Abstract
1. The whole-cell patch clamp technique was employed to study the effects of the beta-agonist isoprenaline (ISO) on the Na(+)=K+ pump current, Ip, in acutely isolated ventricular myocytes from guinea-pig hearts. Propranolol, a beta-adrenergic antagonist, was used to demonstrate that all of the effects of ISO, stimulatory or inhibitory, are mediated by beta-receptors. 2. Below about 150 nM [Ca2+]i, we find that ISO reduces Ip, while above this [Ca2+]i ISO increases Ip. The stimulatory and inhibitory effects of ISO on Ip are independent of either intracellular sodium ([Na+]i) or extracellular potassium ([K+]o). These results suggest that the end-effect of ISO is directly on the maximum pump turnover rate (Vmax) rather than indirectly through changes in [Na+]i or [K+]o or modulatory effects on Na+ or K+ affinity. 3. The maximum effect of ISO increases Ip by 25% when [Ca2+] is buffered at 1.4 microM. A half-maximal effect is reached at roughly 10 nM-ISO and a near-maximal effect by 0.5 microM. 4. The permeabilized patch technique, using amphotericin B (Horn & Marty, 1988; Rae, Cooper, Gates & Watsky, 1991), was employed to minimize changes in the normal second messenger systems and calcium buffers. In these experiments, we used a high intracellular sodium solution (pipette sodium was 50 mM), thus sodium-calcium exchange was depressed and we expected [Ca2+]i to be above 150 nM. ISO increases Ip in these conditions as in the dialysed cells. 5. Our results suggest that beta-stimulation can increase Ip, but only if [Ca2+]i is above about 150 nM. In the beating heart [Ca2+]i rises well above this value during systole and the average [Ca2+]i, which depends on heart rate, is expected to normally be above this level. During beta-stimulation, the increase in Ip along with a concomitant increase in IK (Giles, Nakajima, Ono & Shibata, 1989; Duchatelle-Gourdon, Hartzell & Lagrutta, 1989) helps prevent action potential lengthening and allows an increase in heart rate even in the presence of increased calcium current. Further, beta-stimulation will compensate for the effects on Ip of either hypokalaemia or digitalis toxicity, and so reduce the expected rise in both [Na+]i and [Ca2+]i.
Full text
PDF


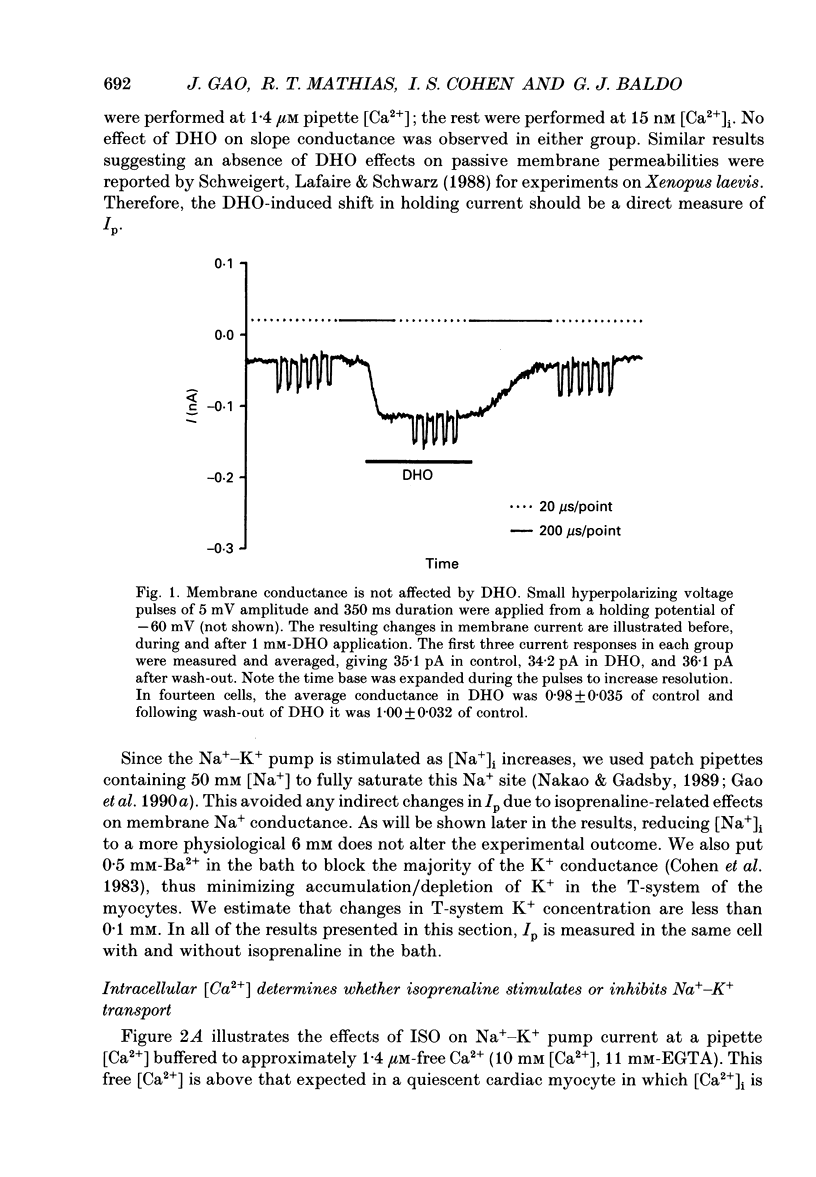
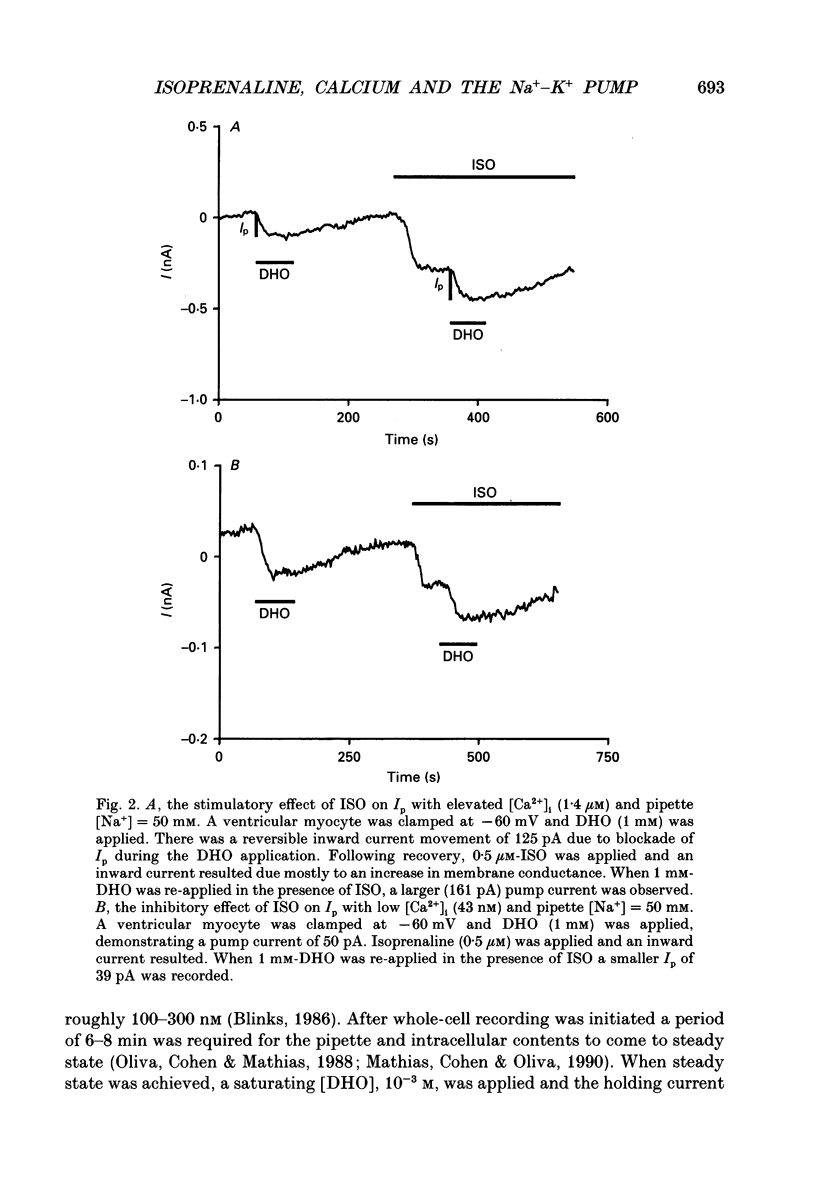
