Abstract
Epithelial cell keratins make up the type I (K9–K20) and type II (K1–K8) intermediate filament proteins. In glandular epithelia, K8 becomes phosphorylated on S73 (71LLpSPL) in human cultured cells and tissues during stress, apoptosis, and mitosis. Of all known proteins, the context of the K8 S73 motif (LLS/TPL) is unique to type II keratins and is conserved in epidermal K5/K6, esophageal K4, and type II hair keratins, except that serine is replaced by threonine. Because knowledge regarding epidermal and esophageal keratin regulation is limited, we tested whether K4–K6 are phosphorylated on the LLTPL motif. K5 and K6 become phosphorylated in vitro on threonine by the stress-activated kinase p38. Site-specific anti-phosphokeratin antibodies to LLpTPL were generated, which demonstrated negligible basal K4–K6 phosphorylation. In contrast, treatment of primary keratinocytes and other cultured cells, and ex vivo skin and esophagus cultures, with serine/threonine phosphatase inhibitors causes a dramatic increase in K4–K6 LLpTPL phosphorylation. This phosphorylation is accompanied by keratin solubilization, filament reorganization, and collapse. K5/K6 LLTPL phosphorylation occurs in vivo during mitosis and apoptosis induced by UV light or anisomycin, and in human psoriatic skin and squamous cell carcinoma. In conclusion, type II keratins of proliferating epithelia undergo phosphorylation at a unique and conserved motif as part of physiological mitotic and stress-related signals.
INTRODUCTION
Epithelial cell keratins consist of noncovalently associated type I (K9–K20) and type II (K1–K8) intermediate filament (IF) proteins that form obligate heteropolymers of at least one type I and one type II keratin (Moll et al., 1982; Herrmann and Aebi, 2000; Coulombe and Omary, 2002). Simple-type (single-layered) epithelia as found in the liver, intestine, and pancreas express unique combinations of K7, K8, K18, K19, and K20, but in all cells the stoichiometry of type I/type II keratins is 1:1 (Moll et al., 1982; Ku et al., 1999). The basal cells of stratified epithelia (e.g., in keratinocytes and esophageal epithelia) express K5/14, whereas the differentiated suprabasal epithelial cells of the esophagus and skin express K4/13 and K1/10, respectively (Moll et al., 1982; Pang et al., 1993; Fuchs, 1997; Ness et al., 1998). K6 is up-regulated in hyperproliferating keratinocytes as seen during wound healing and in psoriasis, and is also expressed basally in the outer sheet of hair follicles as a partner to K16 and K17 (Rao et al., 1996; Coulombe, 1997). Therefore, keratins manifest cell type- and differentiation-specific expression profiles that probably reflect unique functions (Coulombe and Omary, 2002).
Phosphorylation is an important regulatory modification of keratins, and in this regard, K1, K8, K18, and K19 are the best studied of the keratin family (Steinert, 1988; Omary et al., 1998; Zhou et al., 1999). Modulation of keratin phosphorylation occurs upon exposure to multiple contexts, including stress, apoptosis, and mitosis with resultant regulation of keratin filament organization and keratin interaction with its binding proteins (Ku et al., 1999). Serine is by far the major physiological phosphorylated keratin residue (Oshima, 1982; Omary et al., 1998) with minimal tyrosine (Feng et al., 1999) and threonine (Steinert, 1988; Omary et al., 1998) phosphorylation.
Human (h) K8 S73 (S79 in mouse) is a highly dynamic phosphorylation site that becomes phosphorylated in cultured cells and tissues during mitosis and various cell stress conditions, including apoptosis, heat stress, and virus infection (Omary et al., 1998). The context of hK8 S73 is 71LLSPL, which is a highly conserved motif in K4, K5, and K6 of type II keratins except that serine is replaced by threonine as a potential phosphorylation residue: T150 (148LLTPL) in hK5, T145 (143LLTPL) in hK6, and T133 (131LLTPL) in hK4 (Table 1). The LLS/TPL motif in K4–K6 and K8 renders it a probable substrate for phosphorylation by proline-directed stress-regulated and mitogen-activated kinases (Davis, 2000; Ono and Han, 2000; Kyriakis and Avruch, 2001). For K8 S73, it is phosphorylated by p38 stress-activated protein kinase or c-Jun N-terminal kinase (JNK) in a context-dependent manner, and its phosphorylation is important in facilitating filament reorganization in cultured cells (He et al., 2002; Ku et al., 2002).
Table 1.
The LLS/TPL motif in mouse and human type-II keratins
| Keratin | L | L | S/T | P | L | [amino acids] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epithelial cell keratins — “Soft” keratins | |||||||||||||
| hK5 | N | Q | S | L | L | T | P | L | N | L | Q | [149–159] | |
| hK6a-c,e,fa | • | • | • | • | • | • | • | • | • | • | • | [140–150] | |
| hK6hfa | • | • | • | • | • | • | • | • | H | • | • | [126–136] | |
| mK6αb | • | • | • | • | • | • | • | • | • | • | • | [129–139] | |
| mK6βb | • | • | N | • | • | • | • | • | • | V | • | [137–147] | |
| hK4 | • | • | • | • | • | • | • | • | H | V | E | [128–138] | |
| mK4 | • | • | • | • | • | • | • | • | Q | V | E | [123–133] | |
| hK8 | • | • | • | • | • | S | • | • | S | • | E | [68–78] | |
| mK8 | • | • | • | • | • | S | • | • | K | • | E | [74–84] | |
| hK1 | • | • | • | • | • | Q | • | • | • | V | • | [157–167] | |
| Hair keratins — “Hard” keratins | |||||||||||||
| hHb1, hHb6 | • | E | • | • | • | • | • | • | • | • | E | [83–93] | |
| hHb2 | • | E | • | • | • | V | • | • | A | • | E | [97–107] | |
| hHb3 | • | E | • | • | • | • | • | • | • | • | E | [88–99] | |
| hHb4 | • | K | • | • | • | • | • | • | • | • | E | [142–152] | |
| hHb5, mHb5c | • | E | • | • | • | • | • | • | • | • | E | [100–110] | |
Notably, the K5 P151→L mutation (Muller et al., 1998) involves the proline of the LLS/PL motif, and the K1 L160→P mutation (Chipev et al., 1992) creates a proline-directed kinase phosphorylation site (Ku et al., 2002). LLTPL is also present in sheep type II hair keratins (Powell et al., 1992), while LLSPL is also found in a frog type II keratin (Fouquet et al., 1988).
h, human; m, mouse. For complete sequences see references (Knapp et al., 1986; Liao et al., 1997; Takahashi et al., 1998; Winter et al., 1998; Rogers et al., 2000). Dots indicate amino acid residues that are identical to the corresponding K5 residues.
a-c, e, f, hf represent different isoforms of hK6. Human K6d is likely to have an identical LLTPL motif but its full sequence has not been reported.
α, β correspond to mK6 isoforms. A recently discovered K6 isoform expressed in the mouse inner root sheath, K6irs, does not contain the LLTPL consensus sequence (Aoki et al., 2001).
Pubmed accession AAD01692.
Little if any information is available regarding epidermal keratin phosphorylation, particularly relating to physiological phosphorylation sites and their functions. The best-characterized epidermal keratin, in terms of its phosphorylation, is K1 for which eight serine and one threonine phosphorylation sites were biochemically identified after isolation of K1 from human foreskin (Steinert, 1988). Keratin phosphorylation probably involves all epidermal keratins, because several mouse and human keratinocyte keratins in culture become hyperphosphorylated and solubilized in the presence of phosphatase inhibitors (Kasahara et al., 1993; Yatsunami et al., 1993; Paramio, 1999). In addition, K6 and K16 phosphorylation increases after transforming growth factor-β stimulation (Mansbridge and Hanawalt, 1988) and K6e is phosphorylated on S59 (Ku and Omary, 1997).
To further investigate the presence, function, and importance of epidermal and other stratified epithelial keratin physiological phosphorylation, we examined the potential phosphorylation of K4/5/6 at the conserved LLTPL motif during mitosis and cell stress conditions. A rabbit antibody, termed DL15, was generated against the conserved LLTPL motif and was used with the previously described anti-K8 phospho(p)S73 antibody LJ4 (Liao et al., 1997) to study the potential in vivo phosphorylation of K4–K6 at this site. We show that K4, K5, and K6 are phosphorylated on threonine in the LLS/TPL-motif and become solubilized in primary keratinocytes and tissues ex vivo upon treatment with the protein phosphatase inhibitors calyculin A (Cl-A) and okadaic acid (OA). We also show that K5/6 phosphorylation occurs during mitosis and a variety of apoptosis-associated cell stress, and that this site is phosphorylated in squamous cell carcinoma and in dividing keratinocytes of psoriatic skin. Therefore, LLS/TPL is an important and unique physiological phosphorylation motif for type II keratins that becomes engaged during mitotic and stress stimuli.
