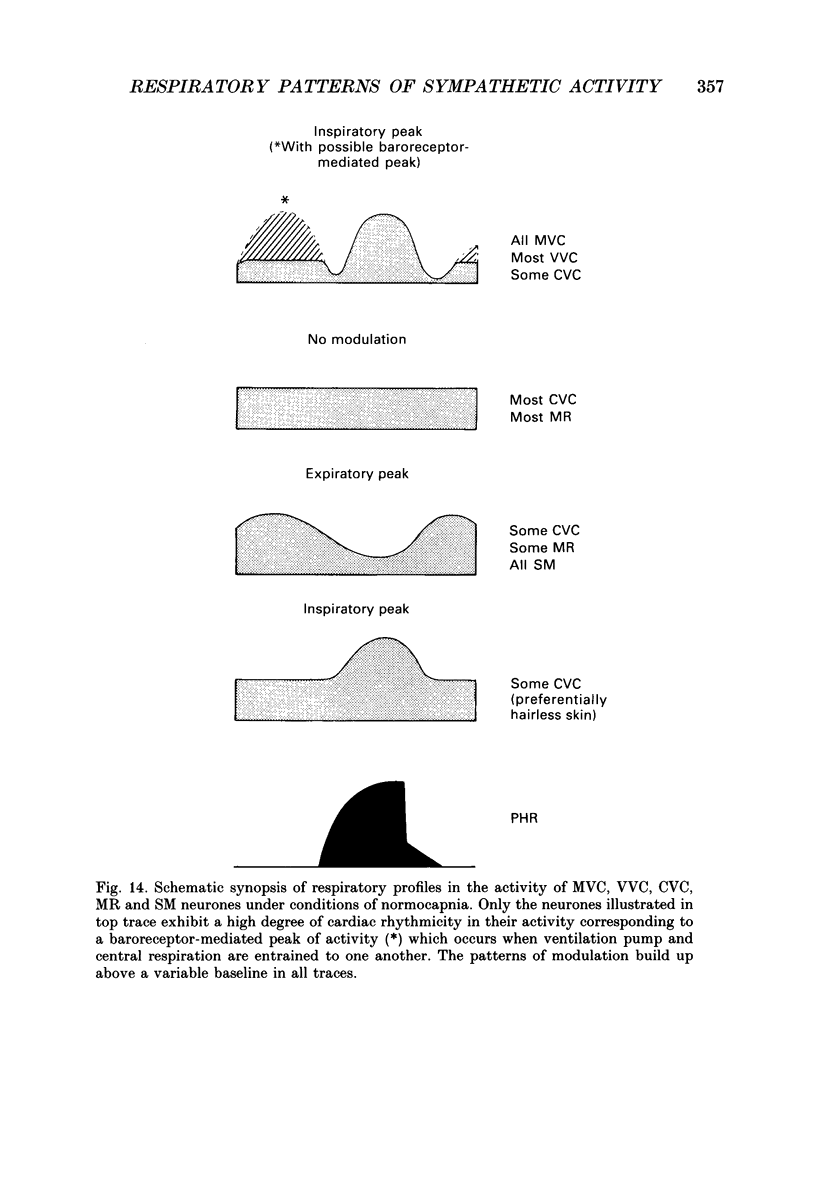
1. The respiratory-related modulation of activity in neurones of the lumbar sympathetic outflow to skeletal muscle, skin and pelvic organs was investigated in anaesthetized, paralysed and artificially ventilated cats, using single- and multi-unit recordings. The activity of the neurones was analysed with respect to the phrenic nerve discharge under various experimental conditions. 2. Neurones tentatively classified as muscle vasoconstrictor and visceral vasoconstrictor neurones exhibited two activity peaks, one caused by baroreceptor unloading during the declining phase of the second order blood pressure waves and a respiratory drive-dependent peak in parallel with inspiration. The two peaks were separated by depressions of activity in early inspiration and post-inspiration. After cutting vagus and buffer nerves the activity peak during inspiration remained and was followed and sometimes preceded by a depression of activity. 3. The majority of the neurones tentatively classified as cutaneous vasoconstrictor neurones exhibited no respiratory modulation in their activity. Others exhibited an activity peak in expiration, an activity peak in inspiration, or a respiratory profile similar to that in muscle vasoconstrictor neurones. During increased respiratory drive (induced by hypercapnia) some neurones with unmodulated activity changed to an inspiratory or an expiratory pattern. Neurones discharging predominantly in inspiration projected preferentially to hairless skin. 4. Neurones which were tentatively classified as sudomotor neurones discharged predominantly in early expiration. 5. Some preganglionic neurones which were tentatively classified as motility-regulating neurones discharged during expiration. The majority of these neurones disclosed no respiratory modulation of their activity. 6. The study shows that different types of neurone of the lumbar sympathetic system exhibit distinct patterns of respiratory modulation in their activity. We conclude that the type and degree of central coupling between respiratory system and sympathetic nervous system may vary according to the destination of the sympathetic neurones.
