Abstract
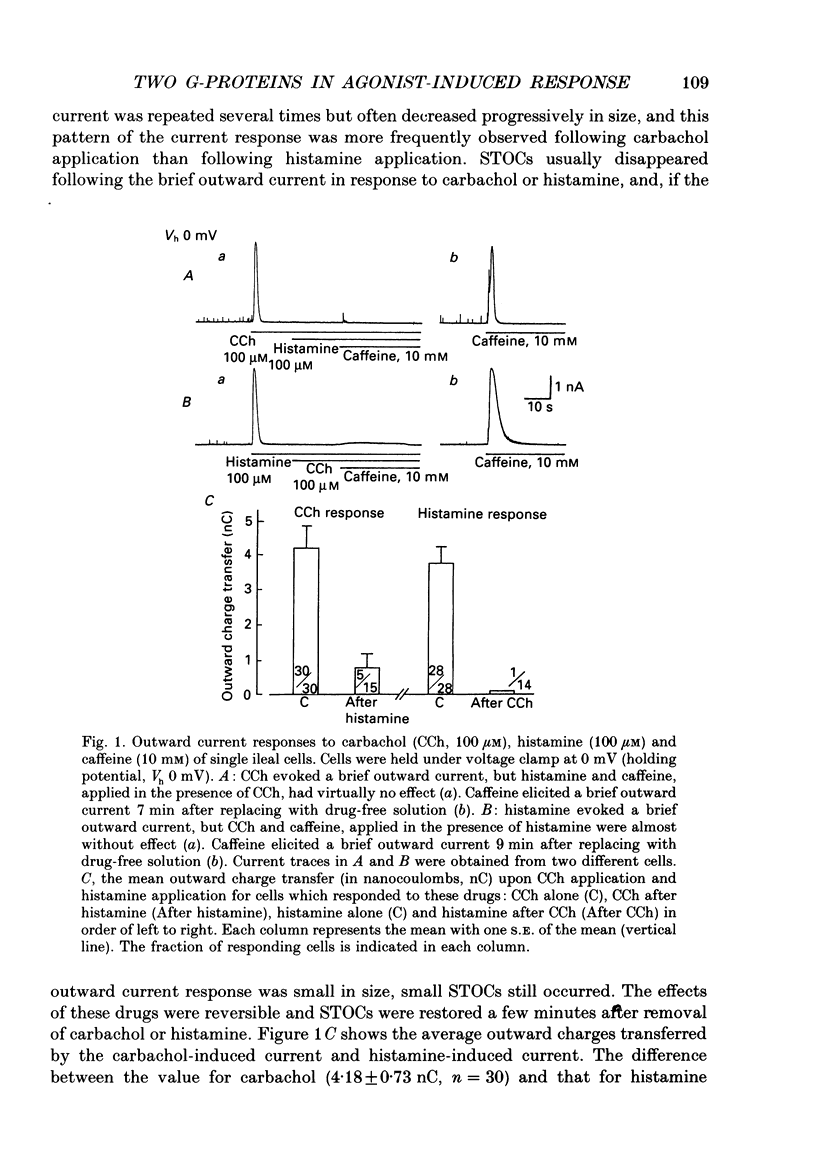