MATERIALS AND METHODS
Cells and Tissues
Primary human foreskin keratinocytes (KCs) were cultured in a 1:1 mixture of Medium 154 supplemented with human keratinocyte growth supplement (Cascade Biologics, Portland, OR) and keratinocyte serum-free medium supplemented with 5 μg/l epidermal growth factor and 50 mg/l bovine pituitary extract (Invitrogen, Carlsbad, CA). The keratinocyte medium was also supplemented with 0.25 μg/ml amphotericin B, 100 μg/ml streptomycin, and 100 U/ml penicillin G. Most experiments were performed using cells that have been passaged 3–8 times. The SCC-13 (human squamous cell carcinoma) line was cultured in DMEM and Ham's F-12 medium (1:1 mixture) and supplemented with 10% fetal bovine serum, 0.4 μg/ml hydrocortisone and penicillin G/streptomycin. HT29 (human colon carcinoma) cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum and penicillin G/streptomycin. Fragments of mouse (m) ear skin and intestine were cultured in the KC and HT29 medium, respectively, whereas mouse esophagus was cultured in DMEM supplemented with 10% fetal bovine serum and penicillin G/streptomycin. Skin tumors and biopsies were obtained from patients with psoriasis, basal cell carcinoma, or squamous cell carcinoma under a protocol that is approved by the Panel of Human Subjects Committee at Stanford University.
Antibodies and Other Reagents
The primary antibodies used were Troma I rat anti-mK8 antibody (Developmental Studies Hybridoma Bank; University of Iowa, Iowa City, IA); monoclonal antibody L2A1, which recognizes hK18 (Ku and Omary, 1997); monoclonal antibody LJ4, which recognizes human pS73 and mouse pS79 K8 (Liao et al., 1997); rabbit anti-K6 antibody (a generous gift from P. Coulombe; Johns Hopkins University, Baltimore, MD); MK5 rabbit anti-K5 antibody (Covance, Richmond, CA); and anti-poly(ADP-ribose) polymerase-1 rabbit antibody (Upstate Biotechnology, Lake Placid, NY). The antibodies M20 (anti-hK8), LHK6B (anti-mK6), and 6B10 (anti-mK4) were obtained from Neomarkers (Fremont, CA). The DL15 rabbit antibody was generated against the peptide NQSLLpTPLNLQC (with the putative phosphorylated keratin threonines corresponding to T150 in hK5, T145 in hK6, and T133 in hK4; Table 1). Phosphopeptide synthesis, coupling of the peptide to keyhole limpet hemocyanin [1:1 using sulfosuccinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate at pH 7.2], and immunization of New Zealand White rabbits with the peptide by using complete and then incomplete Freund's adjuvant was done by Anaspec (San Jose, CA; see http://www.anaspec.com/xantibody_fset.html for details). Other reagents included anisomycin (An) (Calbiochem, San Diego, CA), methylmethane sulfonate (MMS) (Aldrich Chemical, Milwaukee, WI), OA and Cl-A (Alexis Biochemicals, San Diego, CA).
In Vitro Kinase Assays, Phosphoamino Acid Analysis, Protein Phosphatase Inhibition, and Stress Treatment
K8/18 immunoprecipitates were obtained from HT29 cells as previously described (Ku and Omary, 1997; Liao et al., 1997). Purified K5/6/14 and K8 were kindly provided by Drs. Pierre Coulombe (Johns Hopkins University, Baltimore, MD) and Harald Herrmann (German Cancer Research Center, Heidelberg, Germany), respectively. Purified individual keratins or K8/K18 precipitates were phosphorylated with GST-linked p38 stress-activated protein kinase (Calbiochem) for 30 min at 37°C with [γ-32P]ATP in buffer as recommended by the supplier. Proteins were separated using SDS-PAGE (Laemmli et al., 1970), and gels were stained with Coomassie blue then exposed to x-ray film. For phosphoamino acid analysis, radiolabeled proteins were individually cut out from the gel, electroeluted from the gel slices, acetone precipitated, and subjected to HCl hydrolysis and phosphoamino analysis as described (Boyle et al., 1991).
For phosphatase inhibition, the protein phosphatase 1 and 2A (PP1 and PP2A) inhibitors OA (0.25 μM) and Cl-A (0.2 μM) were added to culture cells for 30 min to 5 h, at 37°C. Mouse tissues were cultured in the presence of 1.0 μM Cl-A for 1–5 h at 37°C. For stress treatments, confluent KCs were cultured for 72 h then treated with An (10 μM; 60 min, or 24 h) or MMS (1 mg/ml, 24 h). For irradiation treatment, KC cells were exposed to 400 J/m2 UV light (UV-C; UV Stratalinker 2400; Stratagene, La Jolla, CA) through a thin layer of medium in culture dishes with the lid removed then further cultured for 10 min, 60 min, or 24 h.
Isolation of Cell and Protein Fractions
Total cell lysates were obtained from cells (floater and adherent) after solubilization in Laemmli sample buffer. The Triton X-100 (TX-100) or high salt extract (HSE) fractions were prepared by solubilizing cells for 2 min at 4°C with buffer containing 1% TX-100, 5 mM EDTA, and a protease inhibitor mix (1 mM phenylmethylsulfonyl fluoride, 10 μM leupeptin, 10 μM pepstatin, and 25 μg/ml aprotinin) in phosphate-buffered saline (PBS, pH 7.4), followed by centrifugation (16,000 × g, 10 min). The supernatant was collected as the soluble fraction. The pellet was homogenized in 1 ml of 10 mM Tris-HCl pH 7.6, 140 mM NaCl, 1.5 M KCl, 5 mM EDTA, 0.5% Triton X-100, and the protease inhibitor mix. After 30 min (4°C) the homogenate was pelleted (16,000 × g; 10 min) and the pellet (insoluble fraction) was rehomogenized with 5 mM EDTA in PBS pH 7.4, pelleted (termed HSE), and dissolved in Laemmli sample buffer for subsequent analysis. Freshly isolated mouse intestine, esophagus, and ear skin were incubated with Cl-A or dimethyl sulfoxide (DMSO) then homogenized with buffer (600 μl/25 mg of tissue) consisting of 0.187 M Tris-HCl pH 6.8, 3% SDS, and 5 mM EDTA. Solubilized tissue proteins were diluted to 2 mg/ml (BCA method; Pierce Chemical, Rockford, IL), separated by SDS-PAGE, and then subjected to immunoblotting after protein transfer to polyvinylidene difluoride membranes. Proteins were visualized using an enhanced chemiluminescence system (PerkinElmer Life Sciences, Boston, MA).
Cell and Tissue Staining
Cells grown on coverslips were washed with prewarmed (37°C) PBS and fixed in acetone (−20°C, 10 min). The floater cells that are generated after phosphatase inhibition or upon trypsinization were transferred to slides using a Cytospin (7 min, 700 rpm). Mouse tissues and human biopsies were frozen in “optimum cutting temperature” compound, sectioned (6 μm), and fixed using acetone (−20°C, 10 min). Fixed cells and tissues were processed for immunofluorescence staining as described previously (Liao et al., 1995). Human psoriasis samples were fixed with 10% paraformaldehyde, dehydrated, and embedded in paraffin, deparaffinized at 23°C (xylene 5 min twice, 100% ethanol 5 min twice, 90% ethanol 3 min, 80% ethanol 3 min, 70% ethanol 3 min), rinsed in PBS, treated with 0.2% Nonidet P-40 in PBS pH 7.4 for 5 min, and then stained (Liao et al., 1995). DNA was stained using toto-3 iodide (Molecular Probes, Eugene, OR) after pretreatement with 0.5 mg/ml RNase A. Cells and tissues were mounted using the Prolong antifade kit (Molecular Probes) and fluorescence images were analyzed with a MRC 1024ES confocal microscope (Bio-Rad, Hercules, CA). Apoptotic cells were detected using the TdT-FragEL DNA fragmentation detection kit (Oncogene Research Products, Boston, MA).
RESULTS
K5 and K6b Are Phosphorylated In Vitro on Threonine and Serine Residues by p38 Kinase
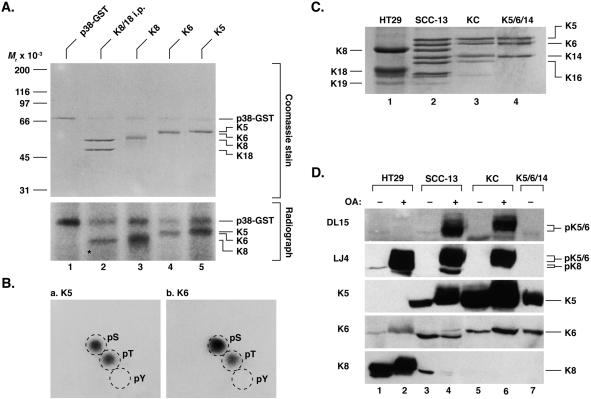
The conserved S73 in the LLS/TPL motif of hK8 is a proline-directed stress-regulated phosphorylation site, which can be phosphorylated in vitro by the mitogen-activated protein kinase extracellular signal-regulated kinase 1 (Liao et al., 1997) and the stress-activated protein kinase p38 (Ku et al., 2002) and JNK (He et al., 2002). To evaluate the potential phosphorylation of the LLS/TPL motif in K5/6 and whether threonine phosphorylation can occur on these proteins by a proline-directed kinase, purified human K5 and K6b were phosphorylated in vitro by using [γ-32P]ATP and glutathione S-transferase (GST)-linked p38 kinase. As shown in Figure 1A, K5 and K6b were phosphorylated in vitro by p38 kinase in a similar manner as noted for purified hK8 or for K8 that is isolated by immunoprecipitation from HT29 cells. In contrast, K18 in the same K8/18 immunoprecipitate was not phosphorylated (Figure 1A, migration position of K18 is highlighted by an asterisk). Phosphoamino acid analysis of K5 and K6b that are phosphorylated by p38 kinase in vitro demonstrated serine and threonine, but not tyrosine, phosphorylation (Figure 1B). In contrast, K8 is phosphorylated exclusively on serine (Chou and Omary, 1993; Toivola et al., 1997). Hence, K5/6 threonine phosphorylation can occur in vitro and may therefore also occur in vivo.