Full text
PDF




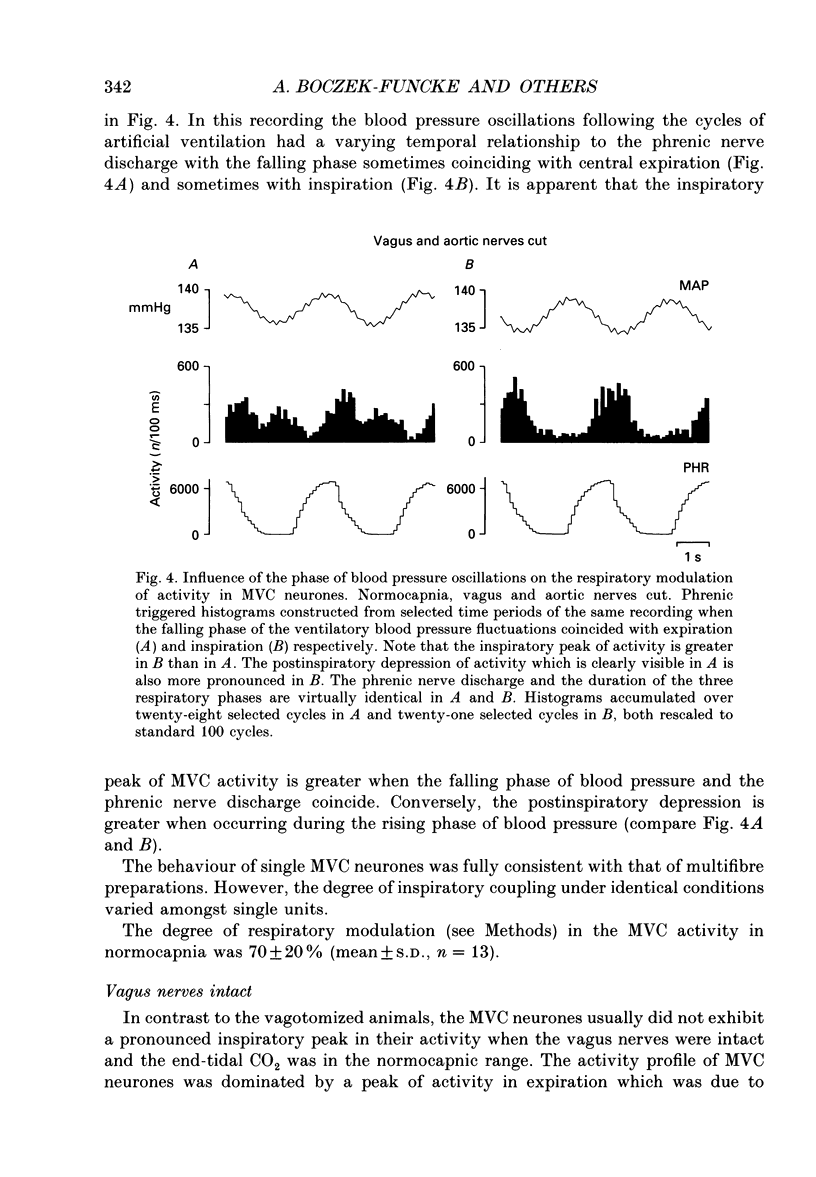
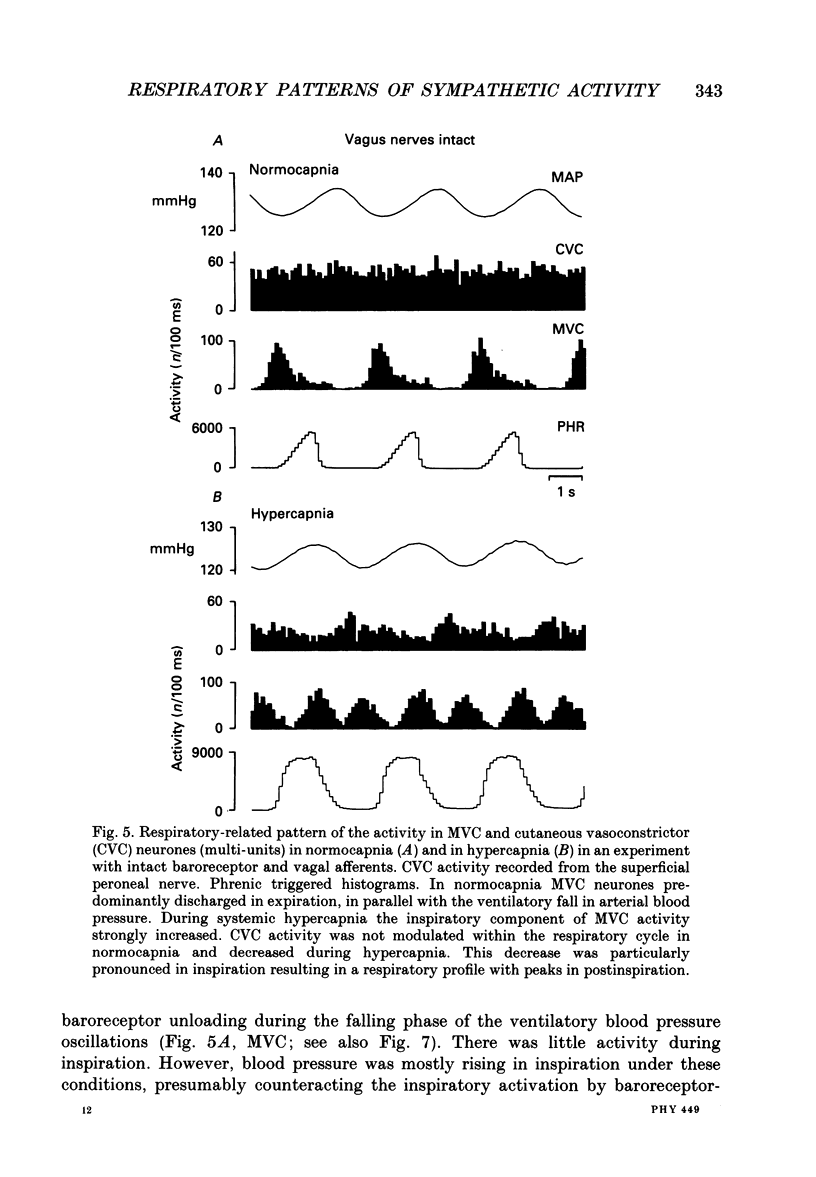
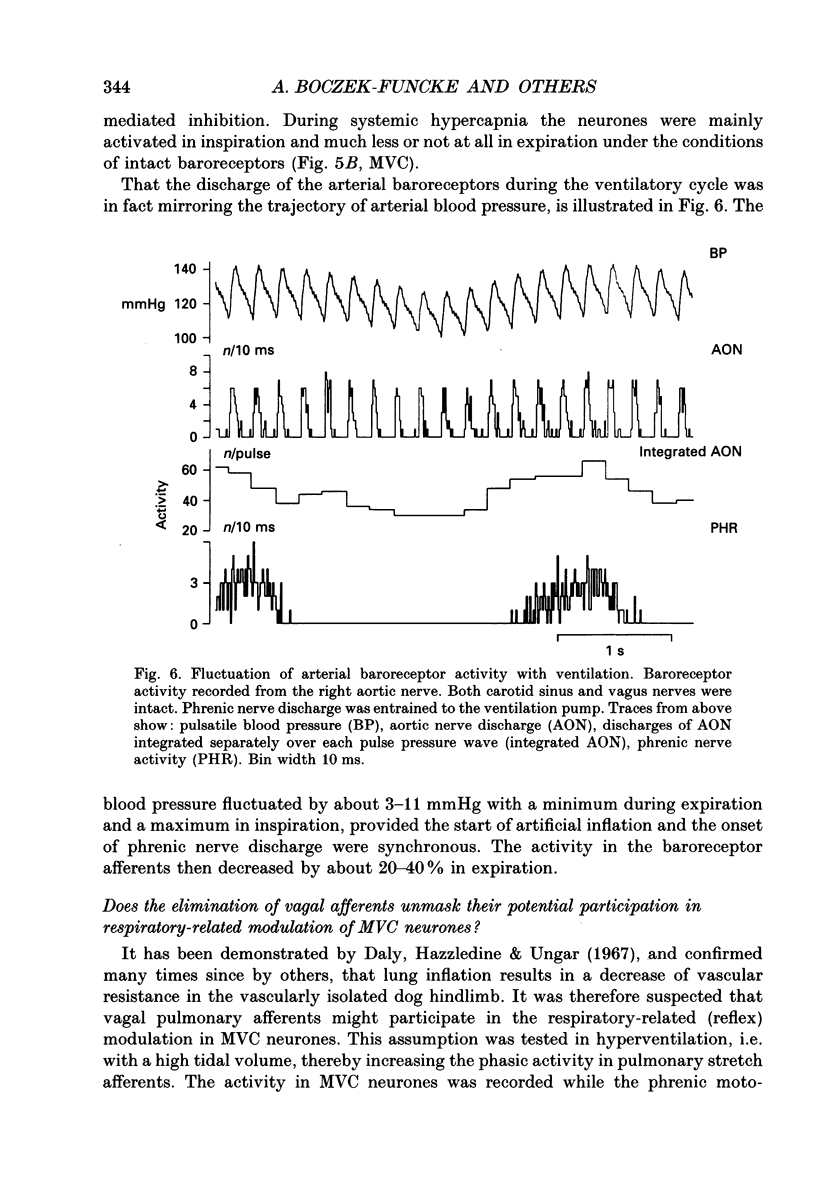
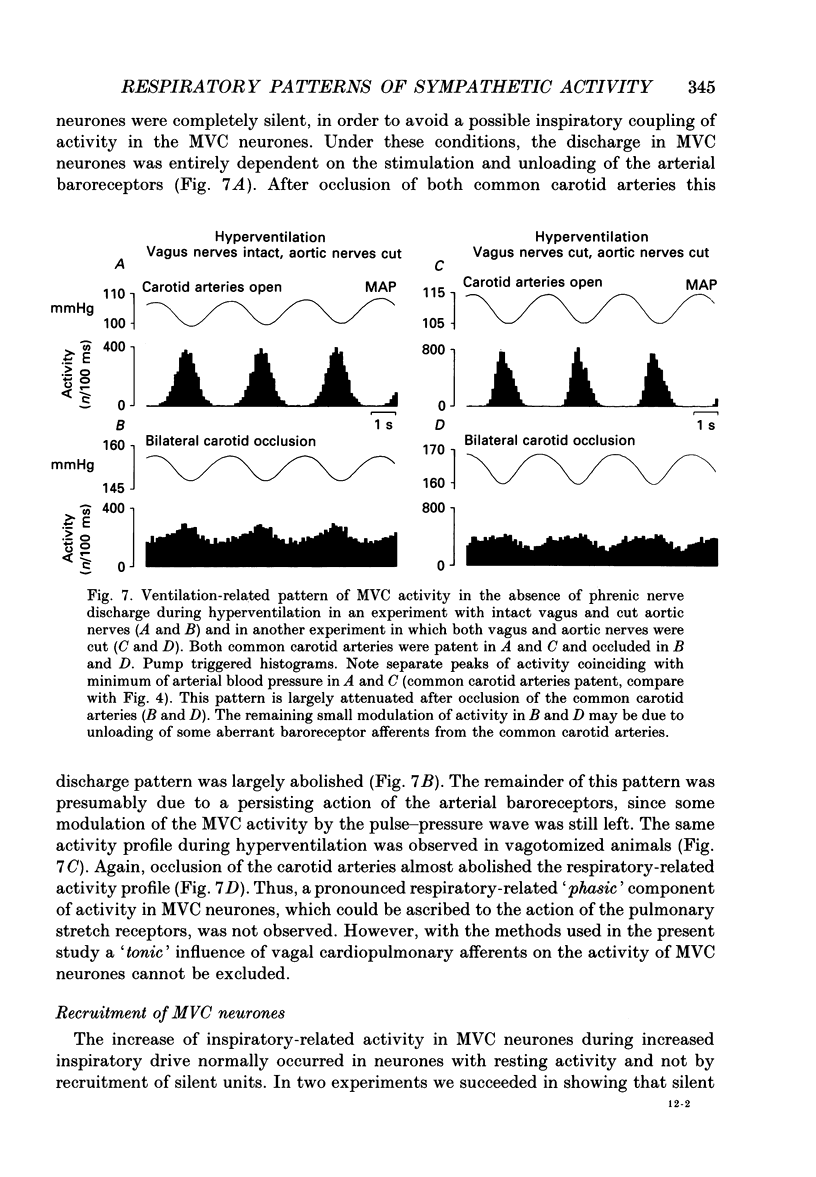





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D., Bronk D. W., Phillips G. Discharges in mammalian sympathetic nerves. J Physiol. 1932 Feb 8;74(2):115–133. doi: 10.1113/jphysiol.1932.sp002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr R., Bartel B., Blumberg H., Jänig W. Functional characterization of preganglionic neurons projecting in the lumbar splanchnic nerves: neurons regulating motility. J Auton Nerv Syst. 1986 Feb;15(2):109–130. doi: 10.1016/0165-1838(86)90008-1. [DOI] [PubMed] [Google Scholar]
- Bahr R., Bartel B., Blumberg H., Jänig W. Functional characterization of preganglionic neurons projecting in the lumbar splanchnic nerves: vasoconstrictor neurons. J Auton Nerv Syst. 1986 Feb;15(2):131–140. doi: 10.1016/0165-1838(86)90009-3. [DOI] [PubMed] [Google Scholar]
- Bahr R., Bartel B., Blumberg H., Jänig W. Secondary functional properties of lumbar visceral preganglionic neurons. J Auton Nerv Syst. 1986 Feb;15(2):141–152. doi: 10.1016/0165-1838(86)90010-x. [DOI] [PubMed] [Google Scholar]
- Bainton C. R., Richter D. W., Seller H., Ballantyne D., Klein J. P. Respiratory modulation of sympathetic activity. J Auton Nerv Syst. 1985 Jan;12(1):77–90. doi: 10.1016/0165-1838(85)90041-4. [DOI] [PubMed] [Google Scholar]
