Abstract
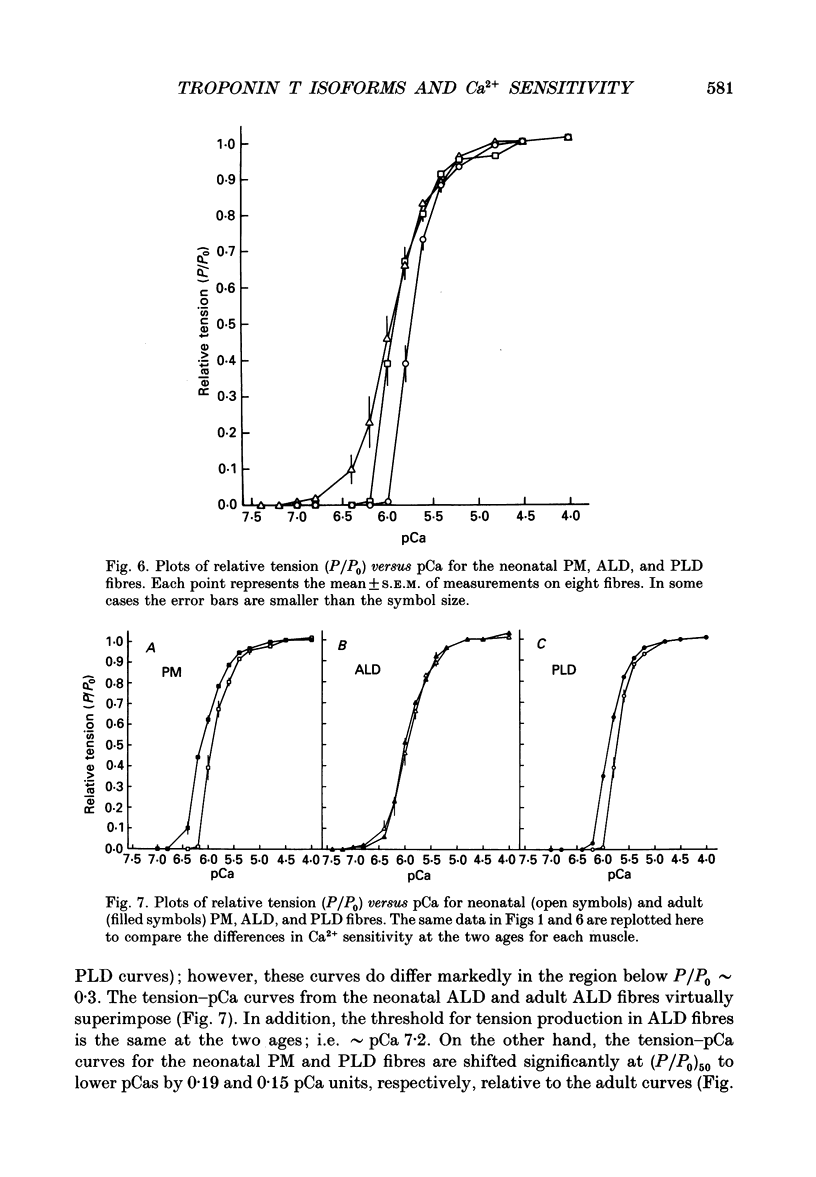
1. The Ca2+ sensitivity of tension development was characterized in single skinned fibres from the slow anterior latissimus dorsi (ALD), fast posterior latissimus dorsi (PLD), and fast pectoralis major (PM) muscles of the chicken at adult and neonatal (2 weeks post-hatch) stages of development. In the adult, the PM was most sensitive, the ALD intermediate, and the PLD least sensitive to Ca2+. 2. PM and PLD fibres were less sensitive to Ca2+ at the neonatal stage of development than in the adult. However, ALD fibres exhibited no age-dependent changes in Ca2+ sensitivity. 3. Characterization of regulatory protein composition indicated that the PM and PLD fibres had identical fast isoforms of troponin C and troponin I at each developmental stage examined, but there were muscle-specific and age-dependent expressions of troponin T isoforms in these fibres. 4. In the ALD fibres, identical slow isoforms of troponin C, troponin I and tropomyosin were found at each stage. In addition, the troponin T isoform that was present did not change with age. 5. The results suggest a relationship between the specific troponin T isoform composition of individual muscle fibres and their calcium sensitivities of tension development.
