Abstract
1. The ionic dependence of current through the 3',5'-cyclic guanosine monophosphate (cyclic GMP)-activated channels of salamander rods was studied in excised inside-out membrane patches from isolated outer segments. Voltage-clamp experiments on transducing rods were performed so that the channels in intact cells could be compared with those in excised patches. 2. The reversal potential of the cyclic GMP-induced patch current was close to the Na+ equilibrium potential when the concentration of NaCl on the cytoplasmic surface of a patch was varied at constant external NaCl concentration. Fitting the Goldman-Hodgkin-Katz equation indicated that the apparent ratio of permeabilities for Na+ and Cl- was at least 50. This confirms a previous report that the channel's Na+ permeability is much larger than its Cl- permeability. 3. Na+ currents through the channel did not obey the independence principle. The outward patch current at large positive potential began to saturate with increasing concentrations of internal Na+, as if permeation required Na+ to bind to a site with an apparent dissociation constant around 180 mM. 4. In symmetrical NaCl solutions containing very low concentrations of divalent cations the current-voltage relation measured from excised patches 50 microseconds after switching the voltage showed mild outward rectification. By 1 ms the rectification was more pronounced. The rectification at 50 microseconds is attributed to voltage dependence of Na+ permeation. The additional rectification at later times is attributed to voltage dependence of the channel's probability of being open, depolarization favouring the open state. 5. In symmetrical Mg2+ solutions the cyclic GMP-induced patch currents were smaller and the outward rectification was more pronounced. 