- Bartel B., Blumberg H., Jänig W. Discharge patterns of motility-regulating neurons projecting in the lumbar splanchnic nerves to visceral stimuli in spinal cats. J Auton Nerv Syst. 1986 Feb;15(2):153–163. doi: 10.1016/0165-1838(86)90011-1. [DOI] [PubMed] [Google Scholar]
- Boczek-Funcke A., Häbler H. J., Jänig W., Michaelis M. Rapid phasic baroreceptor inhibition of the activity in sympathetic preganglionic neurones does not change throughout the respiratory cycle. J Auton Nerv Syst. 1991 Jun 15;34(2-3):185–194. doi: 10.1016/0165-1838(91)90084-g. [DOI] [PubMed] [Google Scholar]
- Cassell J. F., McLachlan E. M., Sittiracha T. The effect of temperature on neuromuscular transmission in the main caudal artery of the rat. J Physiol. 1988 Mar;397:31–49. doi: 10.1113/jphysiol.1988.sp016986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. I., Gootman P. M. Periodicities in efferent discharge of splanchnic nerve of the cat. Am J Physiol. 1970 Apr;218(4):1092–1101. doi: 10.1152/ajplegacy.1970.218.4.1092. [DOI] [PubMed] [Google Scholar]
- Darnall R. A., Guyenet P. Respiratory modulation of pre- and postganglionic lumbar vasomotor sympathetic neurons in the rat. Neurosci Lett. 1990 Nov 13;119(2):148–152. doi: 10.1016/0304-3940(90)90820-y. [DOI] [PubMed] [Google Scholar]
- Day T. J., Lagerlund T. D., Low P. A. Analysis of H2 clearance curves used to measure blood flow in rat sciatic nerve. J Physiol. 