Full text
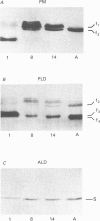
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe H., Komiya T., Obinata T. Expression of multiple troponin T variants in neonatal chicken breast muscle. Dev Biol. 1986 Nov;118(1):42–51. doi: 10.1016/0012-1606(86)90071-0. [DOI] [PubMed] [Google Scholar]
- Allen J. D., Moss R. L. Factors influencing the ascending limb of the sarcomere length-tension relationship in rabbit skinned muscle fibres. J Physiol. 1987 Sep;390:119–136. doi: 10.1113/jphysiol.1987.sp016689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird I. M., Dhoot G. K., Wilkinson J. M. Identification of multiple variants of fast muscle troponin T in the chicken using monoclonal antibodies. Eur J Biochem. 1985 Aug 1;150(3):517–525. doi: 10.1111/j.1432-1033.1985.tb09052.x. [DOI] [PubMed] [Google Scholar]
- Breitbart R. E., Nadal-Ginard B. Complete nucleotide sequence of the fast skeletal troponin T gene. Alternatively spliced exons exhibit unusual interspecies divergence. J Mol Biol. 1986 Apr 5;188(3):313–324. doi: 10.1016/0022-2836(86)90157-9. [DOI] [PubMed] [Google Scholar]
- Briggs M. M., Schachat F. N-terminal amino acid sequences of three functionally different troponin T isoforms from rabbit fast skeletal muscle. J Mol Biol. 1989 Mar 5;206(1):245–249. doi: 10.1016/0022-2836(89)90538-x. [DOI] [PubMed] [Google Scholar]
- Dhoot G. K. Identification and distribution of the fast class of troponin T in the adult and developing avian skeletal muscle. J Muscle Res Cell Motil. 1988 Oct;9(5):446–455. doi: 10.1007/BF01774070. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Everett A. W., Prior G., Clark W. A., Zak R. Quantitation of myosin in muscle. Anal Biochem. 1983 Apr 1;130(1):102–107. doi: 10.1016/0003-2697(83)90655-3. [DOI] [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem. 1983 Mar;129(2):277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Greaser M. L., Gergely J. Purification and properties of the components from troponin. J Biol Chem. 1973 Mar 25;248(6):2125–2133. [PubMed] [Google Scholar]
- Greaser M. L., Gergely J. Reconstitution of troponin activity from three protein components. J Biol Chem. 1971 Jul 10;246(13):4226–4233. [PubMed] [Google Scholar]
- Greaser M. L., Moss R. L., Reiser P. J. Variations in contractile properties of rabbit single muscle fibres in relation to troponin T isoforms and myosin light chains. J Physiol. 1988 Dec;406:85–98. doi: 10.1113/jphysiol.1988.sp017370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrick W. G., Hoar P. E., Malencik D. A., Stamps L., Fischer E. H. Characterization of Ca2+- and Sr2+-activated tension in functionally skinned chicken fibers of normal and dystrophic skeletal and normal cardiac muscle. Pflugers Arch. 1979 Jul;381(1):53–62. doi: 10.1007/BF00582332. [DOI] [PubMed] [Google Scholar]
- Lim S. S., Tu Z. H., Lemanski L. F. Anti-troponin-T monoclonal antibody crossreacts with all muscle types. J Muscle Res Cell Motil. 1984 Oct;5(5):515–526. doi: 10.1007/BF00713258. [DOI] [PubMed] [Google Scholar]
- Mak A. S., Golosinska K., Smillie L. B. Induction of nonpolymerizable tropomyosin binding to F-actin by troponin and its components. J Biol Chem. 1983 Dec 10;258(23):14330–14334. [PubMed] [Google Scholar]
- Mak A. S., Smillie L. B. Structural interpretation of the two-site binding of troponin on the muscle thin filament. J Mol Biol. 1981 Jul 5;149(3):541–550. doi: 10.1016/0022-2836(81)90486-1. [DOI] [PubMed] [Google Scholar]
- Matsuda R., Bandman E., Strohman R. C. Regional differences in the expression of myosin light chains and tropomyosin subunits during development of chicken breast muscle. Dev Biol. 1983 Feb;95(2):484–491. doi: 10.1016/0012-1606(83)90050-7. [DOI] [PubMed] [Google Scholar]
- Matsuda R., Obinata T., Shimada Y. Types of troponin components during development of chicken skeletal muscle. Dev Biol. 1981 Feb;82(1):11–19. doi: 10.1016/0012-1606(81)90424-3. [DOI] [PubMed] [Google Scholar]