Figure 1.
K5 and K6b in vitro phosphorylation by p38 kinase and characterization of antibodies to the LLS/TPL motif. (A) K8/18 immunoprecipitates and purified human K5, K6b, and K8 were phosphorylated in vitro using GST-p38 kinase and [γ-32P]ATP then analyzed by SDS-PAGE. Gels were stained with Coomassie blue and then exposed to x-ray film. Note that K18 is not phosphorylated by p38-GST kinase (asterisk) and that the kinase becomes autophosphorylated. (B) In vitro phosphorylated K5 and K6b were subjected to phosphoamino acid analysis as described in MATERIALS AND METHODS. pS, phospho-serine; pT, phospho-threonine; pY, phospho-tyrosine. (C) Keratin-containing cytoskeletal fractions were isolated by high salt extraction from HT29, SCC-13 and KC cells as described in MATERIALS AND METHODS. The keratin fractions were analyzed by SDS-PAGE then Coomassie blue staining. (D) Cells were cultured in the presence (+) or absence (−) of OA (1.25 μM for HT29 and SCC-13 cells, 0.25 μM for KC cells; 2 h). Total cell lysates were prepared and equal fractions (confirmed by Coomassie blue staining; not shown) were analyzed by using DL15 and LJ4 or antibodies that recognize the total K5, K6, or K8 pools.
Antibodies to the LLpS/TPL Motif Demonstrate K5/6 Hyperphosphorylation and Keratin Filament Reorganization Upon Phosphatase Inhibition
We confirmed the in vivo phosphorylation of the epidermal keratin LLTPL motif by generating a polyclonal phospho-specific antibody (termed DL15) directed to the conserved type II keratin sequence NQLLpTPLNLQC (Table 1). The specificity of DL15 antibody and the previously described LJ4 antibody directed to K8 pS73 (Liao et al., 1997) were tested using primary human KCs, SCC-13, and HT29 cells, which express the keratins indicated in Figure 1C (confirmed by blotting with keratin-specific antibodies; unpublished data). Immunoblotting of total KC, SCC-13, or HT29 cell extracts (isolated from OA-treated and untreated cells), or of purified K5/6/14 keratins, with antibody DL15 showed that it recognized only the OA-treated KC and SCC-13 keratins (Figure 1D). In contrast, antibody LJ4 recognizes K8 as expected after HT29 cell exposure to OA (Liao et al., 1997), and also strongly recognizes K5 and K6 upon OA-induced hyperphosphorylation (Figure 1D). Hence, the DL15 antibody recognizes the K5/6 phospho-threonine LLpTPL motif of K5/6 preferentially, whereas the LJ4 is more promiscuous in that it recognizes the LLpS/TPL motif of K5/6/8.
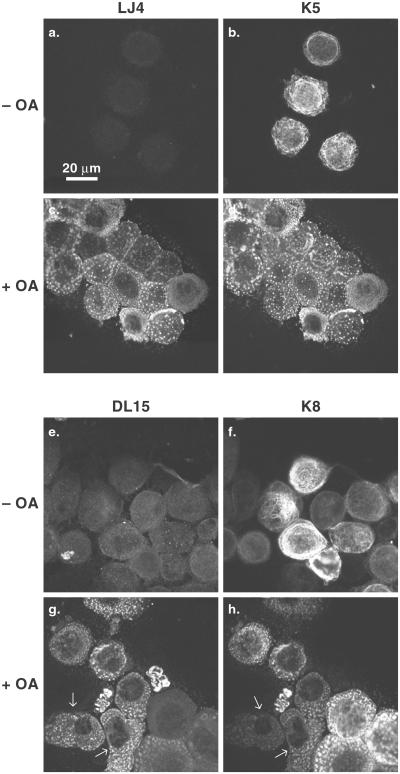
We also examined keratin filament organization in SCC-13 cells by using anti-keratin and anti-phosphokeratin antibodies in the presence or absence of OA. OA leads to cell detachment and disassembly of the keratin filament network into cytoplasmic dots (Figure 2, compare b and f with d and h). These dots stained with the LJ4 and DL15 antibodies (Figure 2, c and g), whereas control cells were essentially nonreactive, and colocalized with dots positive for K5 and K8 (Figure 2) and K6 (unpublished data). Of note, SCC-13 cells are mosaic for K8 expression, whereas most cells express K5. As a consequence, there were OA-treated cells that were positive for DL15 and negative for K8 (Figure 2, g and h, arrows), thereby indicating that the DL15-positive dots stem from K5 or K6 (LLTPL), but not K8 (LLSPL) phosphorylation. Also, OA-treated HT29 cells (which lack K5/6) are strongly positive for LJ4 but not DL15 (unpublished data). Taken together, these data indicate that K5 and K6 are phosphorylated on Thr-150 and Thr-145, respectively, in the LLTPL motif.
Figure 2.
LJ4 and DL15 antibody reactivity with SCC-13 cells upon cell exposure to okadaic acid. SCC-13 cells were treated with 0.2 μM OA (+OA) for 3 h and the detached cells were collected (c, d, g, and h), whereas control cells (treated with DMSO; −OA) were detached by trypsin (a, b, e, and f). Detached cells were fixed then double labeled using LJ4 and anti-K5 antibodies (a–d) or DL15 and anti-K8 antibodies (e–h). Note that cells with low or no expression of K8 manifest stronger staining with DL15 (compare arrows in g and h).
Keratin Solubility Correlates with Its Hyperphosphorylation, and LLpS/TPL Dephosphorylation by a PP1-related Phosphatase
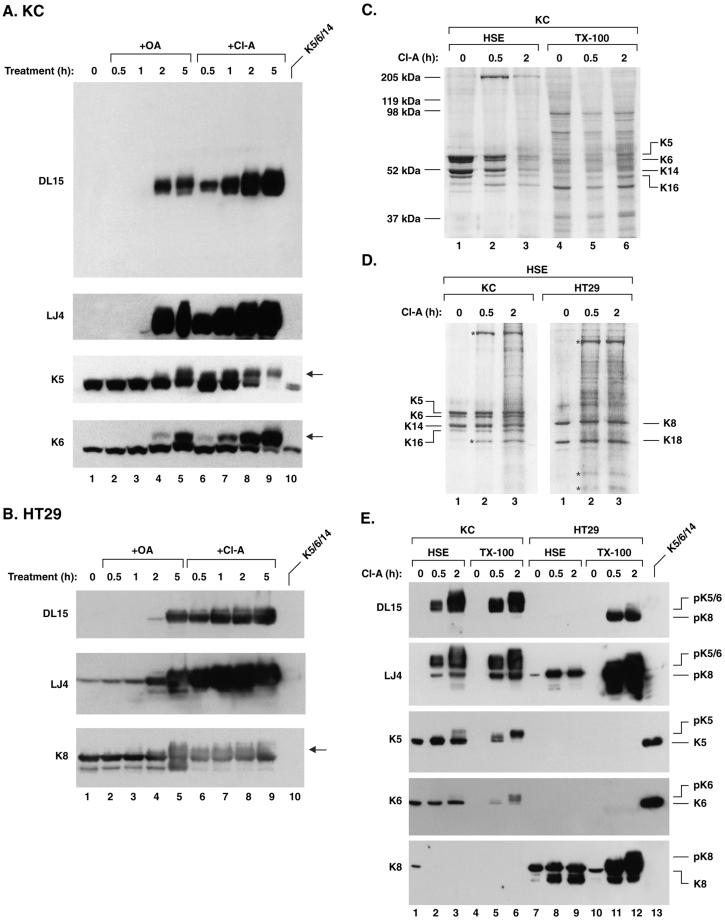
The phosphorylation dynamics of K5 and K6 was studied in KC and HT29 cells treated with OA and Cl-A, two inhibitors with similar potency for PP2A but a higher Cl-A potency toward PP1 (Eriksson et al., 1998). Cl-A and OA resulted in a time-dependent K5 and K6 (LLTPL) phosphorylation and SDS-PAGE migration shifts, as noted for K8 S73 phosphorylation in HT29 cells (Figure 3, A and B). The Cl-A–induced K5/6/8 hyperphosphorylation began much earlier (before 30 min) compared with OA (after 1 h), thereby suggesting a role for PP1 as the LLS/TPL phosphatase (Figure 3, A and B). On prolonged exposure, the DL15 antibody does recognize phosphorylated K8 in HT29 cells weakly (Figure 3B), thereby indicating a slight cross-reactivity with K8 pS73 in the absence of pK5/6 (see support for clear distinction of the two antibodies in Figures 1D and 2).
Figure 3.
