Abstract
1. Influence of temperature on relaxation rate as a function of isometric tetanus duration and on Ca2+ and Mg2+ dissociation rates from purified parvalbumin (PA) was examined to test the hypothesis that PA promotes relaxation in frog skeletal muscle. Single fibres and PA IVB from Rana temporaria skeletal muscle were utilized. 2. The magnitude of slowing of relaxation rate with increasing tetanus duration, relative to the final, steady value of relaxation rate, is 3-fold greater at O than at 10 degrees C. 3. In the 0-10 degrees C range, the Q10 for relaxation rate increases from 2.3 to 3.7 with increasing tetanus duration. 4. Dissociation of Ca2+ and Mg2+ from PA exhibited: (i) rate constants of 1.03 +/- 0.03 s-1 (mean +/- S.D., n = 5) and 3.42 +/- 0.14 s-1 (n = 5) at 20 degrees C and (ii) Q10 values of 2.3 and 1.9 in the 0-20 degrees C range, respectively. 5. Time courses of slowing of relaxation rate with increasing tetanus duration and recovery of relaxation rate with rest after a prolonged tetanus at 10 degrees C are similar to rates of dissociation of Mg2+ and Ca2+ from PA, respectively, as previously reported at 0 degree C. 6. Both the temperature dependence of the relative magnitude of slowing of relaxation rate and the increased Q10 of relaxation rate with increased tetanus duration can be explained if the Q10 for Ca2+ uptake by the sarcoplasmic reticulum is greater than the Q10 for Ca2+ sequestration by PA during relaxation. When Ca2+ and Mg2+ dissociation rates from PA at various temperatures are compared to other proposed indicators of PA function, it is concluded that PA facilitates relaxation of frog skeletal muscle in the 0-20 degrees C range.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT B. C. The heat production associated with the maintenance of a prolonged contraction and the extra heat produced during large shortening. J Physiol. 1951 Feb;112(3-4):438–445. doi: 10.1113/jphysiol.1951.sp004541. [DOI] [PMC free article] [PubMed] [Google Scholar]
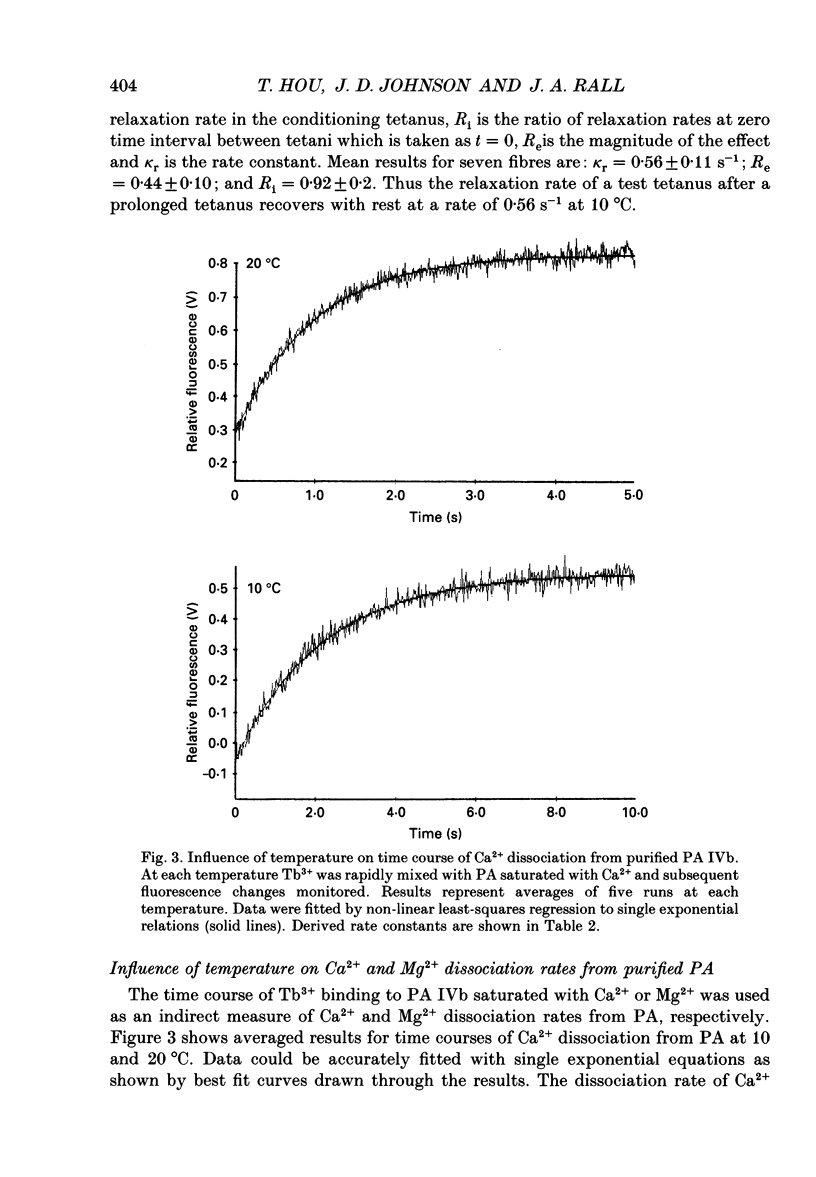
- Cannell M. B. Effect of tetanus duration on the free calcium during the relaxation of frog skeletal muscle fibres. J Physiol. 1986 Jul;376:203–218. doi: 10.1113/jphysiol.1986.sp016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Flitney F. W. Laser diffraction studies of sarcomere dynamics during 'isometric' relaxation in isolated muscle fibres of the frog. J Physiol. 1982 Aug;329:1–20. doi: 10.1113/jphysiol.1982.sp014287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis J. M., Piront A., Gosselin-Rey C. Parvalbumins. Distribution and physical state inside the muscle cell. Biochim Biophys Acta. 1979 Jul 4;585(3):444–450. doi: 10.1016/0304-4165(79)90089-8. [DOI] [PubMed] [Google Scholar]