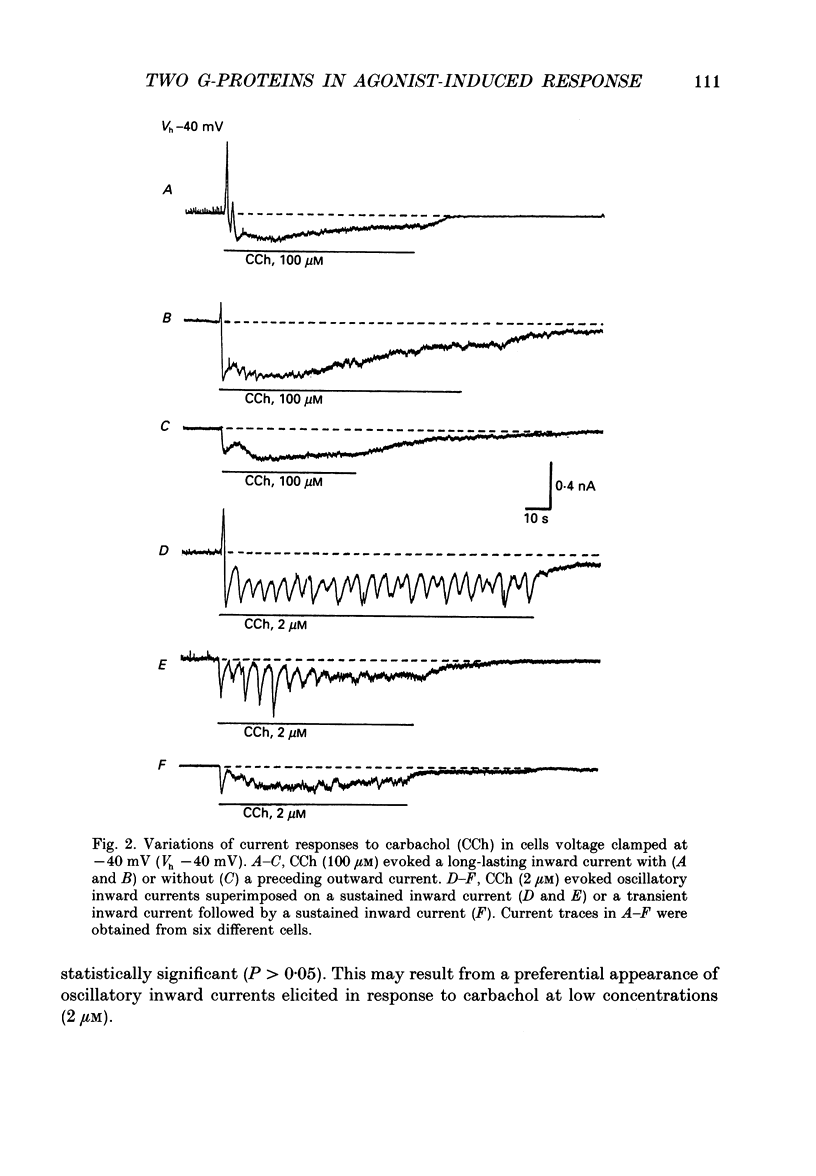
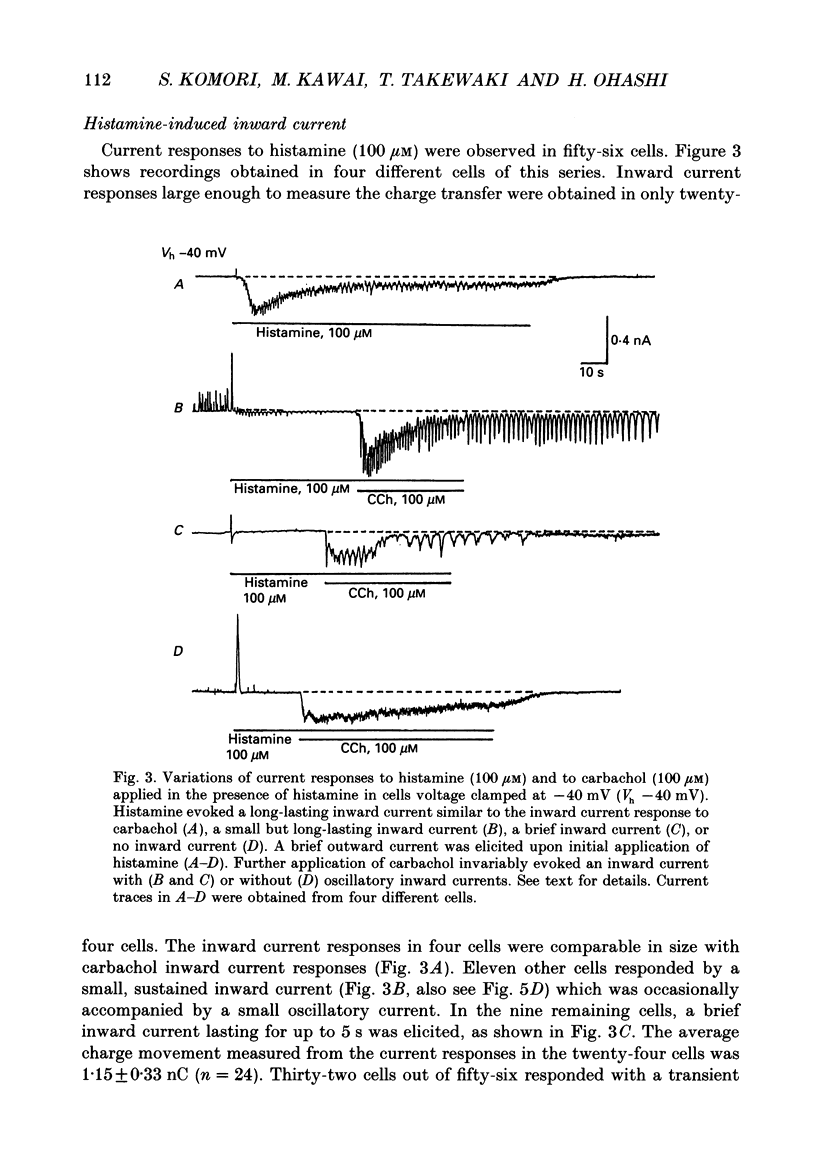