Inhibition of serine/threonine protein phosphatases induces hyperphosphorylation and solubilization of K5/6/8. (A and B) KC or HT29 cells were treated with 0.25 μM OA or 0.20 μM Cl-A for 0.5, 1, 2, and 5 h and total cell lysates were then prepared and analyzed by blotting with DL15 (whole filter shown for KC) and LJ4 or with antibodies to K5, K6, and K8. OA and Cl-A induced hyperphosphorylation and a migration shift of K5/6 and K8 (arrows in A and B, respectively). Equal protein loading was confirmed by Coomassie blue staining (unpublished data). (C and D) KC and HT29 cells were treated with 0.20 μM Cl-A for 0.5 or 2 h followed by isolation of the TX-100 and HSE fractions then SDS-PAGE and Coomassie blue staining as described in MATERIALS AND METHODS. (C) Samples were loaded based on normalization to equal cell numbers (note the increase in keratins in the TX-100 fraction with concurrent decrease of keratins in the HSE fraction as exposure time to Cl-A increased). (D) Normalization of the same sample shown in C was done to show nearly equal amount of keratins in the HSE fractions. Asterisks in D highlight unidentified proteins that partition with the HSE after phosphatase inhibition. (E) Equal amounts of HSE keratins (as shown in D) and TX-100 fractions normalized to cell numbers (as shown in C) were analyzed by blotting with DL15 or LJ4 or antibodies to K5, K6, or K8.
Given that simple epithelial keratin phosphorylation leads to keratin solubilization (Omary et al., 1998) we examined whether epidermal K5/6 phosphorylation causes similar solubilization. Exposure of KC cells and HT29 cells to Cl-A induced a dramatic and time-dependent decrease of keratins in the insoluble HSE pool (e.g., Figure 3C, lanes 1–3) and an increase in the TX-100–soluble keratin pool (Figure 3E, compare lanes 5 and 6 with 4, and lanes 11 and 12 with 10). Concurrently, the DL15- and LJ4-specific phosphorylation increased in the HSE pool (Figure 3E, compare lanes 2 and 3 with 1, and lanes 8 and 9 with 7; which shows HSE keratins after normalizing to nearly equal keratin levels as shown in Figure 3D). K8 becomes much more soluble than K5 and K6 (Figure 3E, contrast band intensity of lane 12 vs. 9 [K8 blot], or lane 6 vs. 3 [K5 and K6 blots]). Notably, the soluble TX-100 fraction contains the hyperphosphorylated keratins preferentially (note the pK5 and pK6 species in the K5 and K6 blots of Figure 3E, lane 6 vs. 3). Similar results were found for K5/6 in SCC-13 cells (unpublished data). Hence, phosphorylation of the LLS/TPL motif correlates with K5/6/8 solubilization and a migration shift on SDS-PAGE gels, although a significant portion of the keratin species that are phosphorylated at this motif remain within the insoluble pool.
Phosphorylation of K4 and K5 LLS/TPL Motif Occurs in Skin, Esophagus, and Intestine Ex Vivo
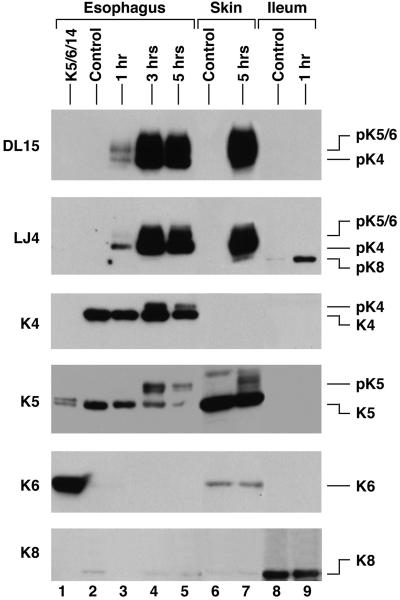
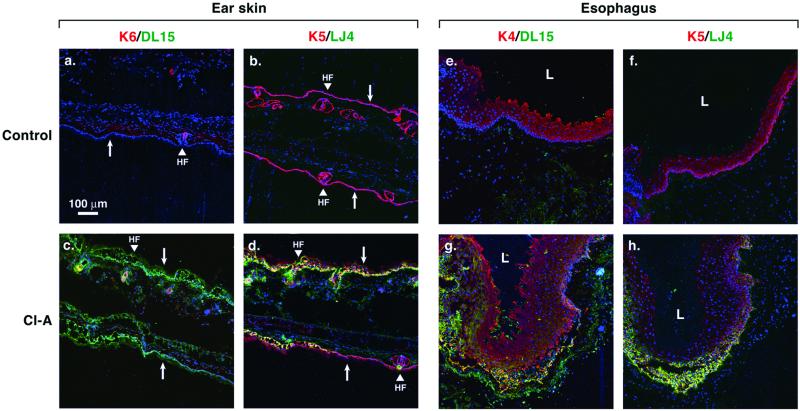
Given that human and mouse K4 contain the same LLTPL motif as K5 and K6, we asked whether K4 can indeed be phosphorylated as determined by DL15 and LJ4 reactivity. Culture of mouse esophagus (which expresses primarily K4 with low levels of K5 type II keratins) ex vivo in the presence of Cl-A led to time-dependent K4 and K5 phosphorylation at LLTPL (Figure 4, lanes 1–4). Similar, but more prominent K5 and K6 LLTPL phosphorylation were also observed in mouse ear skin (Figure 4, lanes 6 and 7), whereas culture of mouse ileum, which expresses K8 and lacks K5/6, showed the expected K8 LLSPL phosphorylation (Figure 4, lanes 8 and 9). The immunoblot data were supported by immunofluorescence staining that showed increased DL15 (Figure 5, c and g; green/yellow) and LJ4 (Figure 5, d and h; green/yellow) staining in Cl-A–treated esophagus and skin. In mouse skin, the increased phosphorylation occurred in hair follicles (which contain K6) and in the basal K5-expressing cells (Figure 5). In the esophagus, LLTPL phosphorylation occurred primarily in the basal cells but did extend into the stratified layer in more exposed parts of the culture fragment (Figure 5, g and h). Cl-A treatment increased the thickness of both epithelia due to tissue edema (unpublished data) and loss of cell-cell contacts. Taken together, K4–K6 can be phosphorylated at the LLTPL motif ex vivo in their respective tissue compartments as recognized by the two independent DL15 and LJ4 antibodies.
Figure 4.
LLS/TPL phosphorylation of K4, K5, and K6 in mouse esophagus, skin, and ileum occurs “ex vivo” upon phosphatase inhibition. Fragments of mouse esophagus, ear skin, and ileum were treated ex vivo with 1 μM Cl-A for 1, 3, and 5 h (esophagus), 5 h (skin), and 1 h (ileum). Control tissues were treated with DMSO for 1–5 h, depending on the tissue. Total tissue homogenates were then prepared and analyzed by immunoblotting with DL15 and LJ4 and antibodies to total K4, K5, K6, and K8. Equal protein loading was confirmed by Coomassie blue staining (unpublished data). The migration positions of K5 and K6 were confirmed by parallel loading of purified proteins (Figure 3; unpublished data).
Figure 5.
Immunofluorescence staining of mouse skin and esophagus after ex vivo inhibition of protein phosphatases. Fragments of mouse ear skin and esophagus were treated for 2 h with 1 μM Cl-A (c, d, g, and h) or DMSO (a, b, e, and f; controls). Tissue sections were triple labeled for DL15/K6/nuclei (a and c), LJ4/K5/nuclei (b, d, f, and h), and DL15/K4/nuclei (e and g). Toto-3 iodide was used to stain nuclei, shown in blue (a–h). DL15 and LJ4 staining is shown in green, whereas K4–K6 staining is shown in red. Arrows point from suprabasal toward the basal epithelial layer. HF, hair follicle; L, lumen.
K5/6 LLTPL Phosphorylation Increases during Mitosis and Stress
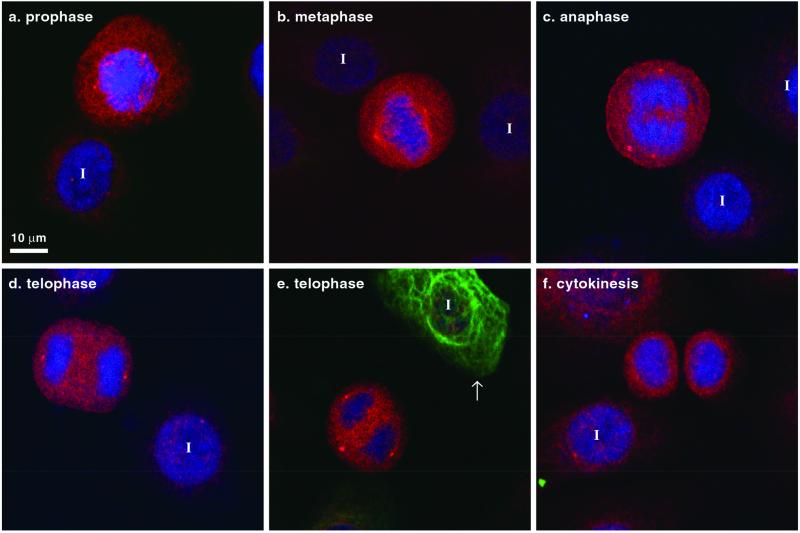
We tested whether LLTPL phosphorylation in K5 and/or K6 occurs during mitosis and stress conditions as previously described for K8 S73 in simple epithelial tissues (Liao et al., 1997). Interphase primary culture keratinocytes manifested a weak background DL15 signal, which increased dramatically at various mitotic stages (Figure 6, a–e; also seen in SCC-13 cells, unpublished data), but begins to decrease during cytokinesis (Figure 6f). Of note, K8 expression is limited to <10% of primary cultured keratinocytes (unpublished data) and was absent in all the mitotic cells shown in Figure 6 (note the single interphase K8-positive cell in Figure 6e highlighted by arrow). Similar staining results to those of DL15 were also obtained using the LJ4 antibody (unpublished data). The DL15 antibody (but not LJ4) also binds to centrosomes (e.g., the two prominent dots at the poles of the dividing cells in Figure 6d) as confirmed by double staining with antibodies to γ-tubulin (unpublished data) but the significance of this cross-reactivity is unclear because keratins are the only known proteins in the GenBank database that have the LLS/TPL motif. Hence, phosphorylation of the LLS/TPL motif of K5, K6, and K8 occurs during mitosis as an on/off switch.