6. Addition of Mg2+ or Ca2+ to an internal Na+ solution blocked the cyclic GMP-induced Na+ current through the channels, as if by occupying a single binding site with an affinity in the 0.1-2 mM range. Block by Mg2+ was voltage dependent, suggesting that the binding site was within the channel's transmembrane electric field. Raising the Mg2+ concentration on the external surface of the patch increased the apparent dissociation constant of block by internal Mg2+, as expected if external and internal Mg2+ compete for the same binding site. 7. Block by internal Ca2+ had an opposite and weaker voltage dependence than block by internal Mg2+. 8. In symmetrical solutions containing both Na+ and Mg2+ the outward rectification was more pronounced than in solutions containing Na+ alone. In solutions thought to be close to physiological the outward patch current increased e-fold for a depolarization of 24-30 mV.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
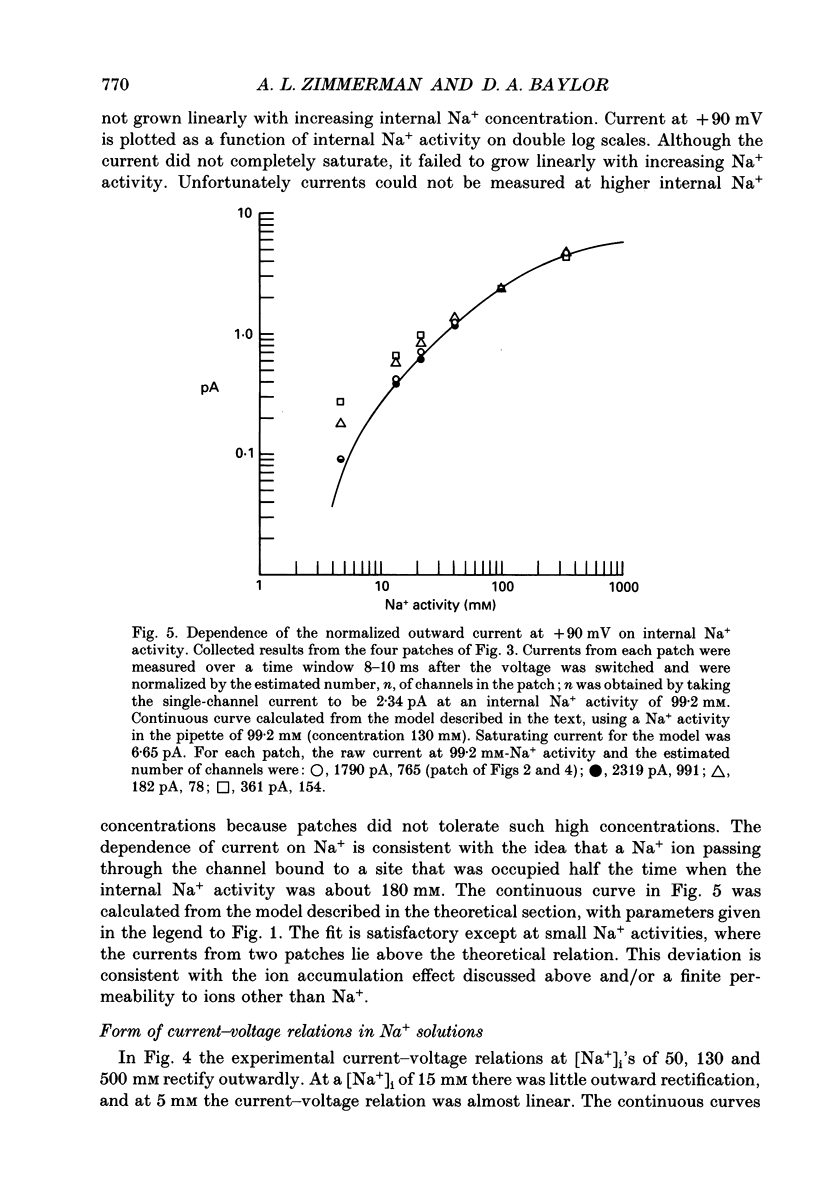
PDF



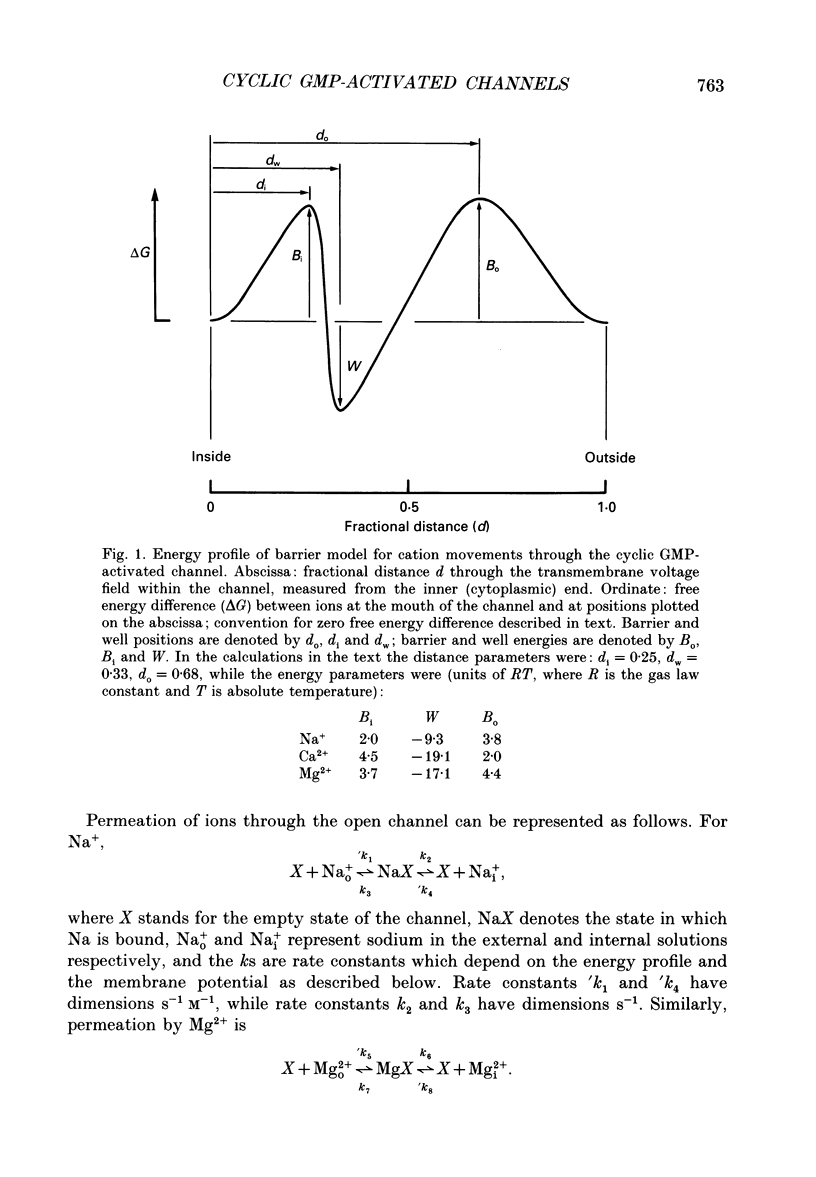


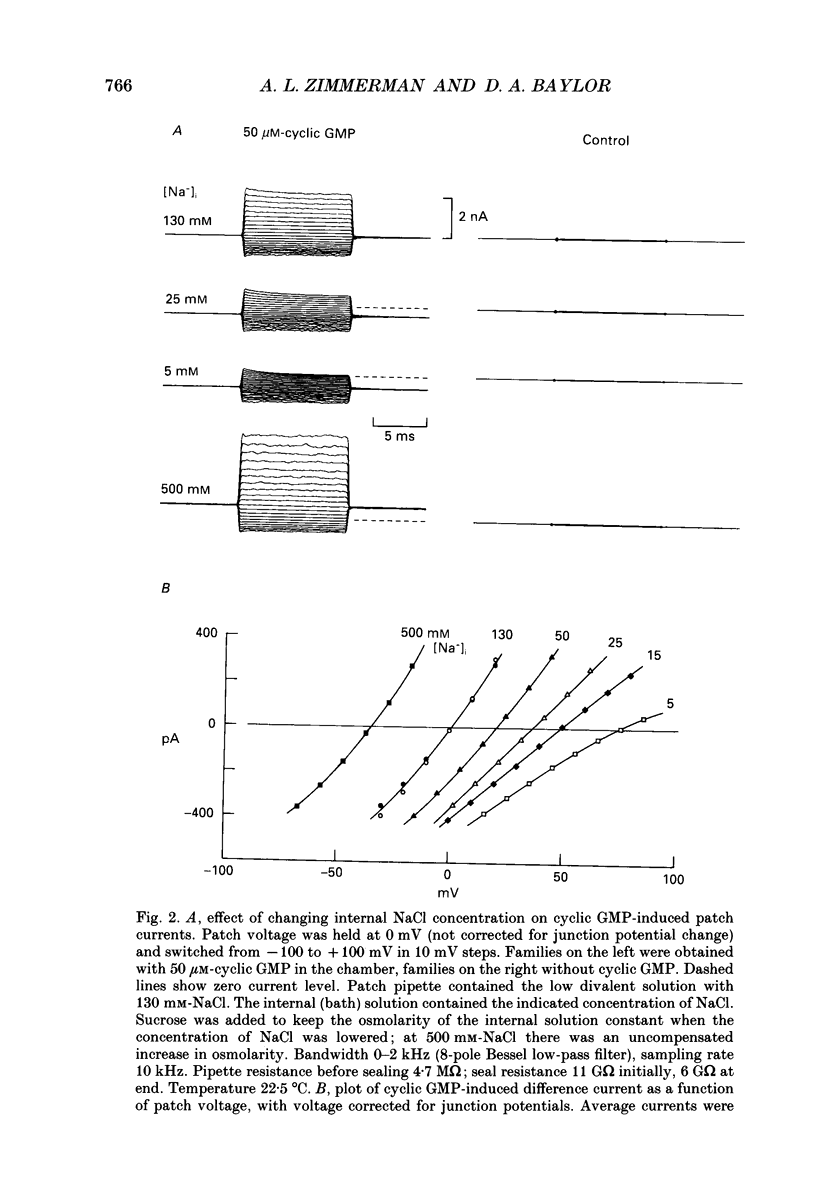

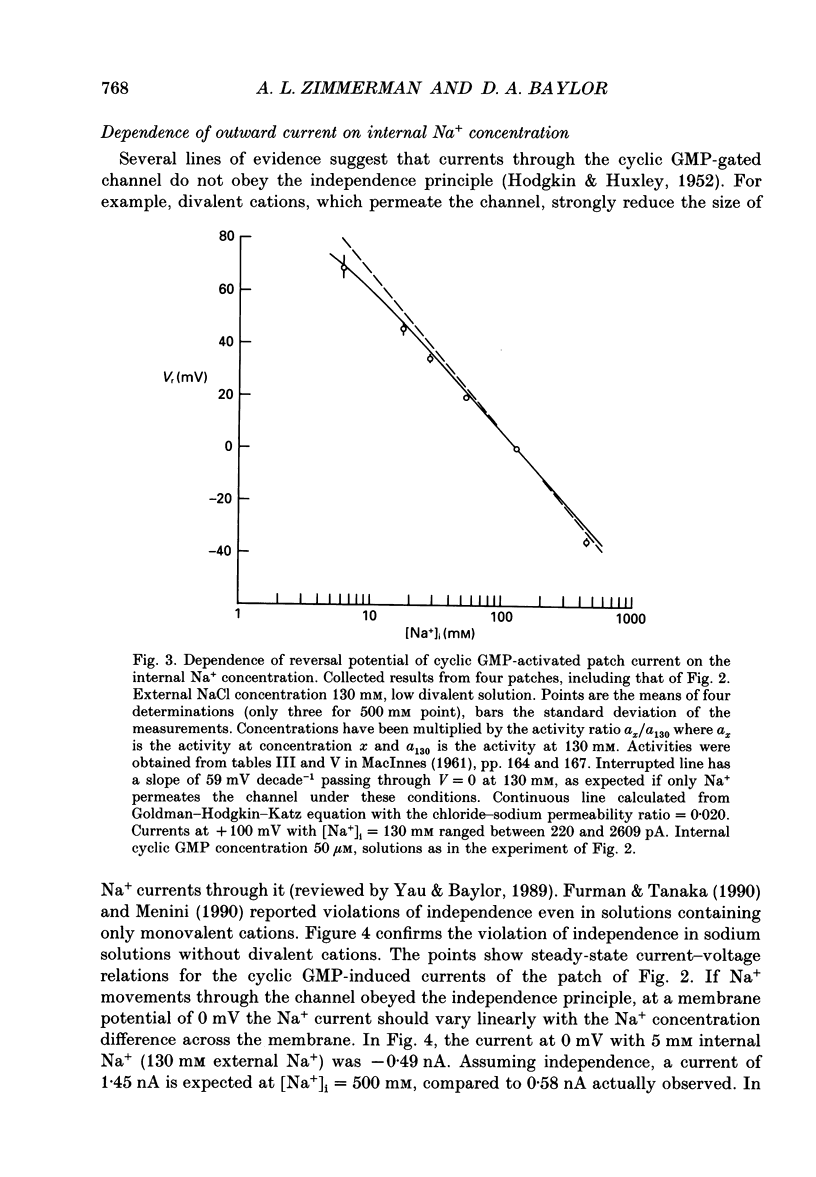
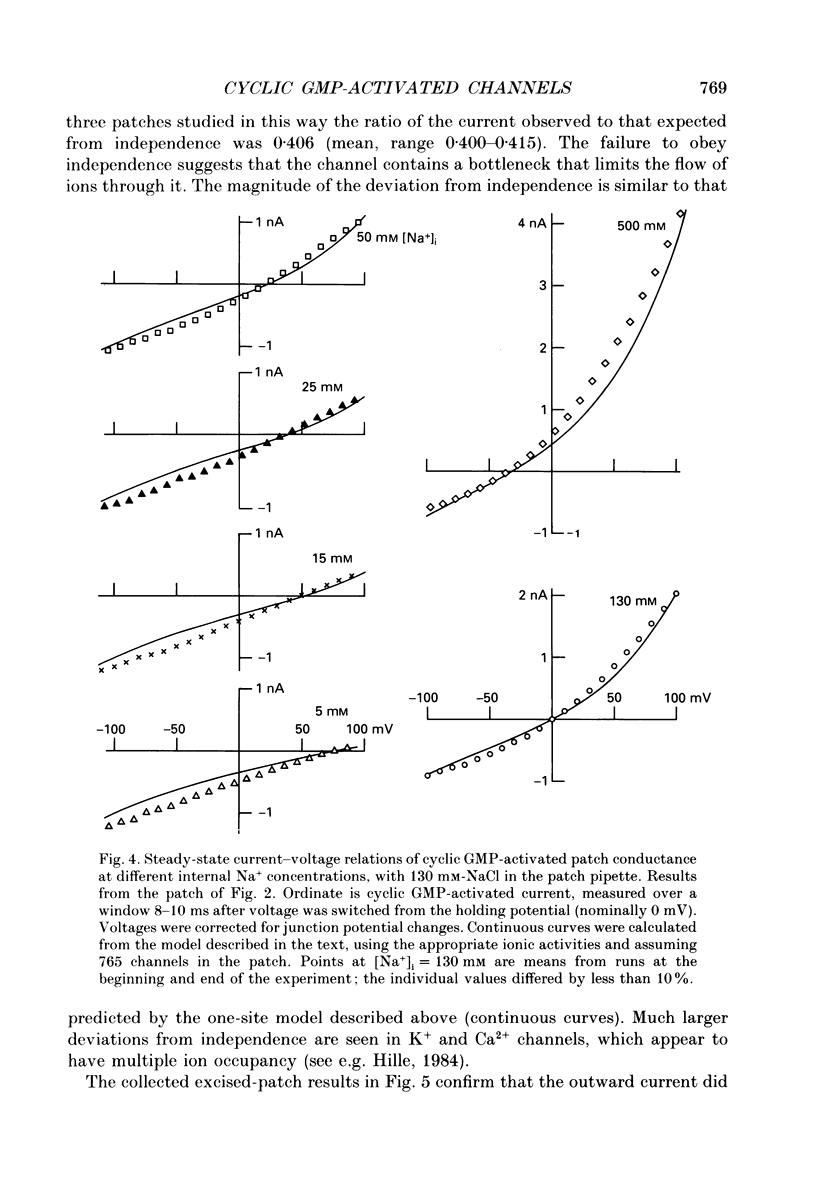
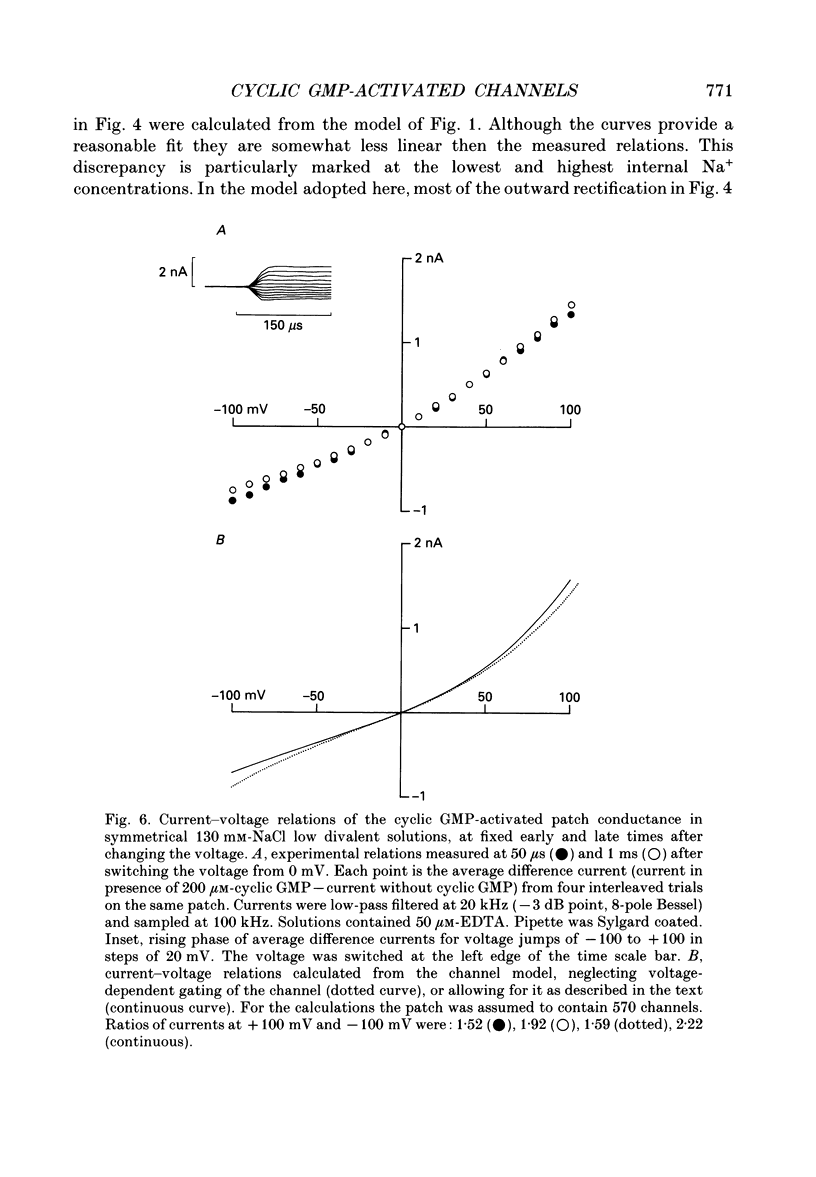

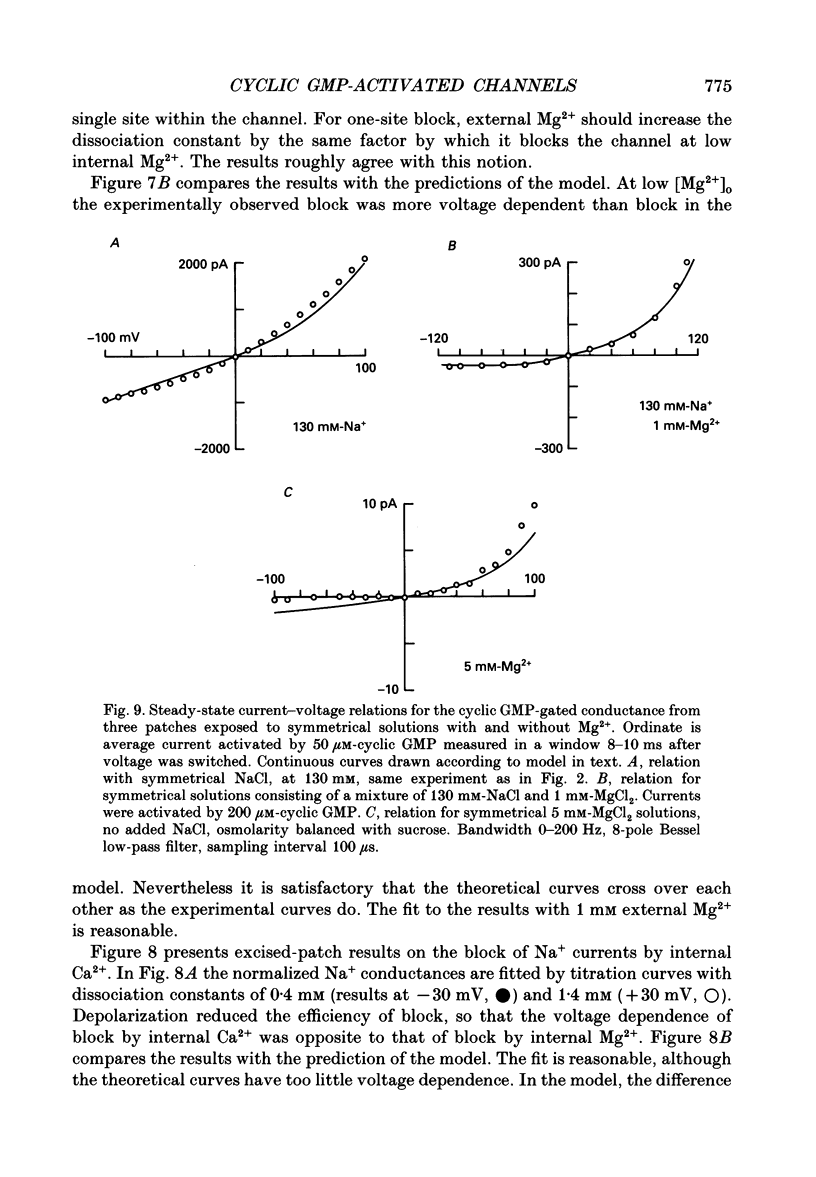

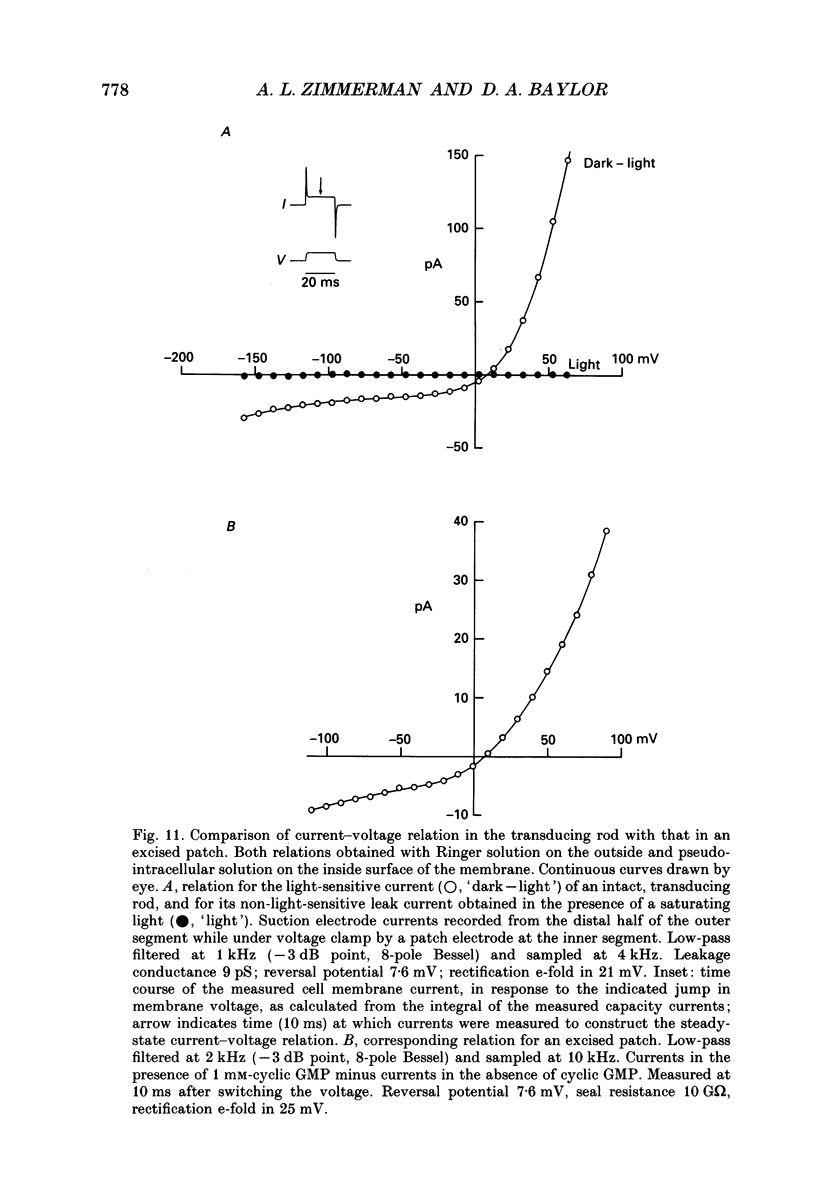




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
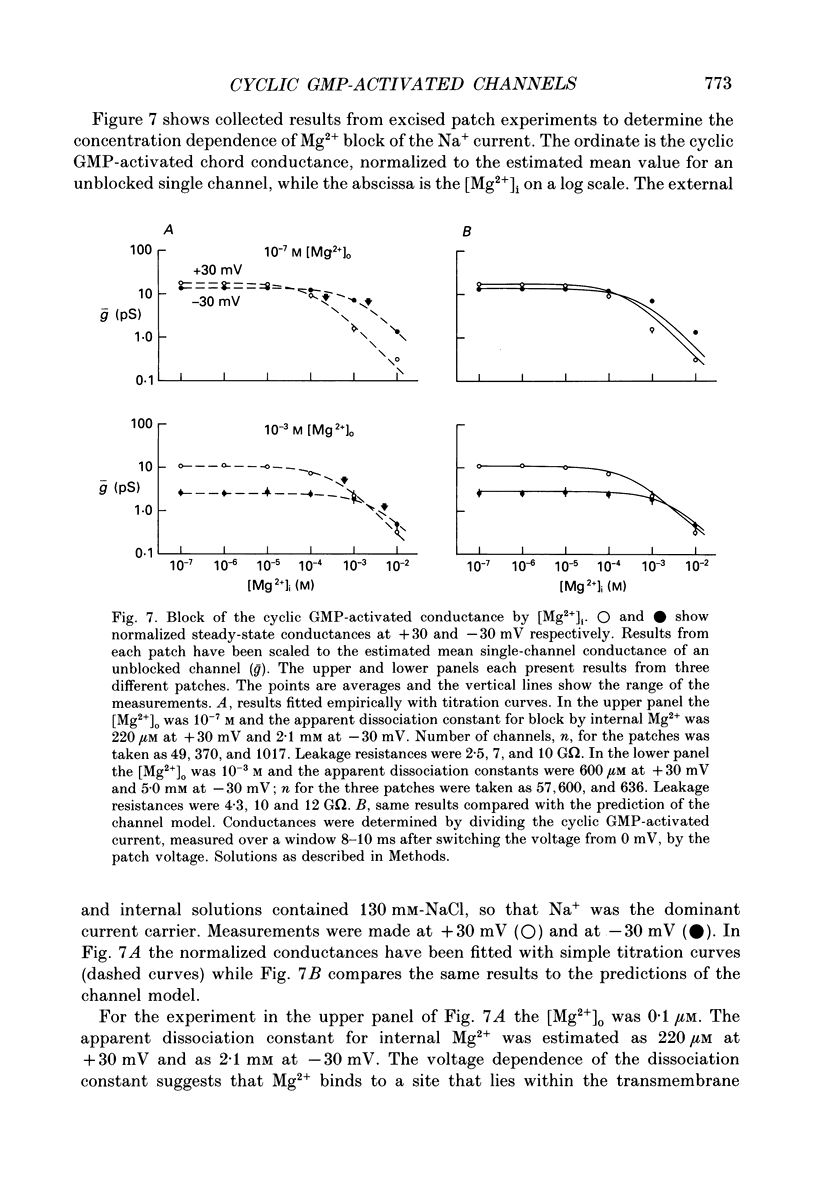
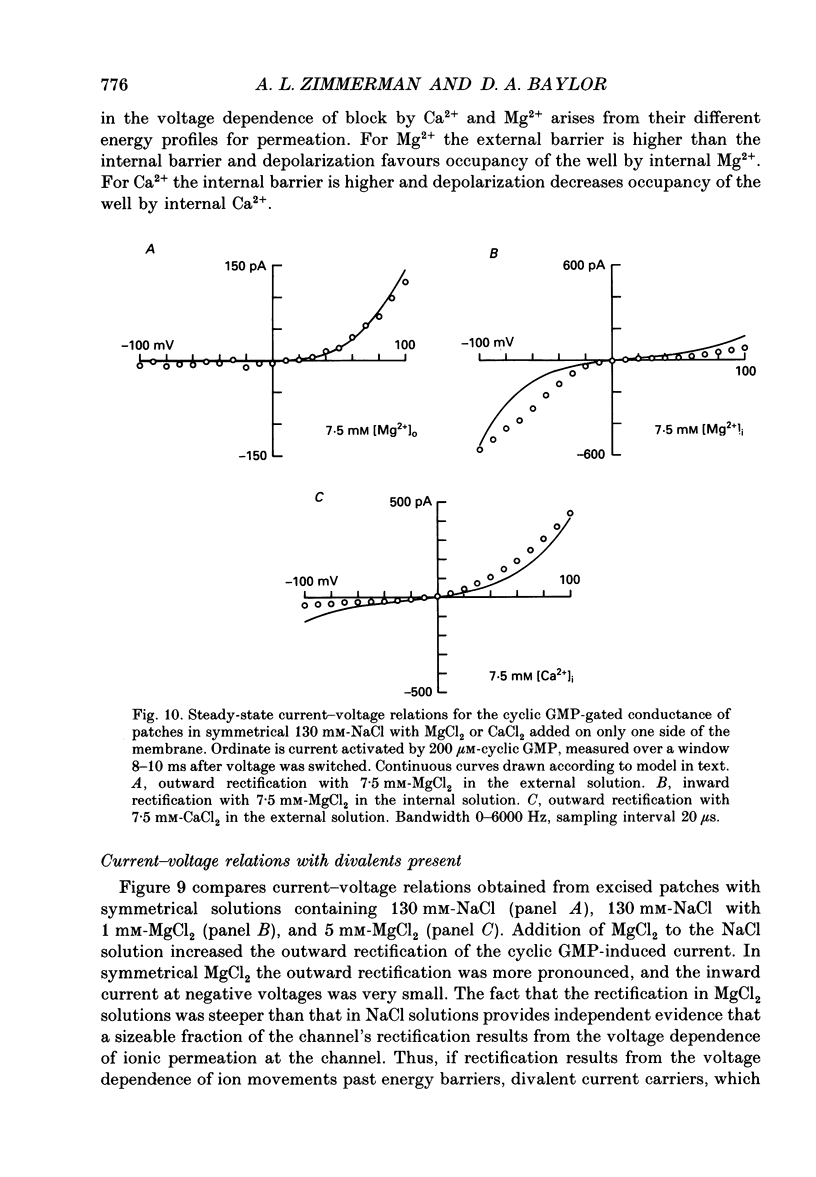
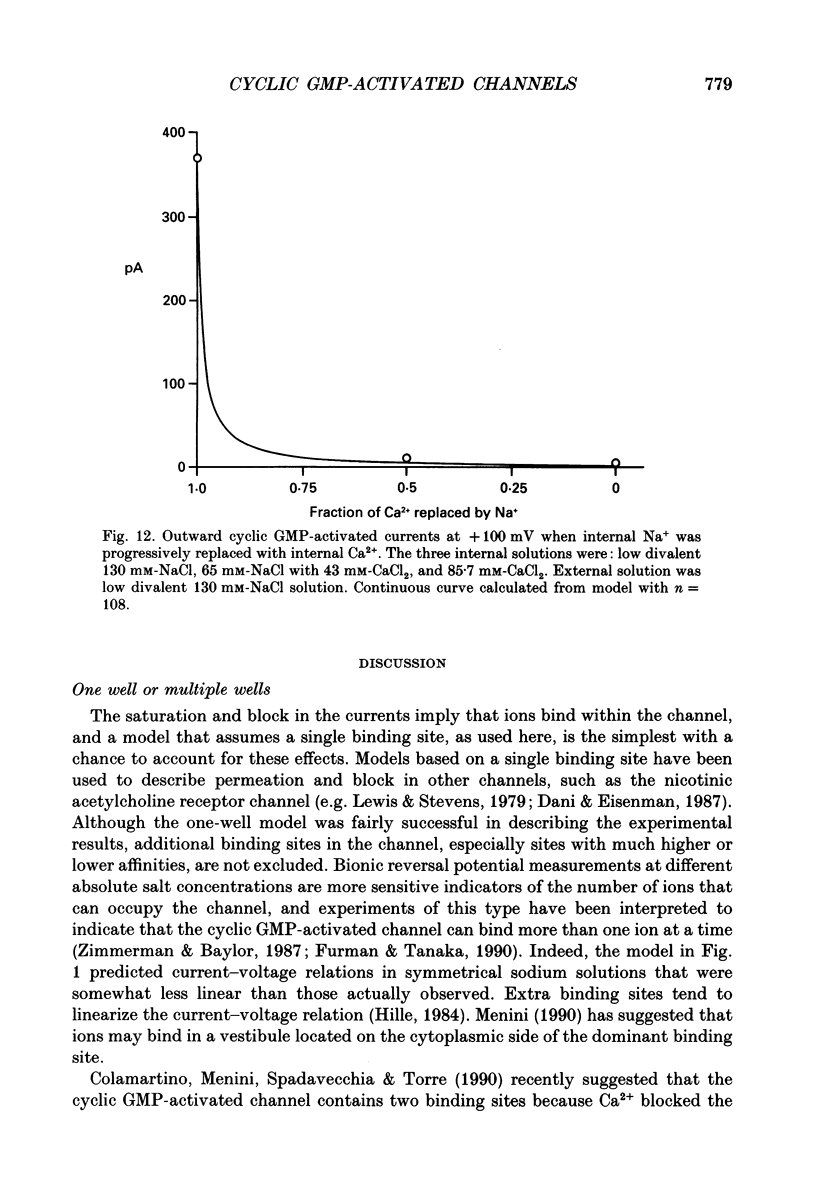
- Almers W., McCleskey E. W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984 Aug;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
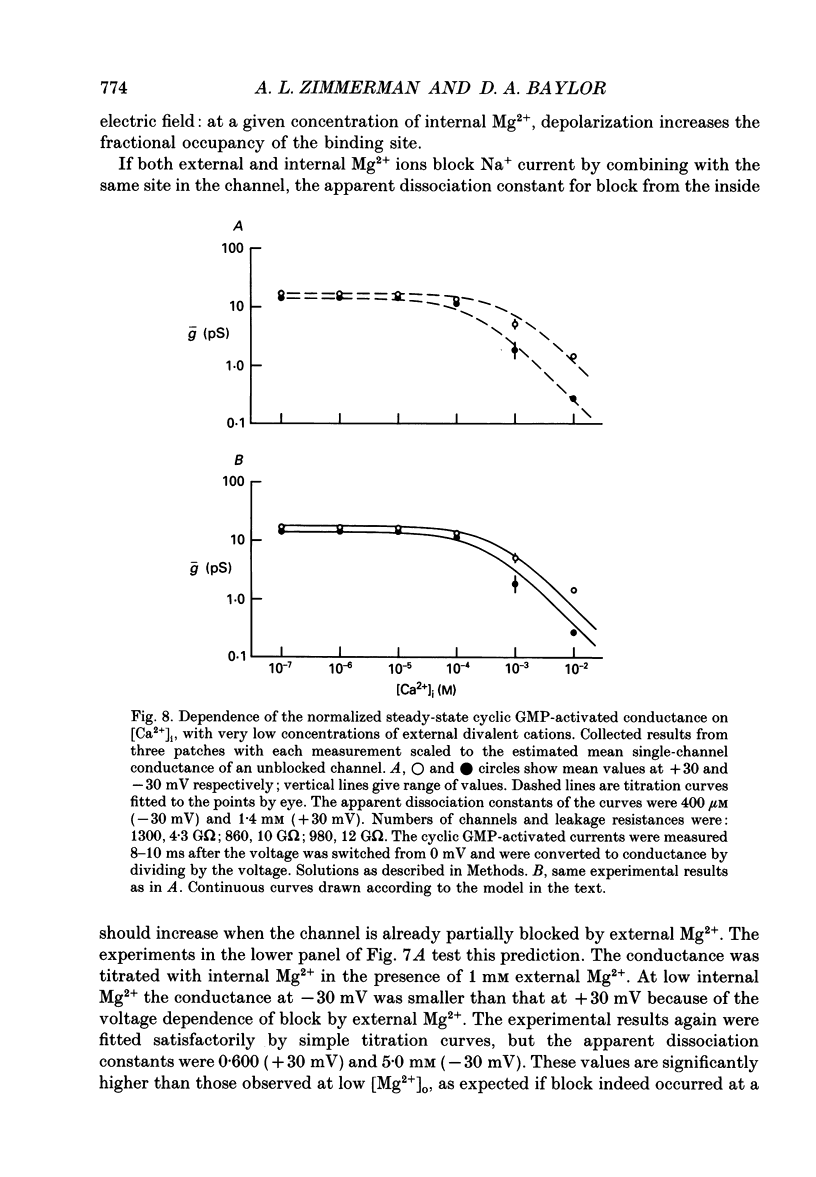
- Almers W., McCleskey E. W., Palade P. T. A non-selective cation conductance in frog muscle membrane blocked by micromolar external calcium ions. J Physiol. 