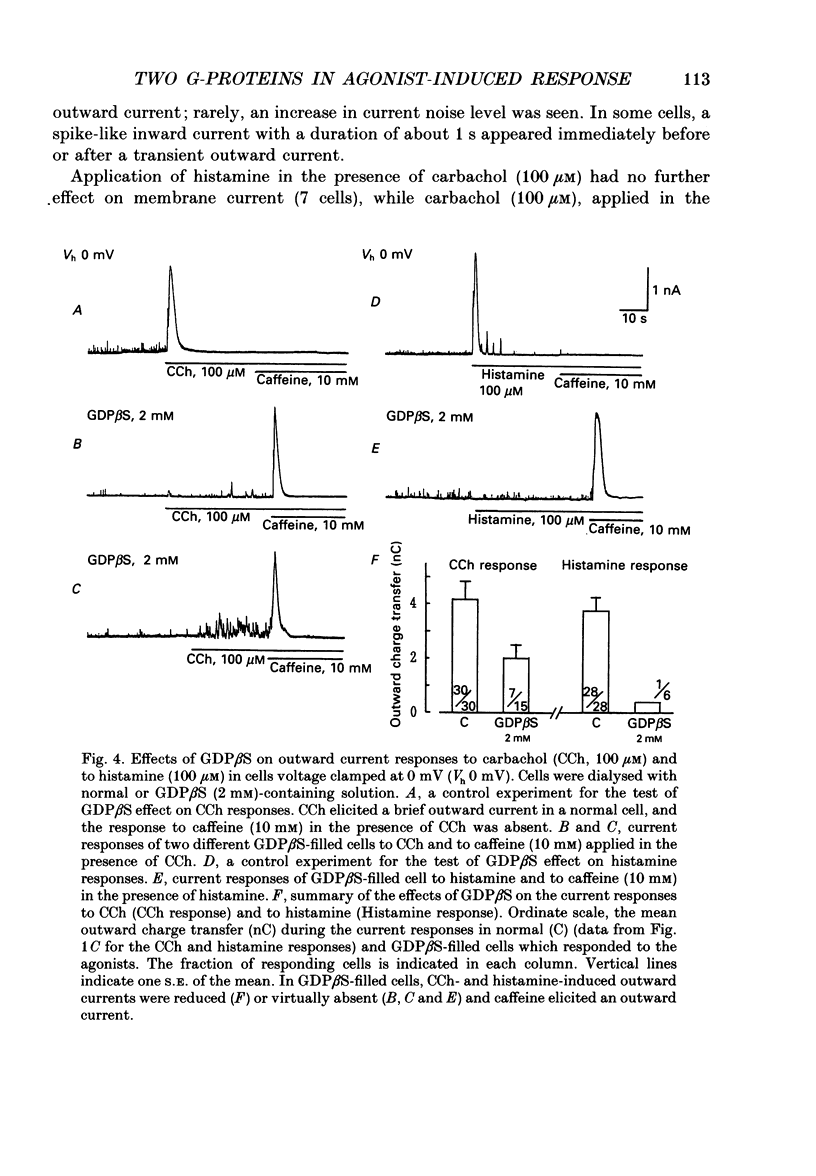