Figure 6.
K5 and K6 LLTPL motif is phosphorylated during mitosis. Primary cultured keratinocytes were triple stained with DL15 (red), toto-3 iodide for DNA (blue), and anti-K8 antibody (green). Most of the cells are K8-negative (except for the cell highlighted by an arrow in e) and K5-positive (unpublished data but similar to Figure 2). DL15 staining increased during mitosis in cells lacking K8. I, interphase cell.
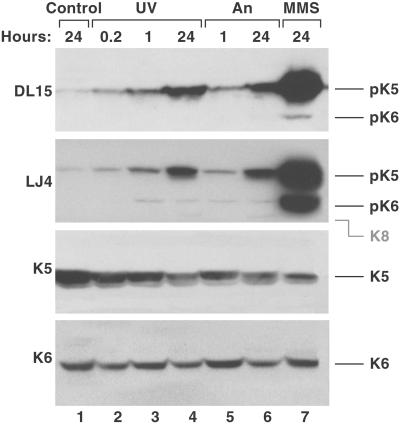
We also investigated whether stress induces phosphorylation of the epidermal K5/K6 LLTPL motif. Exposure of primary keratinocytes to the stress and apoptosis-inducing conditions UV light (Geilen et al., 1996), anisomycin (Cano et al., 1996), and the alkylating agent MMS (a known p38 kinase and JNK activator; Wilhelm et al., 1997) resulted in time-dependent increase in LJ4 and DL15 reactivity (Figure 7). Rounding of cells and cleavage of poly(ADP-ribose) polymerase-1 were also noted upon An and UV treatment (unpublished data), thereby confirming that some of the cells were undergoing apoptosis. Taken together, LLTPL of K5/6 in KC cells becomes phosphorylated similarly to LLSPL of K8 in cultured colonocytes in the presence of several apoptotic and stress signals.
Figure 7.
K5 and K6 LLTPL motif is phosphorylated during cell stress. Confluent primary cultured keratinocytes were treated with UV-C (400 J/m2; 0.2, 1, and 24 h), An (10 μM; 1 and 24 h), or MMS (1 mg/ml; 24 h). Control cells (24 h) were cultured without any manipulations. Total cell lysates were separated on SDS-PAGE, transferred to membranes, and then blotted using DL15, LJ4, and antibodies to K5 and K6. Equal protein loading was confirmed by Coomassie blue staining (unpublished data). The predicted K8 migration position is indicated by gray marking.
Phosphorylation of K5/6 LLTPL Motif in Human Psoriatic Skin and Squamous Cell Carcinoma
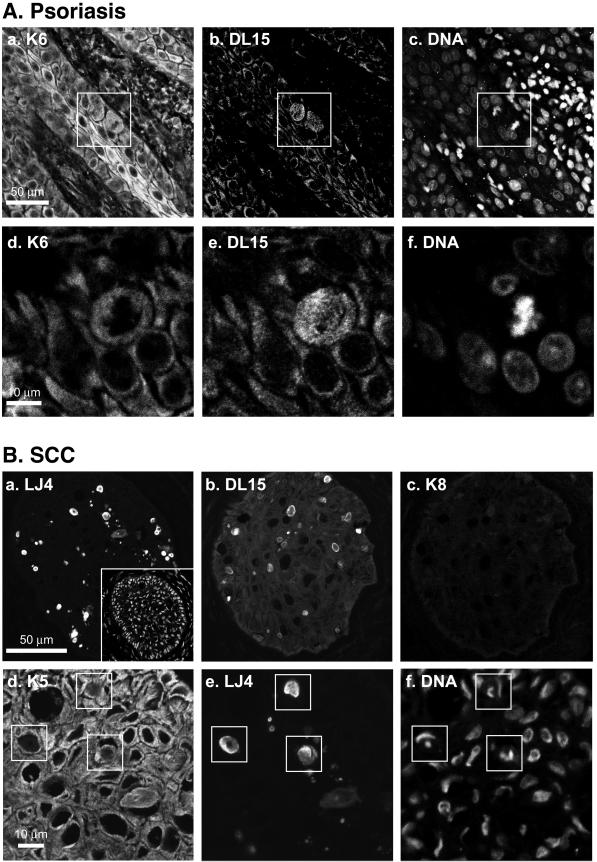
We investigated whether human diseases related to abnormal epidermal keratin expression, such as psoriasis (Rao et al., 1996) and epidermal tumors (Yoshikawa et al., 1998), manifest phosphorylation of the LLTPL motif. Immunofluorescence staining of skin biopsies from patients with psoriasis by using antibody DL15 (Figure 8A) or antibody LJ4 (unpublished data) displayed positive staining of several mitotic cells. Notably, we observed occasional yet rare mitotic cell staining in normal skin biopsies, whereas psoriatic skin had easily discernible DL15/LJ4-positive cell staining that is consistent with the known increased mitotic activity of keratinocytes in psoriasis (Gelfant et al., 1982). K6 was not detected in normal skin (unpublished data) but its expression was up-regulated in psoriatic keratinocytes (Figure 8A, a) as reported previously (Leigh et al., 1995).
Figure 8.
Epidermal keratin LLTPL motif is phosphorylated in psoriatic skin and in squamous cell carcinoma. (A) Human psoriatic skin biopsies were triple stained for K6 (a and d), DL15 (b and e), and DNA (c and f). Images a–c and d–f represent two independent triple stainings. The boxes in a–c highlight mitotic cells. (B) Squamous cell carcinoma (SCC) biopsies were double or triple stained for LJ4 (a and e), DL15 (b), K8 (c), K5 (d), and DNA (f, and insert in a). Double staining of DNA (a, inset) and LJ4, and a double staining with anti-K8 antibody (which shows background binding) and DL15 (b and c) are shown. Triple staining (K5/LJ4/DNA) with overlapping LJ4-positive areas enclosed with boxes (d–f, note nuclear fragmentation of the LJ4-positive cells). No background staining is seen if anti-mouse or anti-rabbit secondary antibody is used alone (unpublished data).
We also examined phosphorylation of the LLTPL keratin motif in squamous and basal cell carcinomas. We did not observe any significant LLTPL phosphorylation in two independent basal cell tumors (except for few sporadic mitotic cells; unpublished data). However, one of two examined squamous tumors had multiple foci of DL15- and LJ4-positive cells that also stained with antibodies to K5 (Figure 8B). The DL15/LJ4-positive cells did not stain with anti-K8 antibody, and some of these cells did show positive K6 staining (unpublished data). DNA costaining (Figure 8B, d–f) indicated that most of the DL15/LJ4-positive cells are apoptotic based on the presence of condensed/fragmented nuclei in these cells (Figure 8B, d–f). DNA fragmentation staining also confirmed the presence of apoptotic cells in this tumor (unpublished data). Therefore, LLTPL K5 and/or K6 phosphorylation increases in dividing keratinocytes from psoriatic patients and in some squamous cell tumors in association with apoptosis.
DISCUSSION
LLS/TPL Is a Conserved Type II Keratin Stress and Mitosis Phosphorylation Motif
We have identified and characterized a conserved type II keratin motif that is phosphorylated in K4, K5, K6, and K8, within tissues that express these keratins, in a mitogen- and stress-regulated manner (Figure 9). The context and phosphorylation of the LLS/TPL motif, located at the N-terminal “head” domain (within the H1 subdomain) outside the central coiled-coil α-helical IF rod domain, is exclusive for the type II keratins (both soft and hard keratins; Table 1) that harbor it, because it is not found in other proteins upon a search of sequence databases. Evidence for K4/5/6 LLTPL phosphorylation is based on the following observations: 1) K5 and K6 are phosphorylated on threonine in vitro by the proline-directed p38 mitogen-activated protein kinase (Figure 1). 2) Serine/threonine protein phosphatase inhibition leads to K4/5/6 phosphorylation at LLTPL, in association with keratin solubilization in cells and tissues (Figures 3 and 4). The hyperphosphorylated K4/5/6 are recognized by two independent phosphospecific antibodies (Figures 1–5). 3) K5/6 LLTPL is phosphorylated during mitosis and apoptosis in cell culture, in dividing keratinocytes of psoriatic skin, and apoptotic cells of some squamous cell carcinomas.