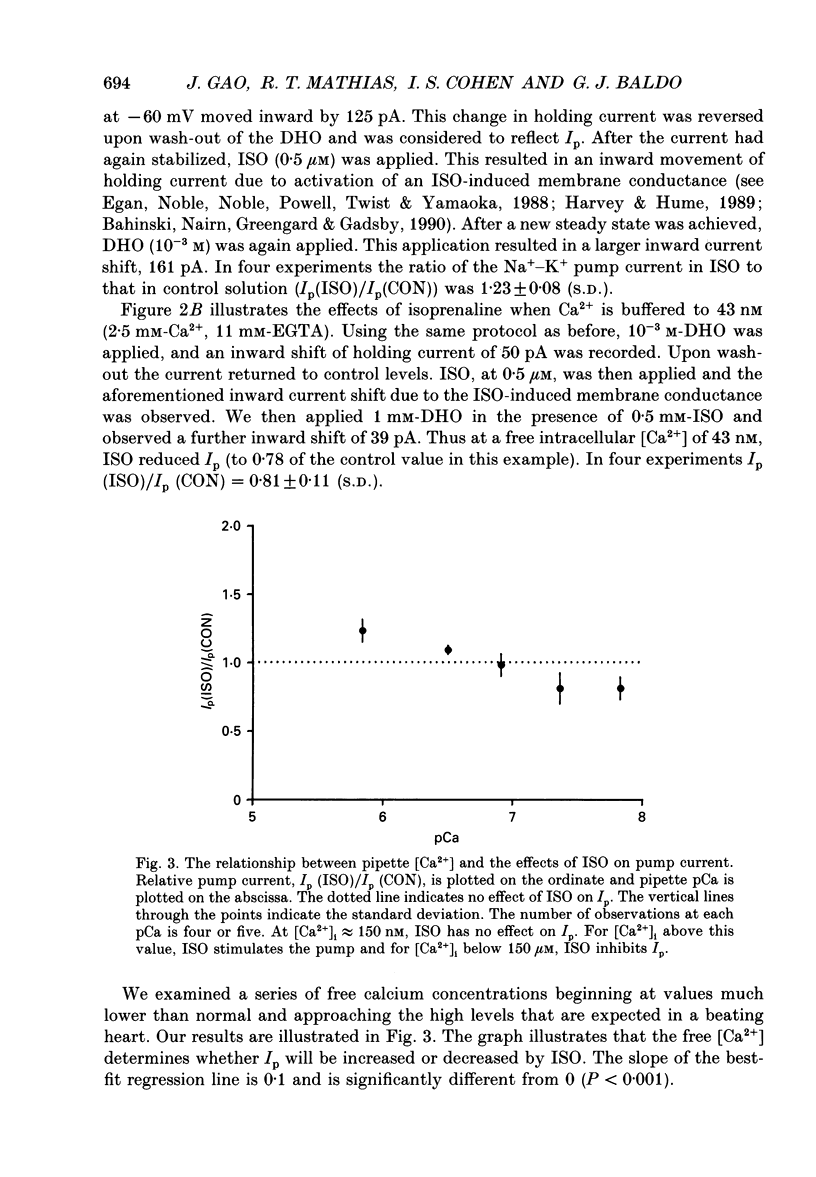
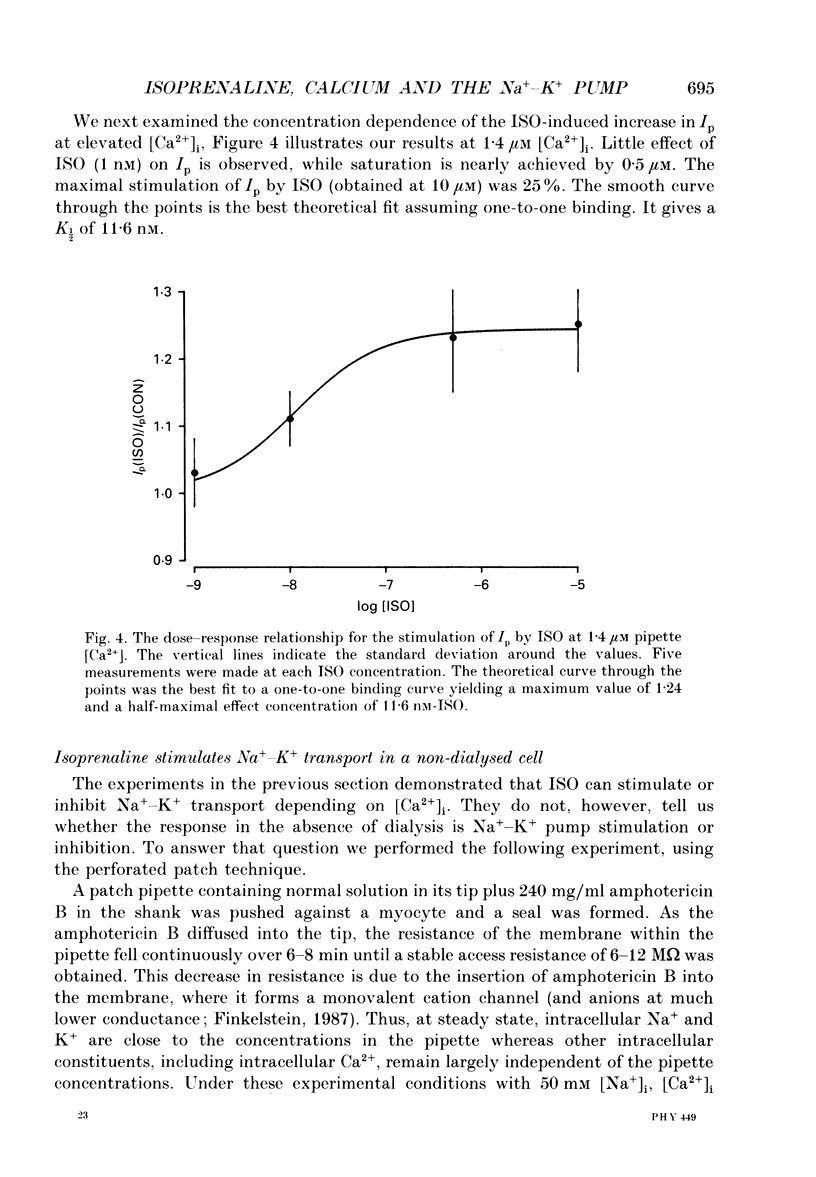
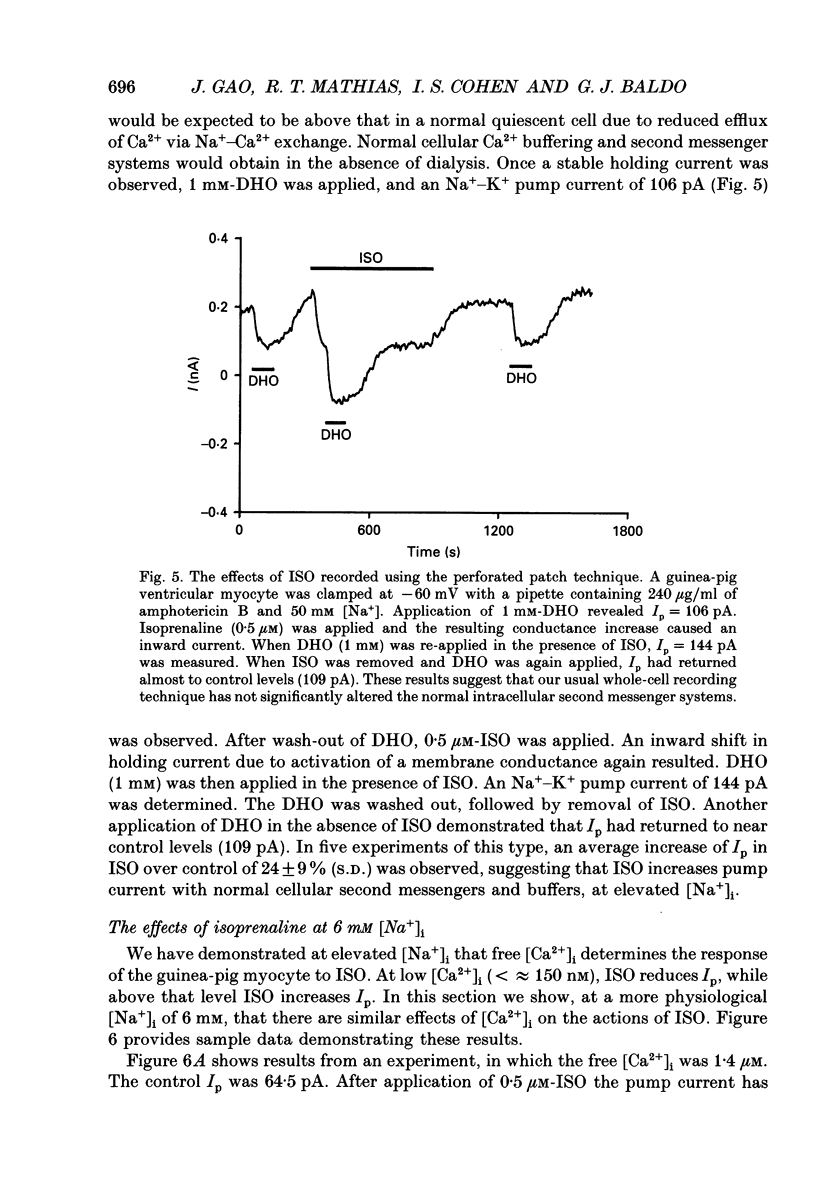
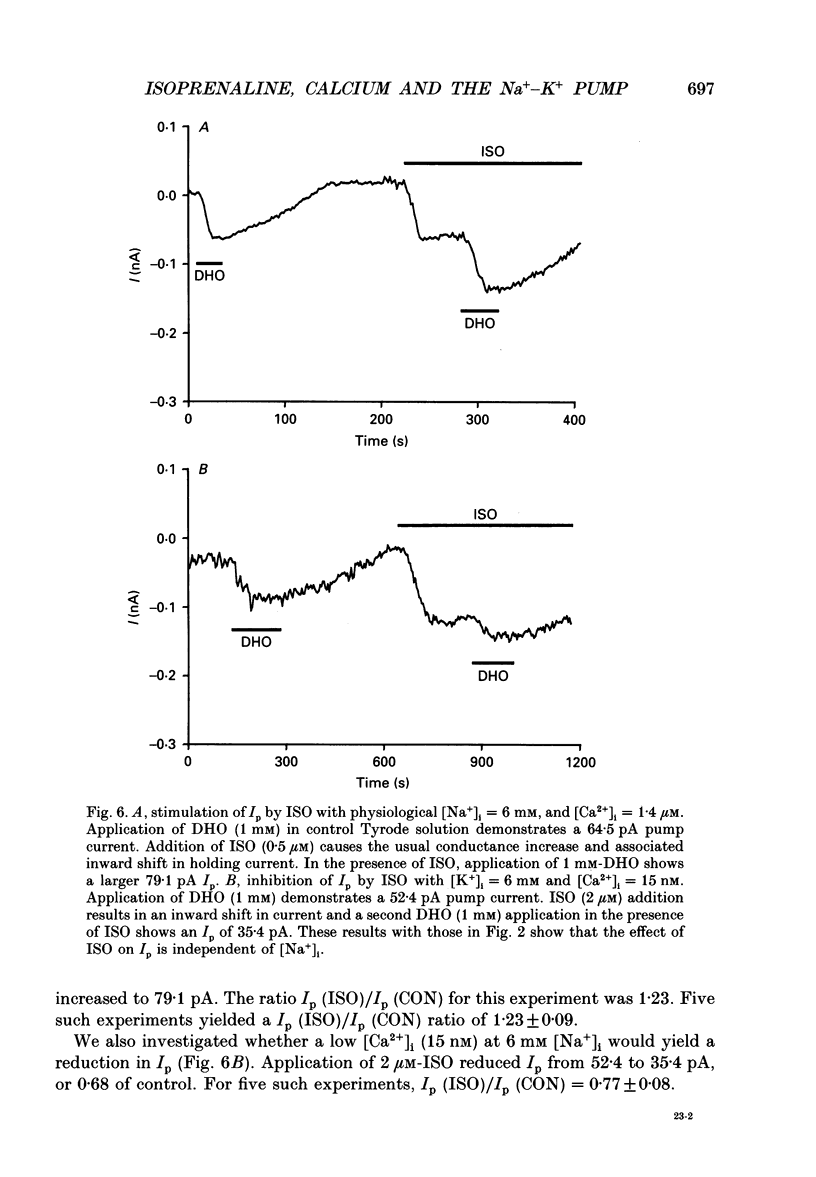
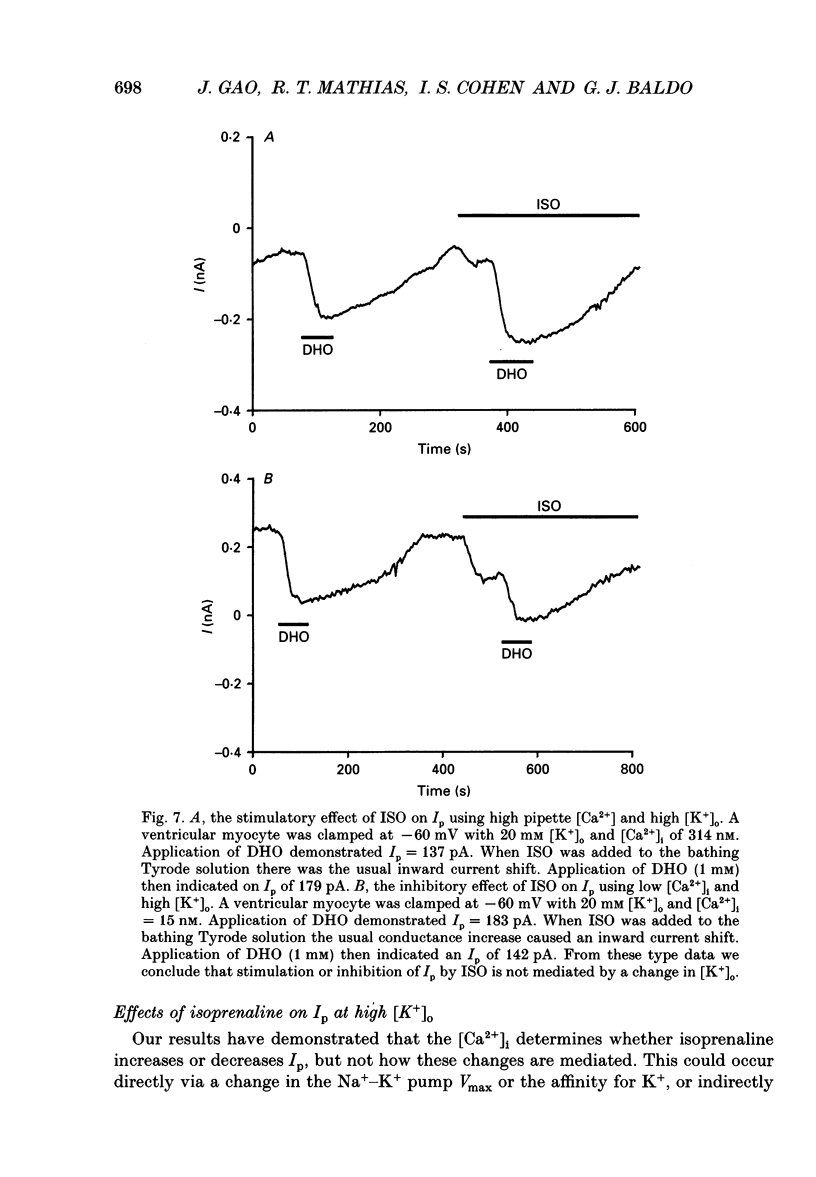

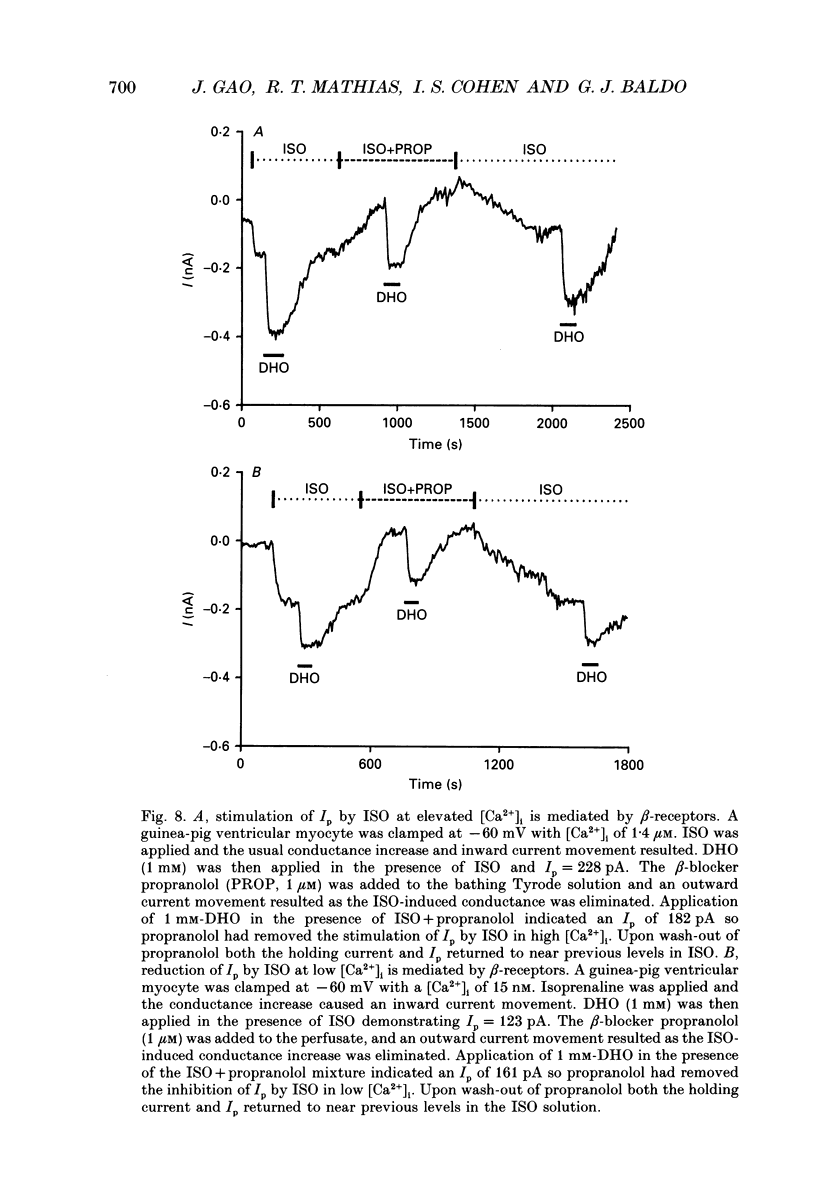




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahinski A., Nairn A. C., Greengard P., Gadsby D. C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989 Aug 31;340(6236):718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- Berthon B., Capiod T., Claret M. Effects of noradrenaline, vasopressin and angiotensin on the Na-K pump in rat isolated liver cells. Br J Pharmacol. 1985 Sep;86(1):151–161. doi: 10.1111/j.1476-5381.1985.tb09445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum G., Osterrieder W., Trautwein W. Beta-adrenergic increase in the calcium conductance of cardiac myocytes studied with the patch clamp. Pflugers Arch. 1984 Jun;401(2):111–118. doi: 10.1007/BF00583870. [DOI] [PubMed] [Google Scholar]