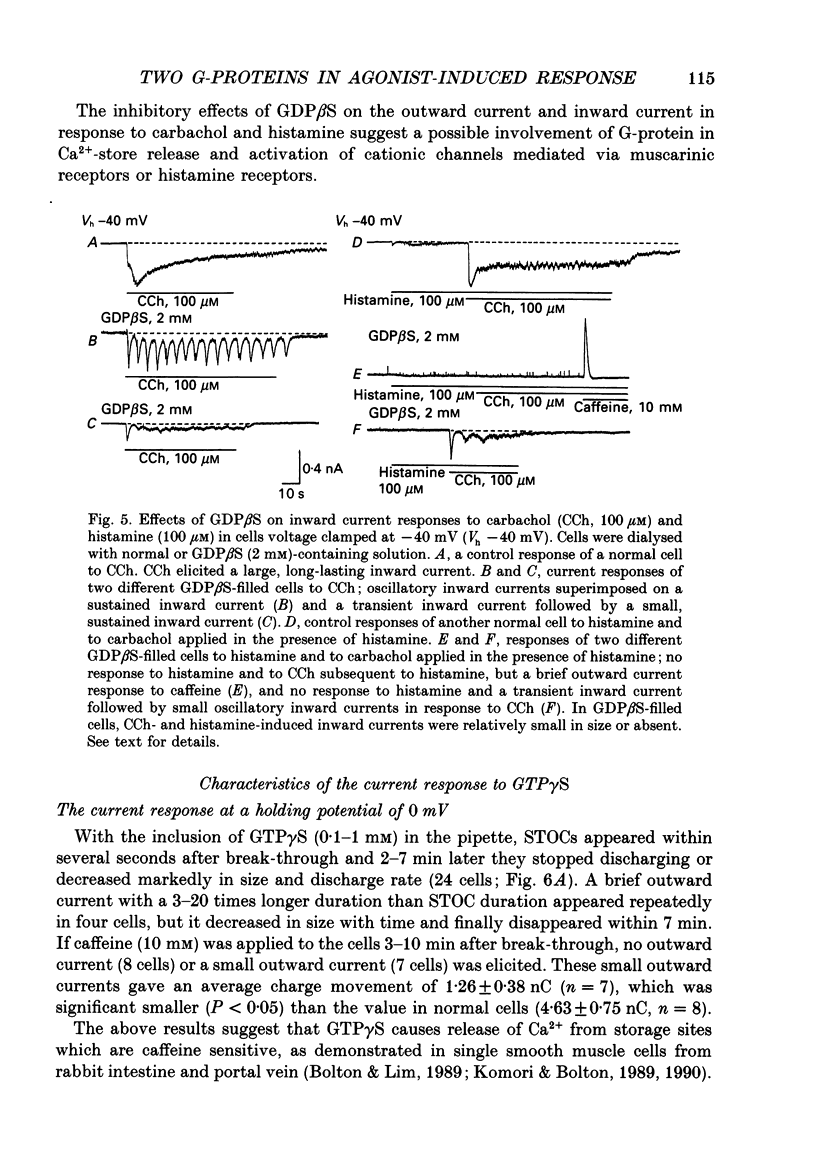