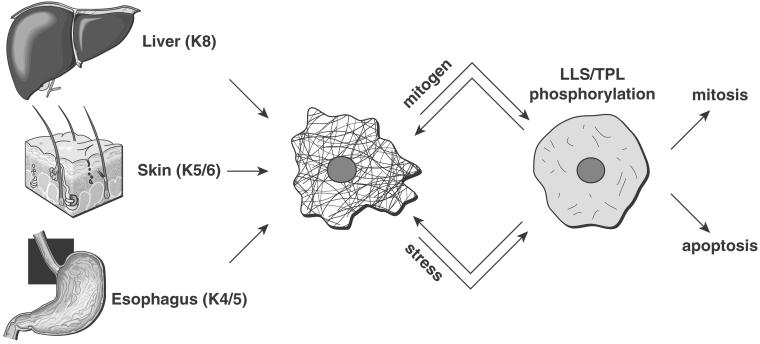
Figure 9.
LLS/TPL motif of type II keratins undergoes phosphorylation in several tissues in response to stress or mitogenic stimulation. The results reported herein for K4/5/6, and previously reported for K8 (Liao et al., 1997) are summarized schematically. Representative organs that express the indicated keratins are shown. Upon mitogenic (e.g., mitosis during psoriasis or tumor growth) or stress-mediated stimulation (e.g., UV irradiation and apoptosis), four type II keratins (K4, K5, K6, and K8) undergo phosphorylation at the LLS/TPL motif. These four keratins share the property of expression in epithelia with regenerative potential. Phosphorylation at the LLS/TPL motif is associated with keratin solubilization, filament reorganization and collapse, which may be reversible.
To study LLTPL phosphorylation of keratins, we characterized a new phospho-specific antibody (termed DL15) that binds to the LLpTPL motif of human and mouse K4, K5, and K6 (human K4 was not tested). DL15 exhibits minimal cross-reactivity with pK8, which is noted primarily when pK4/pK5/pK6 are absent. We also demonstrated that the previously characterized phosphospecific anti-K8 pS73 antibody (termed LJ4; Liao et al., 1997) has high affinity for K4/5/6 that are phosphorylated on the conserved threonine. Therefore, the LJ4 antibody is promiscuous in that it recognizes LLpS/TPL, whereas the DL15 antibody recognizes LLpTPL preferentially. In addition, both anti-phosphokeratin antibodies are remarkably keratin specific, probably due to the uniqueness of the LLS/TPL sequence.
IF protein physiological threonine phosphorylation is relatively uncommon, compared with serine phosphorylation, but does occur in K1 (Steinert, 1988), desmin (Inada et al., 1999), glial fibrillary acidic protein (Yasui et al., 1998), and nestin (Sahlgren et al., 2001). Human K1 threonine phosphorylation occurs on threonine-31, the amino acid context of which is very different (28RRTTSS) from the LLS/TPL motif. To date, all of the identified K8 and K18 in vivo phosphorylation sites are serines (Omary et al., 1998), although calmodulin-dependent protein kinase II does phosphorylate rat K8 and K18 on threonine (and serine) in vitro (Yano et al., 1991). Hence, although threonines are relatively abundant potential phosphorylation sites in keratins (e.g., K8 has 59 serines and 21 threonines and K18 has 37 serines and 30 threonines), their in vivo phosphorylation has not been documented despite being part of several kinase-recognition consensus sequences.
The threonine in the LLS/TPL motif in K4/5/6 represents a potential target for proline-directed kinases, including the stress/mitogen-activated (Schaeffer and Weber, 1999; Davis, 2000; Ono and Han, 2000; Kyriakis and Avruch, 2001) and the cyclin-dependent (Nurse, 2000; Maccioni et al., 2001) kinases. For example, K5 and K6 can be phosphorylated in vitro by the stress-activated protein kinase p38, which is likely to also be an in vivo kinase given the biological context of LLTPL phosphorylation in K5 and K6. In support of this, p38 kinase is a K8 S73 in vivo kinase during stress conditions based on exclusive in vitro phosphorylation of that site by p38 kinase, inhibition of K8 S73 phosphorylation in cultured cells by the selective inhibitor SB203580, and association of p38 kinase with K8/18 immunoprecipitated in transfected cells and with K8 by using an in vitro overlay assay (Ku et al., 2002). K8 S73 is also a substrate for, and binds to, JNK upon cell stimulation with the proapoptotic Fas receptor in a caspase-independent manner (He et al., 2002).
Several IF proteins undergo phosphorylation during mitosis at proline-directed sites, including lamin serine-22 (Heald and McKeon, 1990), vimentin serine-55 (Tsujimura et al., 1994), nestin threonine-316 (Sahlgren et al., 2001), and neurofilament-H (Grant et al., 2001). The cell cycle-regulated kinase cdc2 is the likely in vivo kinase in these cases except for neurofilament-H where cdk5 is implicated (Grant et al., 2001). Dephosphorylation of the LLpS/TPL motif in K4, K5, K6, and K8 is likely to involve a PP1-related phosphatase based on the preferential effect of Cl-A, compared with OA (Figure 2). Interestingly, a K5 P151→L mutation (the residue was reported as amino acid P156; Table 1) has been described in a family with epidermolysis bullosa simplex (Muller et al., 1998). Predictably, this mutation converts the conserved keratin phosphorylation motif into LLTLL and thereby should significantly blunt if not abolish K5 threonine phosphorylation at that motif. Future studies should help define the regulation of the LLS/TPL motif by dephosphorylation, confirm its kinase(s), and determine whether phosphorylation rather than a structural feature contributes to the epidermolysis phenotype in patients with keratin mutations that affect phosphorylation at this motif.
Physiologial Relevance of Keratin LLS/TPL Phosphorylation
The phosphorylation of the K5/6 LLTPL motif during mitosis in exponentially growing keratinocytes and in dividing keratinocytes of human psoriatic skin is similar to the K8 S73 phosphorylation that occurs in dividing crypt cell colonocytes and regenerating posthepatectomy hepatocytes (Liao et al., 1997). The in vivo reorganization into fine punctate/disrupted K5/6-containing filaments (Figure 6) is similar to the observed “speckled” keratin filament networks during cell division in epithelial cell lines from several species (Lane et al., 1982). However, the K5/6 punctate distribution is different than the filamentous staining of pK8 that is retained in regenerating hepatocytes (Liao et al., 1997). These differences of in vivo filament organization in simple vs. stratified epithelia are probably related to other posttranslational modifications and/or regulation by interactions with associated proteins (Coulombe and Omary, 2002). One common feature among K4, K5, K6, and K8 is their expression in epithelial subcompartments that have a proliferative capacity (in contrast to e.g., K1 and K2e, which lack this motif and are expressed in differentiated cells), thereby providing evolutionary and physiological support for the mitosis-associated phosphorylation of the LLS/TPL motif in these keratins.
Further evidence for the physiological relevance of the epidermal LLTPL motif is its phosphorylation during cell stress and apoptosis in primary keratinocytes, as described for K8 S73 phosphorylation in HT29 cells (Liao et al., 1997). UV-induced apoptosis is an established physiologically relevant model system in keratinocytes (Geilen et al., 1996), and we demonstrate herein that LLTPL becomes phosphorylated in apoptotic cells (Figure 8). The basal K5/6/8 LLS/TPL in vivo phosphorylation is negligible but becomes prominent during mitosis, apoptosis, and cell stress (Figure 9). This renders LLS/TPL phosphorylation behavior as a switch “off” basally, but “on” during stress or mitogen activation in contrast to other known well-characterized K8/18 phosphorylation sites (Omary et al., 1998).
The presence of the hyperphosphorylated and slower migrating K5 and K6 species preferentially in the soluble pool of Cl-A–treated keratinocytes supports the strong in vivo and in vitro observed association between keratin phosphorylation and solubilization (Omary et al., 1998). A similar correlation was made previously in mouse and human keratinocyte cell lines (Kasahara et al., 1993; Yatsunami et al., 1993; Paramio, 1999), thereby indicating that epidermal keratins are phosphorylated and solubilized in a similar manner to simple epithelial keratins. However, the LLS/TPL-phosphorylated keratins have significant partitioning within the insoluble pool (Figure 3) in contrast with keratins that are phosphorylated at other sites (Omary et al., 1998). Also, K5 and K6 are less soluble after phosphatase inhibitor treatment, compared with K8 (Figure 3), which reflects the general relative insolubility of epidermal keratins compared with simple epithelial keratins (Lowthert et al., 1995). The LLS/TPL motif is located in proximity to the center of the H1 subdomain, which is likely to be important for registration of the nearest neighboring molecules at the three- to four-molecule level (Steinert and Parry, 1993). For example, peptides containing the K1 H1 subdomain Leu160→Pro mutation (157NQSLLQPL→157NQSPLQPL found in epidermolytic hyperkeratosis patients) interact less efficiently with assembled keratin filaments in vitro compared with wild-type peptides (Chipev et al., 1992). The K1 Leu160→Pro mutation introduces a new potential proline-directed kinase phosphorylation site, and indeed, mutating the corresponding Leu in K8 (Leu71→Pro) leads to K8 hyperphosphorylation and affects keratin filament reorganization (Ku et al., 2002). Taken together, disruption of the H1 subdomain by LLTPL phosphorylation in K4/5/6 probably interferes with keratin filament assembly, which may consequently alter keratin solubilization.