- Chang D. C., Reese T. S. Changes in membrane structure induced by electroporation as revealed by rapid-freezing electron microscopy. Biophys J. 1990 Jul;58(1):1–12. doi: 10.1016/S0006-3495(90)82348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. S., Falk R. T., Mulrine N. K. Actions of barium and rubidium on membrane currents in canine Purkinje fibres. J Physiol. 1983 May;338:589–612. doi: 10.1113/jphysiol.1983.sp014691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J., Rüdel R. The electrogenic sodium pump in guinea-pig ventricular muscle: inhibition of pump current by cardiac glycosides. J Physiol. 1982 Sep;330:243–264. doi: 10.1113/jphysiol.1982.sp014339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weer P., Gadsby D. C., Rakowski R. F. Voltage dependence of the Na-K pump. Annu Rev Physiol. 1988;50:225–241. doi: 10.1146/annurev.ph.50.030188.001301. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. Characterization of single pacemaker channels in cardiac sino-atrial node cells. Nature. 1986 Dec 4;324(6096):470–473. doi: 10.1038/324470a0. [DOI] [PubMed] [Google Scholar]
- Duchatelle-Gourdon I., Hartzell H. C., Lagrutta A. A. Modulation of the delayed rectifier potassium current in frog cardiomyocytes by beta-adrenergic agonists and magnesium. J Physiol. 