- Gillis J. M., Thomason D., Lefèvre J., Kretsinger R. H. Parvalbumins and muscle relaxation: a computer simulation study. J Muscle Res Cell Motil. 1982 Dec;3(4):377–398. doi: 10.1007/BF00712090. [DOI] [PubMed] [Google Scholar]
- Haiech J., Derancourt J., Pechère J. F., Demaille J. G. Magnesium and calcium binding to parvalbumins: evidence for differences between parvalbumins and an explanation of their relaxing function. Biochemistry. 1979 Jun 26;18(13):2752–2758. doi: 10.1021/bi00580a010. [DOI] [PubMed] [Google Scholar]
- Hou T. T., Johnson J. D., Rall J. A. Parvalbumin content and Ca2+ and Mg2+ dissociation rates correlated with changes in relaxation rate of frog muscle fibres. J Physiol. 1991 Sep;441:285–304. doi: 10.1113/jphysiol.1991.sp018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving M., Maylie J., Sizto N. L., Chandler W. K. Simultaneous monitoring of changes in magnesium and calcium concentrations in frog cut twitch fibers containing antipyrylazo III. J Gen Physiol. 1989 Apr;93(4):585–608. doi: 10.1085/jgp.93.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y., Tanokura M. Kinetic studies of calcium binding to parvalbumins from bullfrog skeletal muscle. J Biochem. 1986 Jan;99(1):81–89. doi: 10.1093/oxfordjournals.jbchem.a135482. [DOI] [PubMed] [Google Scholar]
- Pechère J. F., Derancourt J., Haiech J. The participation of parvalbumins in the activation-relaxation cycle of vertebrate fast skeletal-muscle. FEBS Lett. 1977 Mar 15;75(1):111–114. doi: 10.1016/0014-5793(77)80064-1. [DOI] [PubMed] [Google Scholar]
- Peckham M., Woledge R. C. Labile heat and changes in rate of relaxation of frog muscles. J Physiol. 1986 May;374:123–135. doi: 10.1113/jphysiol.1986.sp016070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permyakov E. A., Ostrovsky A. V., Kalinichenko L. P. Stopped-flow kinetic studies of Ca(II) and Mg(II) dissociation in cod parvalbumin and bovine alpha-lactalbumin. Biophys Chem. 1987 Dec;28(3):225–233. doi: 10.1016/0301-4622(87)80093-5. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., McClellan G., Gonzalez-Serratos H., Somlyo A. P. Electron probe X-ray microanalysis of post-tetanic Ca2+ and Mg2+ movements across the sarcoplasmic reticulum in situ. J Biol Chem. 1985 Jun 10;260(11):6801–6807. [PubMed] [Google Scholar]