1989 Jul;414:35–54. doi: 10.1113/jphysiol.1989.sp017675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Burgh Daly M., Hazzledine J. L., Ungar A. The reflex effects of alterations in lung volume on systemic vascular resistance in the dog. J Physiol. 1967 Feb;188(3):331–351. doi: 10.1113/jphysiol.1967.sp008142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg D. L., Nerhed C., Wallin B. G. Respiratory modulation of muscle sympathetic and vagal cardiac outflow in man. J Physiol. 1985 Aug;365:181–196. doi: 10.1113/jphysiol.1985.sp015766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L., Millhorn D. E., Waldrop T. G. Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science. 1981 Feb 20;211(4484):844–846. doi: 10.1126/science.7466362. [DOI] [PubMed] [Google Scholar]
- FUXE K., SEDVALL G. THE DISTRIBUTION OF ADRENERGIC NERVE FIBRES TO THE BLOOD VESSELS IN SKELETAL MUSCLE. Acta Physiol Scand. 1965 May-Jun;64:75–86. doi: 10.1111/j.1748-1716.1965.tb04155.x. [DOI] [PubMed] [Google Scholar]
- Gilbey M. P., Jordan D., Richter D. W., Spyer K. M. Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurones in the cat. J Physiol. 1984 Nov;356:65–78. doi: 10.1113/jphysiol.1984.sp015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbey M. P., Numao Y., Spyer K. M. Discharge patterns of cervical sympathetic preganglionic neurones related to central respiratory drive in the rat. J Physiol. 1986 Sep;378:253–265. doi: 10.1113/jphysiol.1986.sp016218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbey M. P., Stein R. D. Characteristics of sympathetic preganglionic neurones in the lumbar spinal cord of the cat. J Physiol. 