The increase in K5/6 LLTPL phosphorylation in psoriasis and squamous cell carcinoma are the first described observations of increased epidermal keratin phosphorylation in association with human disease. The increase involves few apoptotic and/or dividing cells although a more generalized disease-related hyperphosphorylation is also possible as occurs in K8/18 of liver disease-associated Mallory bodies (Stumptner et al., 2000) and in neurofilament hyperphosphorylation in association with amyotrophic lateral sclerosis (Julien and Mushynski, 1998; Nguyen et al., 2001). The increase in keratin and neurofilament protein phosphorylation is also observed in several transgenic mouse models of liver and neuronal diseases and may serve to either protect from and/or promote injury in a site-specific manner (Julien and Mushynski, 1998; Grant et al., 2001; Nguyen et al., 2001; Coulombe and Omary, 2002; Omary et al., 2002). Keratinocytes in disease states such as psoriasis are activated by stress or mitogenic signals that use similar signaling pathways to those active in mitotic and wound healing keratinocytes (Freedberg et al., 2001). It is thus likely that the LLTPL motif is a target for such signals. Future studies with the aid of the phosphospecific antibodies should clarify the potential role of in vivo LLS/TPL phosphorylation in wound healing and during embryonic development, two epithelial biological contexts when cell division and apoptosis are relatively common.
ACKNOWLEDGMENTS
We thank numerous colleagues who provided important reagents: Paul Khavari and Qun Lin for psoriasis, basal cell, and squamous carcinoma blocks; Peter Marinkovich, Linda Millman, and Ngon Nguyen for primary human keratinocytes (Stanford University Program for Epithelial Biology); Pierre Coulombe (Johns Hopkins University) for anti-K6 antibody and for purified K5, K6, and K14; and Harald Herrmann (German Cancer Research Center) for purified K8. We also thank Evelyn Resurreccion for assistance with sectioning and immunofluorescence staining, Li Feng for technical assistance, and Kris Morrow for preparing the figures. This work was supported by a Veterans Affairs Career Development Award, National Institutes of Health grant DK-52951, and Digestive Disease Center grant DK-56339. D.M.T. was partially supported by a Postdoctoral Fellowship from The Academy of Finland, and The McCormick Foundation and Dean's Fellowships at Stanford University.
Abbreviations used:
- An
anisomycin
- Cl-A
calyculin A
- h
human
- HSE
high salt extraction
- IF
intermediate filament
- JNK
c-Jun N-terminal kinase
- K
keratin
- KC
primary human keratinocyte
- m
mouse
- MMS
methyl methanesulfonate
- OA
okadaic acid
- PP
protein phosphatase
- TX-100
Triton X-100
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–12–0591. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–12–0591.
REFERENCES
- Aoki N, Sawada S, Rogers MA, Schweizer J, Shimomura Y, Tsujimoto T, Ito K, Ito M. A novel type II cytokeratin, mK6irs, is expressed in the Huxley and Henle layers of the mouse inner root sheath. J Invest Dermatol. 2001;116:359–365. doi: 10.1046/j.1523-1747.2001.01226.x. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Cano E, Doza YN, Ben-Levy R, Cohen P, Mahadevan LC. Identification of anisomycin-activated kinases p45 and p55 in murine cells as MAPKAP kinase-2. Oncogene. 1996;12:805–812. [PubMed] [Google Scholar]
- Chipev CC, Korge BP, Markova N, Bale SJ, DiGiovanna JJ, Compton JG, Steinert PM. A leucine→proline mutation in the H1 subdomain of keratin 1 causes epidermolytic hyperkeratosis. Cell. 1992;70:821–828. doi: 10.1016/0092-8674(92)90315-4. [DOI] [PubMed] [Google Scholar]
- Chou CF, Omary MB. Mitotic arrest-associated enhancement of O-linked glycosylation and phosphorylation of human keratins 8 and 18. J Biol Chem. 1993;268:4465–4472. [PubMed] [Google Scholar]
- Coulombe PA. Towards a molecular definition of keratinocyte activation after acute injury to stratified epithelia. Biochem Biophys Res Commun. 1997;236:231–238. doi: 10.1006/bbrc.1997.6945. [DOI] [PubMed] [Google Scholar]
- Coulombe PA, Omary MB. “Hard” and “soft” principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol. 2002;14:110–122. doi: 10.1016/s0955-0674(01)00301-5. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Eriksson JE, Toivola DM, Sahlgren C, Mikhailov A, Harmala-Brasken AS. Strategies to assess phosphoprotein phosphatase and protein kinase-mediated regulation of the cytoskeleton. Methods Enzymol. 1998;298:542–569. doi: 10.1016/s0076-6879(98)98044-2. [DOI] [PubMed] [Google Scholar]
- Feng L, Zhou X, Liao J, Omary MB. Pervanadate-mediated tyrosine phosphorylation of keratins 8 and 19 via a p38 mitogen-activated protein kinase-dependent pathway. J Cell Sci. 1999;112:2081–2090. doi: 10.1242/jcs.112.13.2081. [DOI] [PubMed] [Google Scholar]
- Fouquet B, Herrmann H, Franz JK, Franke WW. Expression of intermediate filament proteins during development of Xenopus laevis. III. Identification of mRNAs encoding cytokeratins typical of complex epithelia. Development. 1988;104:533–548. doi: 10.1242/dev.104.4.533. [DOI] [PubMed] [Google Scholar]
- Freedberg IM, Tomic-Canic M, Komine M, Blumenberg M. Keratins and the keratinocyte activation cycle. J Invest Dermatol. 2001;116:633–640. doi: 10.1046/j.1523-1747.2001.01327.x. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Keith R. Porter Lecture, 1996. Of mice and men: genetic disorders of the cytoskeleton. Mol Biol Cell. 1997;8:189–203. doi: 10.1091/mbc.8.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geilen CC, Wieprecht M, Orfanos CE. The mitogen-activated protein kinases system (MAP kinase cascade): its role in skin signal transduction. A review. J Dermatol Sci. 1996;12:255–262. doi: 10.1016/0923-1811(95)00481-5. [DOI] [PubMed] [Google Scholar]
- Gelfant S, Ozawa A, Chalker DK, Smith JG., Jr Circadian rhythms and differences in epidermal and in dermal cel proliferation in uninvolved and involved psoriatic skin in vivo. J Invest Dermatol. 1982;78:58–62. doi: 10.1111/1523-1747.ep12497933. [DOI] [PubMed] [Google Scholar]
- Grant P, Sharma P, Pant HC. Cyclin-dependent protein kinase 5 (Cdk5) and the regulation of neurofilament metabolism. Eur J Biochem. 2001;268:1534–1546. [PubMed] [Google Scholar]
- He T, Stepulak A, Holmstrom TH, Omary MB, Eriksson JE. The intermediate filament protein keratin 8 is a novel cytoplasmic substrate for c-jun-N-terminal kinase. J Biol Chem. 2002;277:10767–10774. doi: 10.1074/jbc.M111436200. [DOI] [PubMed] [Google Scholar]
- Heald R, McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 1990;61:579–589. doi: 10.1016/0092-8674(90)90470-y. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Aebi U. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opin Cell Biol. 2000;12:79–90. doi: 10.1016/s0955-0674(99)00060-5. [DOI] [PubMed] [Google Scholar]
- Inada H, Togashi H, Nakamura Y, Kaibuchi K, Nagata K, Inagaki M. Balance between activities of Rho kinase and type 1 protein phosphatase modulates turnover of phosphorylation and dynamics of desmin/vimentin filaments. J Biol Chem. 1999;274:34932–34939. doi: 10.1074/jbc.274.49.34932. [DOI] [PubMed] [Google Scholar]
- Julien JP, Mushynski WE. Neurofilaments in health and disease. Prog Nucleic Acid Res Mol Biol. 1998;61:1–23. doi: 10.1016/s0079-6603(08)60823-5. [DOI] [PubMed] [Google Scholar]
- Kasahara K, Kartasova T, Ren XQ, Ikuta T, Chida K, Kuroki T. Hyperphosphorylation of keratins by treatment with okadaic acid of BALB/MK-2 mouse keratinocytes. J Biol Chem. 1993;268:23531–23537. [PubMed] [Google Scholar]
- Knapp B, Rentrop M, Schweizer J, Winter H. Nonepidermal members of the keratin multigene family: cDNA sequences and in situ localization of the mRNAs. Nucleic Acids Res. 1986;14:751–763. doi: 10.1093/nar/14.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Azhar S, Omary MB. Keratin 8 phosphorylation by p38 kinase regulates cellular keratin filament reorganization: modulation by a keratin 1-like disease-causing mutation. J Biol Chem. 2002;277:10775–10782. doi: 10.1074/jbc.M107623200. [DOI] [PubMed] [Google Scholar]
- Ku NO, Omary MB. Phosphorylation of human keratin 8 in vivo at conserved head domain serine 23 and at epidermal growth factor-stimulated tail domain serine 431. J Biol Chem. 1997;272:7556–7564. doi: 10.1074/jbc.272.11.7556. [DOI] [PubMed] [Google Scholar]