1989 Aug;415:251–274. doi: 10.1113/jphysiol.1989.sp017721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Désilets M., Baumgarten C. M. Isoproterenol directly stimulates the Na+-K+ pump in isolated cardiac myocytes. Am J Physiol. 1986 Jul;251(1 Pt 2):H218–H225. doi: 10.1152/ajpheart.1986.251.1.H218. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Twist V. W., Yamaoka K. On the mechanism of isoprenaline- and forskolin-induced depolarization of single guinea-pig ventricular myocytes. J Physiol. 1988 Jun;400:299–320. doi: 10.1113/jphysiol.1988.sp017121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Falk R. T., Cohen I. S. Membrane current following activity in canine cardiac Purkinje fibers. J Gen Physiol. 1984 May;83(5):771–799. doi: 10.1085/jgp.83.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C. Beta-adrenoceptor agonists increase membrane K+ conductance in cardiac Purkinje fibres. Nature. 1983 Dec 15;306(5944):691–693. doi: 10.1038/306691a0. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C., Nakao M. Steady-state current-voltage relationship of the Na/K pump in guinea pig ventricular myocytes. J Gen Physiol. 1989 Sep;94(3):511–537. doi: 10.1085/jgp.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W., Nakajima T., Ono K., Shibata E. F. Modulation of the delayed rectifier K+ current by isoprenaline in bull-frog atrial myocytes. J Physiol. 1989 Aug;415:233–249. doi: 10.1113/jphysiol.1989.sp017720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Krahn T., Pusch H., Suleymanian M. Effect of isoprenaline on active Na transport in sheep cardiac Purkinje fibres. Pflugers Arch. 1989 Oct;415(1):88–94. doi: 10.1007/BF00373145. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch. 1982 Oct;395(1):30–41. doi: 10.1007/BF00584965. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Trautwein W. The effect of dihydro-ouabain and lithium-ions on the outward current in cardiac Purkinje fibers. Evidence for electrogenicity of active transport. Pflugers Arch. 1974;350(1):41–54. doi: 10.1007/BF00586737. [DOI] [PubMed] [Google Scholar]
- Mogul D. J., Rasmussen H. H., Singer D. H., Ten Eick R. E. Inhibition of Na-K pump current in guinea pig ventricular myocytes by dihydroouabain occurs at high- and low-affinity sites. Circ Res. 1989 Jun;64(6):1063–1069. doi: 10.1161/01.res.64.6.1063. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Hemmings H. C., Jr, Greengard P. Protein kinases in the brain. Annu Rev Biochem. 1985;54:931–976. doi: 10.1146/annurev.bi.54.070185.004435. [DOI] [PubMed] [Google Scholar]
- Nakao M., Gadsby D. C. [Na] and [K] dependence of the Na/K pump current-voltage relationship in guinea pig ventricular myocytes. J Gen Physiol. 1989 Sep;94(3):539–565. doi: 10.1085/jgp.94.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Palfrey C., Fozzard H. A. Modulation of the cardiac transient outward current by catecholamines. J Mol Cell Cardiol. 1989 Feb;21 (Suppl 1):109–118. doi: 10.1016/0022-2828(89)90845-6. [DOI] [PubMed] [Google Scholar]
- Oliva C., Cohen I. S., Mathias R. T. Calculation of time constants for intracellular diffusion in whole cell patch clamp configuration. Biophys J. 1988 Nov;54(5):791–799. doi: 10.1016/S0006-3495(88)83017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Schubert B., VanDongen A. M., Kirsch G. E., Brown A. M. Beta-adrenergic inhibition of cardiac sodium channels by dual G-protein pathways. Science. 1989 Aug 4;245(4917):516–519. doi: 10.1126/science.2547248. [DOI] [PubMed] [Google Scholar]