1991 Jan;432:427–443. doi: 10.1113/jphysiol.1991.sp018392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor M., Jänig W., Wiprich L. Cardiac and respiratory rhythmicities in cutaneous and muscle vasoconstrictor neurones to the cat's hindlimb. Pflugers Arch. 1977 Sep 16;370(3):299–302. doi: 10.1007/BF00585543. [DOI] [PubMed] [Google Scholar]
- Hagbarth K. E., Hallin R. G., Hongell A., Torebjörk H. E., Wallin B. G. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand. 1972 Feb;84(2):164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Hagbarth K. E., Vallbo A. B. Pulse and respiratory grouping of sympathetic impulses in human muscle-nerves. Acta Physiol Scand. 1968 Sep-Oct;74(1):96–108. doi: 10.1111/j.1748-1716.1968.tb04218.x. [DOI] [PubMed] [Google Scholar]
- Herbert D. A., Mitchell R. A. Blood gas tensions and acid-base balance in awake cats. J Appl Physiol. 1971 Mar;30(3):434–436. doi: 10.1152/jappl.1971.30.3.434. [DOI] [PubMed] [Google Scholar]
- Hirst G. D. Neuromuscular transmission in arterioles of guinea-pig submucosa. J Physiol. 1977 Dec;273(1):263–275. doi: 10.1113/jphysiol.1977.sp012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., Kümmel H. Functional discrimination of postganglionic neurones to the cat's hindpaw with respect to the skin potentials recorded from the hairless skin. Pflugers Arch. 1977 Nov 23;371(3):217–225. doi: 10.1007/BF00586261. [DOI] [PubMed] [Google Scholar]