1984 Aug;353:565–583. doi: 10.1113/jphysiol.1984.sp015351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfai T. Preparation of metal-chelate complexes and the design of steady-state kinetic experiments involving metal nucleotide complexes. Adv Cyclic Nucleotide Res. 1979;10:219–242. [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J. Electrical properties of the light-sensitive conductance of rods of the salamander Ambystoma tigrinum. J Physiol. 1986 Feb;371:115–145. doi: 10.1113/jphysiol.1986.sp015964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodoia R. D., Detwiler P. B. Patch-clamp recordings of the light-sensitive dark noise in retinal rods from the lizard and frog. J Physiol. 1985 Oct;367:183–216. doi: 10.1113/jphysiol.1985.sp015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Menini A., Rispoli G., Torre V. The modulation of the ionic selectivity of the light-sensitive current in isolated rods of the tiger salamander. J Physiol. 1988 Dec;406:181–198. doi: 10.1113/jphysiol.1988.sp017375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani J. A., Eisenman G. Monovalent and divalent cation permeation in acetylcholine receptor channels. Ion transport related to structure. J Gen Physiol. 1987 Jun;89(6):959–983. doi: 10.1085/jgp.89.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Direct action of cGMP on the conductance of retinal rod plasma membrane. Biochim Biophys Acta. 1986 Apr 25;856(3):661–671. doi: 10.1016/0005-2736(86)90162-8. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Furman R. E., Tanaka J. C. Monovalent selectivity of the cyclic guanosine monophosphate-activated ion channel. J Gen Physiol. 1990 Jul;96(1):57–82. doi: 10.1085/jgp.96.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity, saturation, and block in sodium channels. A four-barrier model. J Gen Physiol. 