- Schweigert B., Lafaire A. V., Schwarz W. Voltage dependence of the Na-K ATPase: measurements of ouabain-dependent membrane current and ouabain binding in oocytes of Xenopus laevis. Pflugers Arch. 1988 Oct;412(6):579–588. doi: 10.1007/BF00583758. [DOI] [PubMed] [Google Scholar]
- Tromba C., Cohen I. S. A novel action of isoproterenol to inactivate a cardiac K+ current is not blocked by beta and alpha adrenergic blockers. Biophys J. 1990 Sep;58(3):791–795. doi: 10.1016/S0006-3495(90)82422-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W. Effects of epinephrine on the pacemaker potassium current of cardiac Purkinje fibers. J Gen Physiol. 1974 Sep;64(3):293–319. doi: 10.1085/jgp.64.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalle M., Barnabei O. Norepinephrine and potassium fluxes in cardiac Purkinje fibers. Pflugers Arch. 1971;322(4):287–303. doi: 10.1007/BF00587747. [DOI] [PubMed] [Google Scholar]
- Yingst D. R. Modulation of the Na,K-ATPase by Ca and intracellular proteins. Annu Rev Physiol. 1988;50:291–303. doi: 10.1146/annurev.ph.50.030188.001451. [DOI] [PubMed] [Google Scholar]