- Jänig W., McLachlan E. M. Organization of lumbar spinal outflow to distal colon and pelvic organs. Physiol Rev. 1987 Oct;67(4):1332–1404. doi: 10.1152/physrev.1987.67.4.1332. [DOI] [PubMed] [Google Scholar]
- Jänig W. Organization of the lumbar sympathetic outflow to skeletal muscle and skin of the cat hindlimb and tail. Rev Physiol Biochem Pharmacol. 1985;102:119–213. doi: 10.1007/BFb0034086. [DOI] [PubMed] [Google Scholar]
- Jänig W., Räth B. Electrodermal reflexes in the cat's paws elicited by natural stimulation of skin. Pflugers Arch. 1977 May 6;369(1):27–32. doi: 10.1007/BF00580806. [DOI] [PubMed] [Google Scholar]
- Jänig W. Spinal cord integration of visceral sensory systems and sympathetic nervous system reflexes. Prog Brain Res. 1986;67:255–277. doi: 10.1016/s0079-6123(08)62767-3. [DOI] [PubMed] [Google Scholar]
- Kendrick E., Oberg B., Wennergren G. Vasoconstrictor fibre discharge to skeletal muscle, kidney, intestine and skin at varying levels of arterial baroreceptor activity in the cat. Acta Physiol Scand. 1972 Aug;85(4):464–476. doi: 10.1111/j.1748-1716.1971.tb05284.x. [DOI] [PubMed] [Google Scholar]
- Koizumi K., Seller H., Kaufman A., Brooks C. M. Pattern of sympathetic discharges and their relation to baroreceptor and respiratory activities. Brain Res. 1971 Apr 2;27(2):281–294. doi: 10.1016/0006-8993(71)90254-x. [DOI] [PubMed] [Google Scholar]