- Montarras D., Fiszman M. Y., Gros F. Characterization of the tropomyosin present in various chick embryo muscle types and in muscle cells differentiated in vitro. J Biol Chem. 1981 Apr 25;256(8):4081–4086. [PubMed] [Google Scholar]
- Moore G., Johnston I. A., Goldspink G. The pCa-tension characteristics of single skinned fibres isolated from the anterior and posterior latissimus dorsi muscles of the chicken. J Exp Biol. 1983 Jul;105:411–416. doi: 10.1242/jeb.105.1.411. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Giulian G. G., Greaser M. L. The effects of partial extraction of TnC upon the tension-pCa relationship in rabbit skinned skeletal muscle fibers. J Gen Physiol. 1985 Oct;86(4):585–600. doi: 10.1085/jgp.86.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979 Jul;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Swinford A. E., Greaser M. L. Alterations in the Ca2+ sensitivity of tension development by single skeletal muscle fibers at stretched lengths. Biophys J. 1983 Jul;43(1):115–119. doi: 10.1016/S0006-3495(83)84329-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki I., Maruyama K., Ebashi S. Regulatory and cytoskeletal proteins of vertebrate skeletal muscle. Adv Protein Chem. 1986;38:1–67. doi: 10.1016/s0065-3233(08)60525-2. [DOI] [PubMed] [Google Scholar]
- Rall J. A., Schottelius B. A. Energetics of contraction in phasic and tonic skeletal muscles of the chicken. J Gen Physiol. 1973 Sep;62(3):303–323. doi: 10.1085/jgp.62.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risnik V. V., Verin A. D., Gusev N. B. Comparison of the structure of two cardiac troponin T isoforms. Biochem J. 1985 Jan 15;225(2):549–552. doi: 10.1042/bj2250549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R. K., Sreter F. A., Sarkar S. Changes in tropomyosin subunits and myosin light chains during development of chicken and rabbit striated muscles. Dev Biol. 1979 Mar;69(1):15–30. doi: 10.1016/0012-1606(79)90271-9. [DOI] [PubMed] [Google Scholar]
- Schachat F. H., Diamond M. S., Brandt P. W. Effect of different troponin T-tropomyosin combinations on thin filament activation. J Mol Biol. 1987 Dec 5;198(3):551–554. doi: 10.1016/0022-2836(87)90300-7. [DOI] [PubMed] [Google Scholar]
- Shimizu N., Shimada Y. Immunochemical analysis of troponin T isoforms in adult, embryonic, regenerating, and denervated chicken fast skeletal muscles. Dev Biol. 1985 Oct;111(2):324–334. doi: 10.1016/0012-1606(85)90487-7. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B. Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev. 1986 Jul;66(3):710–771. doi: 10.1152/physrev.1986.66.3.710. [DOI] [PubMed] [Google Scholar]
- Tobacman L. S., Lee R. Isolation and functional comparison of bovine cardiac troponin T isoforms. J Biol Chem. 1987 Mar 25;262(9):4059–4064. [PubMed] [Google Scholar]
- Tobacman L. S. Structure-function studies of the amino-terminal region of bovine cardiac troponin T. J Biol Chem. 1988 Feb 25;263(6):2668–2672. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. P., Cohen C., Phillips G. N., Jr Structure of co-crystals of tropomyosin and troponin. 1987 Feb 26-Mar 4Nature. 325(6107):826–828. doi: 10.1038/325826a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. M. The components of troponin from chicken fast skeletal muscle. A comparison of troponin T and troponin I from breast and leg muscle. Biochem J. 1978 Jan 1;169(1):229–238. doi: 10.1042/bj1690229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates L. D., Greaser M. L. Quantitative determination of myosin and actin in rabbit skeletal muscle. J Mol Biol. 1983 Jul 25;168(1):123–141. doi: 10.1016/s0022-2836(83)80326-x. [DOI] [PubMed] [Google Scholar]
- Yates L. D., Greaser M. L. Troponin subunit stoichiometry and content in rabbit skeletal muscle and myofibrils. J Biol Chem. 1983 May 10;258(9):5770–5774. [PubMed] [Google Scholar]
- el-Saleh S. C., Warber K. D., Potter J. D. The role of tropomyosin-troponin in the regulation of skeletal muscle contraction. J Muscle Res Cell Motil. 1986 Oct;7(5):387–404. doi: 10.1007/BF01753582. [DOI] [PubMed] [Google Scholar]