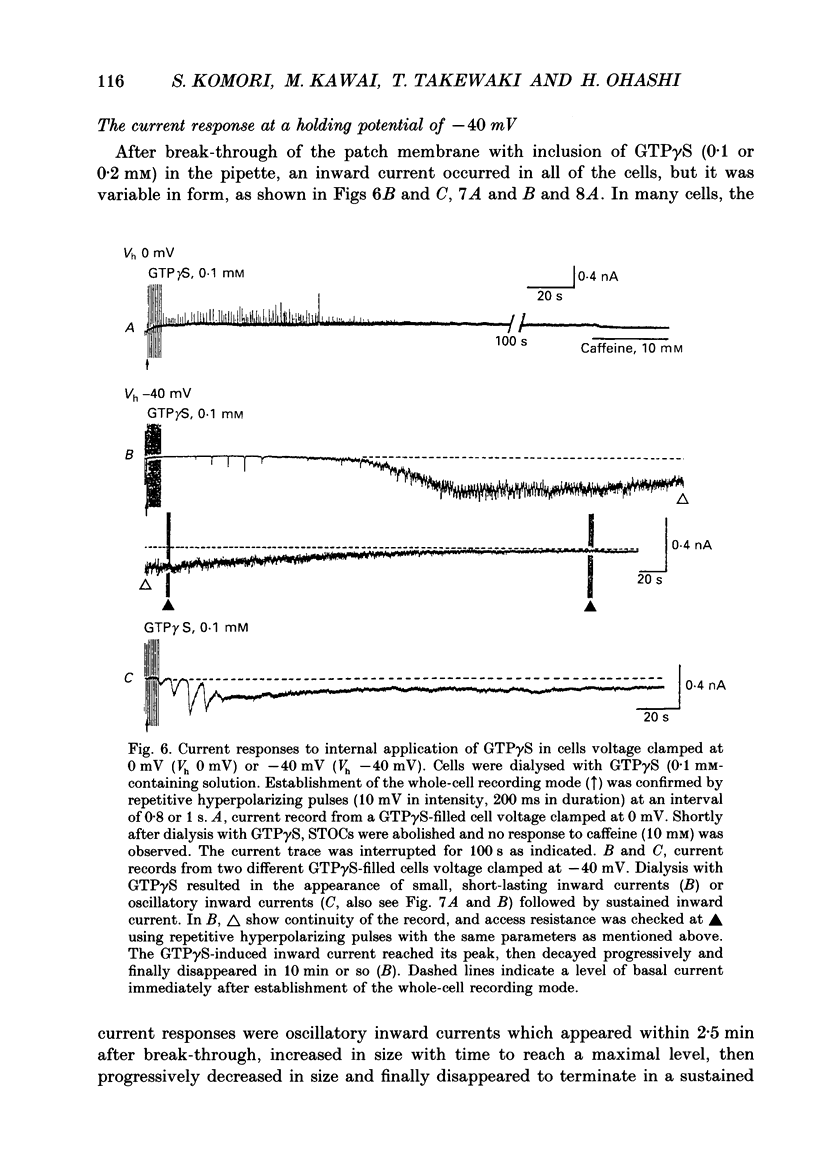
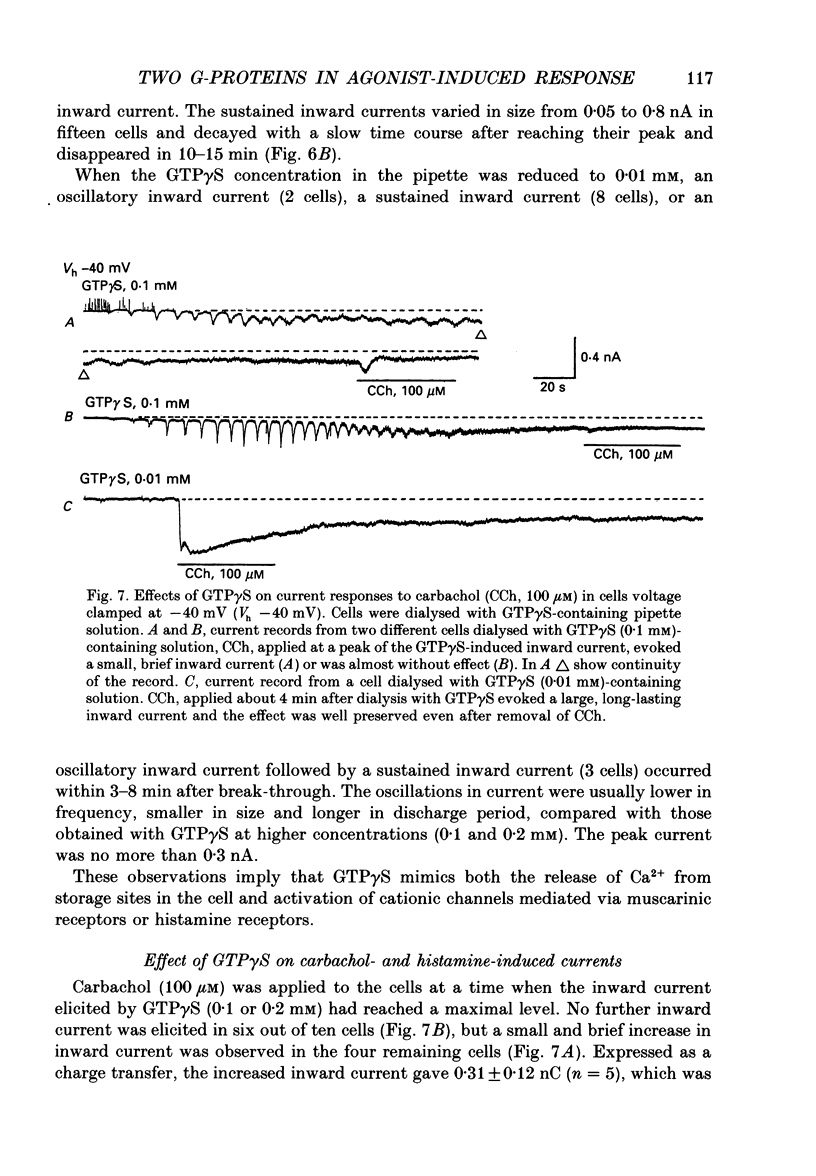