- Lacroix J. S., Stjärne P., Anggård A., Lundberg J. M. Sympathetic vascular control of the pig nasal mucosa: (I). Increased resistance and capacitance vessel responses upon stimulation with irregular bursts compared to continuous impulses. Acta Physiol Scand. 1988 Jan;132(1):83–90. doi: 10.1111/j.1748-1716.1988.tb08301.x. [DOI] [PubMed] [Google Scholar]
- Nilsson H., Ljung B., Sjöblom N., Wallin B. G. The influence of the sympathetic impulse pattern on contractile responses of rat mesenteric arteries and veins. Acta Physiol Scand. 1985 Mar;123(3):303–309. doi: 10.1111/j.1748-1716.1985.tb07592.x. [DOI] [PubMed] [Google Scholar]
- Okada H., Fox I. J. Respiratory grouping of abdominal sympathetic activity in the dog. Am J Physiol. 1967 Jul;213(1):48–56. doi: 10.1152/ajplegacy.1967.213.1.48. [DOI] [PubMed] [Google Scholar]
- Preiss G., Kirchner F., Polosa C. Patterning of sympathetic preganglionic neuron firing by the central respiratory drive. Brain Res. 1975 Apr 11;87(2-3):363–374. doi: 10.1016/0006-8993(75)90434-5. [DOI] [PubMed] [Google Scholar]
- Seller H., Langhorst P., Richter D., Koepchen H. P. Uber die Abhängigkeit der pressorectptorischen Hemmung des Sympathicus von der Atemphase und ihre Auswirkung in der Vasomotorik. Pflugers Arch. 1968;302(4):300–314. doi: 10.1007/BF00592730. [DOI] [PubMed] [Google Scholar]
- TANG P. C., MAIRE F. W., AMASSIAN V. E. Respiratory influence on the vasomotor center. Am J Physiol. 1957 Nov;191(2):218–224. doi: 10.1152/ajplegacy.1957.191.2.218. [DOI] [PubMed] [Google Scholar]