- Ku NO, Zhou X, Toivola DM, Omary MB. The cytoskeleton of digestive epithelia in health and disease. Am J Physiol. 1999;277:G1108–G1137. doi: 10.1152/ajpgi.1999.277.6.G1108. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Laemmli UK, Molbert E, Showe M, Kellenberger E. Form-determining function of the genes required for the assembly of the head of bacteriophage T4. J Mol Biol. 1970;49:99–113. doi: 10.1016/0022-2836(70)90379-7. [DOI] [PubMed] [Google Scholar]
- Lane EB, Goodman SL, Trejdosiewicz LK. Disruption of the keratin filament network during epithelial cell division. EMBO J. 1982;1:1365–1372. doi: 10.1002/j.1460-2075.1982.tb01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh IM, Navsaria H, Purkis PE, McKay IA, Bowden PE, Riddle PN. Keratins (K16 and K17) as markers of keratinocyte hyperproliferation in psoriasis in vivo and in vitro. Br J Dermatol. 1995;133:501–511. doi: 10.1111/j.1365-2133.1995.tb02696.x. [DOI] [PubMed] [Google Scholar]
- Liao J, Ku NO, Omary MB. Stress, apoptosis, and mitosis induce phosphorylation of human keratin 8 at Ser-73 in tissues and cultured cells. J Biol Chem. 1997;272:17565–17573. doi: 10.1074/jbc.272.28.17565. [DOI] [PubMed] [Google Scholar]
- Liao J, Lowthert LA, Ku NO, Fernandez R, Omary MB. Dynamics of human keratin 18 phosphorylation: polarized distribution of phosphorylated keratins in simple epithelial tissues. J Cell Biol. 1995;131:1291–1301. doi: 10.1083/jcb.131.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowthert LA, Ku NO, Liao J, Coulombe PA, Omary MB. Empigen BB: a useful detergent for solubilization and biochemical analysis of keratins. Biochem Biophys Res Commun. 1995;206:370–379. doi: 10.1006/bbrc.1995.1051. [DOI] [PubMed] [Google Scholar]
- Maccioni RB, Otth C, Concha II, Munoz JP. The protein kinase Cdk5. Structural aspects, roles in neurogenesis and involvement in Alzheimer's pathology. Eur J Biochem. 2001;268:1518–1527. doi: 10.1046/j.1432-1033.2001.02024.x. [DOI] [PubMed] [Google Scholar]
- Mansbridge JN, Hanawalt PC. Role of transforming growth factor beta in the maturation of human epidermal keratinocytes. J Invest Dermatol. 1988;90:336–341. doi: 10.1111/1523-1747.ep12456286. [DOI] [PubMed] [Google Scholar]
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Muller FB, Kuster W, Bruckner-Tuderman L, Korge BP. Novel K5 and K14 mutations in German patients with the Weber-Cockayne variant of epidermolysis bullosa simplex. J Invest Dermatol. 1998;111:900–902. doi: 10.1046/j.1523-1747.1998.00374.x. [DOI] [PubMed] [Google Scholar]
- Ness SL, Edelmann W, Jenkins TD, Liedtke W, Rustgi AK, Kucherlapati R. Mouse keratin 4 is necessary for internal epithelial integrity. J Biol Chem. 1998;273:23904–23911. doi: 10.1074/jbc.273.37.23904. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Lariviere RC, Julien JP. Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron. 2001;30:135–147. doi: 10.1016/s0896-6273(01)00268-9. [DOI] [PubMed] [Google Scholar]
- Nurse P. A long twentieth century of the cell cycle and beyond. Cell. 2000;100:71–78. doi: 10.1016/s0092-8674(00)81684-0. [DOI] [PubMed] [Google Scholar]
- Omary MB, Ku NO, Liao J, Price D. Keratin modifications and solubility properties in epithelial cells and in vitro. Subcell Biochem. 1998;31:105–140. [PubMed] [Google Scholar]
- Omary MB, Ku N-O, Toivola DM. Keratins. Guardians of the liver. Hepatology. 2002;35:251–257. doi: 10.1053/jhep.2002.31165. [DOI] [PubMed] [Google Scholar]
- Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Oshima RG. Developmental expression of murine extra-embryonic endodermal cytoskeletal proteins. J Biol Chem. 1982;257:3414–3421. [PubMed] [Google Scholar]
- Pang YY, Schermer A, Yu J, Sun TT. Suprabasal change and subsequent formation of disulfide-stabilized homo- and hetero-dimers of keratins during esophageal epithelial differentiation. J Cell Sci. 1993;104:727–740. doi: 10.1242/jcs.104.3.727. [DOI] [PubMed] [Google Scholar]
- Paramio JM. A role for phosphorylation in the dynamics of keratin intermediate filaments. Eur J Cell Biol. 1999;78:33–43. doi: 10.1016/S0171-9335(99)80005-3. [DOI] [PubMed] [Google Scholar]
- Powell B, Crocker L, Rogers G. Hair follicle differentiation: expression, structure and evolutionary conservation of the hair type II keratin intermediate filament gene family. Development. 1992;114:417–433. doi: 10.1242/dev.114.2.417. [DOI] [PubMed] [Google Scholar]
- Rao KS, Babu KK, Gupta PD. Keratins and skin disorders. Cell Biol Int. 1996;20:261–274. [PubMed] [Google Scholar]
- Rogers MA, Winter H, Langbein L, Wolf C, Schweizer J. Characterization of a 300 kbp region of human DNA containing the type II hair keratin gene domain. J Invest Dermatol. 2000;114:464–472. doi: 10.1046/j.1523-1747.2000.00910.x. [DOI] [PubMed] [Google Scholar]
- Sahlgren CM, Mikhailov A, Hellman J, Chou YH, Lendahl U, Goldman RD, Eriksson JE. Mitotic reorganization of the intermediate filament protein nestin involves phosphorylation by cdc2 kinase. J Biol Chem. 2001;276:16456–16463. doi: 10.1074/jbc.M009669200. [DOI] [PubMed] [Google Scholar]
- Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert PM. The dynamic phosphorylation of the human intermediate filament keratin 1 chain. J Biol Chem. 1988;263:13333–13339. [PubMed] [Google Scholar]
- Steinert PM, Parry DA. The conserved H1 domain of the type II keratin 1 chain plays an essential role in the alignment of nearest neighbor molecules in mouse and human keratin 1/keratin 10 intermediate filaments at the two- to four-molecule level of structure. J Biol Chem. 1993;268:2878–2887. [PubMed] [Google Scholar]
- Stumptner C, Omary MB, Fickert P, Denk H, Zatloukal K. Hepatocyte cytokeratins are hyperphosphorylated at multiple sites in human alcoholic hepatitis and in a mallory body mouse model. Am J Pathol. 2000;156:77–90. doi: 10.1016/S0002-9440(10)64708-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yan B, Yamanishi K, Imamura S, Coulombe PA. The two functional keratin 6 genes of mouse are differentially regulated and evolved independently from their human orthologs. Genomics. 1998;53:170–183. doi: 10.1006/geno.1998.5476. [DOI] [PubMed] [Google Scholar]
- Toivola DM, Goldman RD, Garrod DR, Eriksson JE. Protein phosphatases maintain the organization and structural interactions of hepatic keratin intermediate filaments. J Cell Sci. 1997;110:23–33. doi: 10.1242/jcs.110.1.23. [DOI] [PubMed] [Google Scholar]
- Tsujimura K, Ogawara M, Takeuchi Y, Imajoh-Ohmi S, Ha MH, Inagaki M. Visualization and function of vimentin phosphorylation by cdc2 kinase during mitosis. J Biol Chem. 1994;269:31097–31106. [PubMed] [Google Scholar]
- Wilhelm D, Bender K, Knebel A, Angel P. The level of intracellular glutathione is a key regulator for the induction of stress-activated signal transduction pathways including Jun N-terminal protein kinases and p38 kinase by alkylating agents. Mol Cell Biol. 1997;17:4792–4800. doi: 10.1128/mcb.17.8.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Langbein L, Praetzel S, Jacobs M, Rogers MA, Leigh IM, Tidman N, Schweizer J. A novel human type II cytokeratin, K6hf, specifically expressed in the companion layer of the hair follicle. J Invest Dermatol. 1998;111:955–962. doi: 10.1046/j.1523-1747.1998.00456.x. [DOI] [PubMed] [Google Scholar]
- Yano T, Tokui T, Nishi Y, Nishizawa K, Shibata M, Kikuchi K, Tsuiki S, Yamauchi T, Inagaki M. Phosphorylation of keratin intermediate filaments by protein kinase C, by calmodulin-dependent protein kinase and by cAMP-dependent protein kinase. Eur J Biochem. 1991;197:281–290. doi: 10.1111/j.1432-1033.1991.tb15909.x. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Amano M, Nagata K, Inagaki N, Nakamura H, Saya H, Kaibuchi K, Inagaki M. Roles of Rho-associated kinase in cytokinesis; mutations in Rho-associated kinase phosphorylation sites impair cytokinetic segregation of glial filaments. J Cell Biol. 1998;143:1249–1258. doi: 10.1083/jcb.143.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunami J, Komori A, Ohta T, Suganuma M, Yuspa SH, Fujiki H. Hyperphosphorylation of cytokeratins by okadaic acid class tumor promoters in primary human keratinocytes. Cancer Res. 1993;53:992–996. [PubMed] [Google Scholar]
- Yoshikawa K, Katagata Y, Kondo S. Biochemical and immunohistochemical analyses of keratin expression in basal cell carcinoma. J Dermatol Sci. 1998;17:15–23. doi: 10.1016/s0923-1811(97)00065-0. [DOI] [PubMed] [Google Scholar]
- Zhou X, Liao J, Hu L, Feng L, Omary MB. Characterization of the major physiologic phosphorylation site of human keratin 19 and its role in filament organization. J Biol Chem. 1999;274:12861–12866. doi: 10.1074/jbc.274.18.12861. [DOI] [PubMed] [Google Scholar]