1975 Nov;66(5):535–560. doi: 10.1085/jgp.66.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen J. W., Zimmerman A. L., Stryer L., Baylor D. A. Gating kinetics of the cyclic-GMP-activated channel of retinal rods: flash photolysis and voltage-jump studies. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1287–1291. doi: 10.1073/pnas.85.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen J. W., Zimmerman A. L., Stryer L., Baylor D. A. Molecular mechanics of the cyclic-GMP-activated channel of retinal rods. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):325–332. doi: 10.1101/sqb.1988.053.01.039. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Niidome T., Tanabe T., Terada S., Bönigk W., Stühmer W., Cook N. J., Kangawa K., Matsuo H., Hirose T. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989 Dec 14;342(6251):762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Matthews G. Single-channel recordings demonstrate that cGMP opens the light-sensitive ion channel of the rod photoreceptor. Proc Natl Acad Sci U S A. 1987 Jan;84(1):299–302. doi: 10.1073/pnas.84.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menini A. Currents carried by monovalent cations through cyclic GMP-activated channels in excised patches from salamander rods. J Physiol. 1990 May;424:167–185. doi: 10.1113/jphysiol.1990.sp018061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and magnesium fluxes across the plasma membrane of the toad rod outer segment. J Physiol. 1988 Jan;395:695–729. doi: 10.1113/jphysiol.1988.sp016942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Karpen J. W., Baylor D. A. Hindered diffusion in excised membrane patches from retinal rod outer segments. Biophys J. 1988 Aug;54(2):351–355. doi: 10.1016/S0006-3495(88)82966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