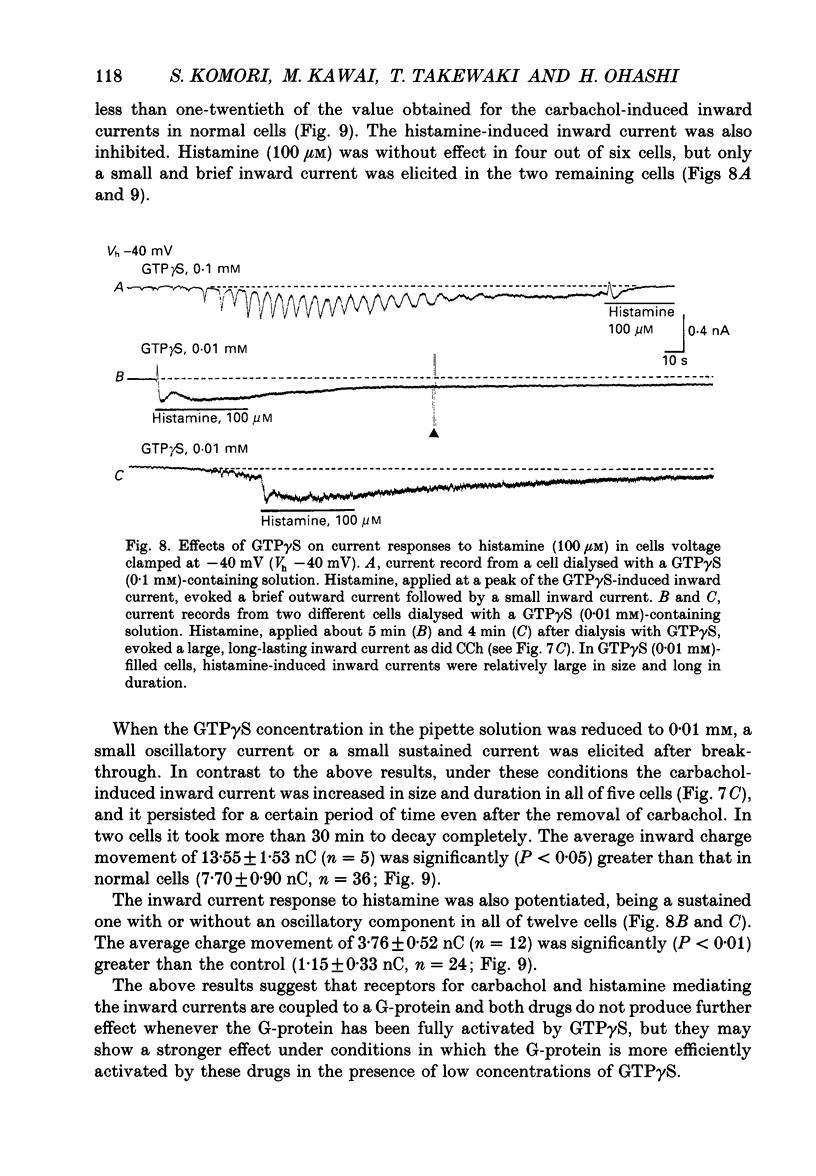
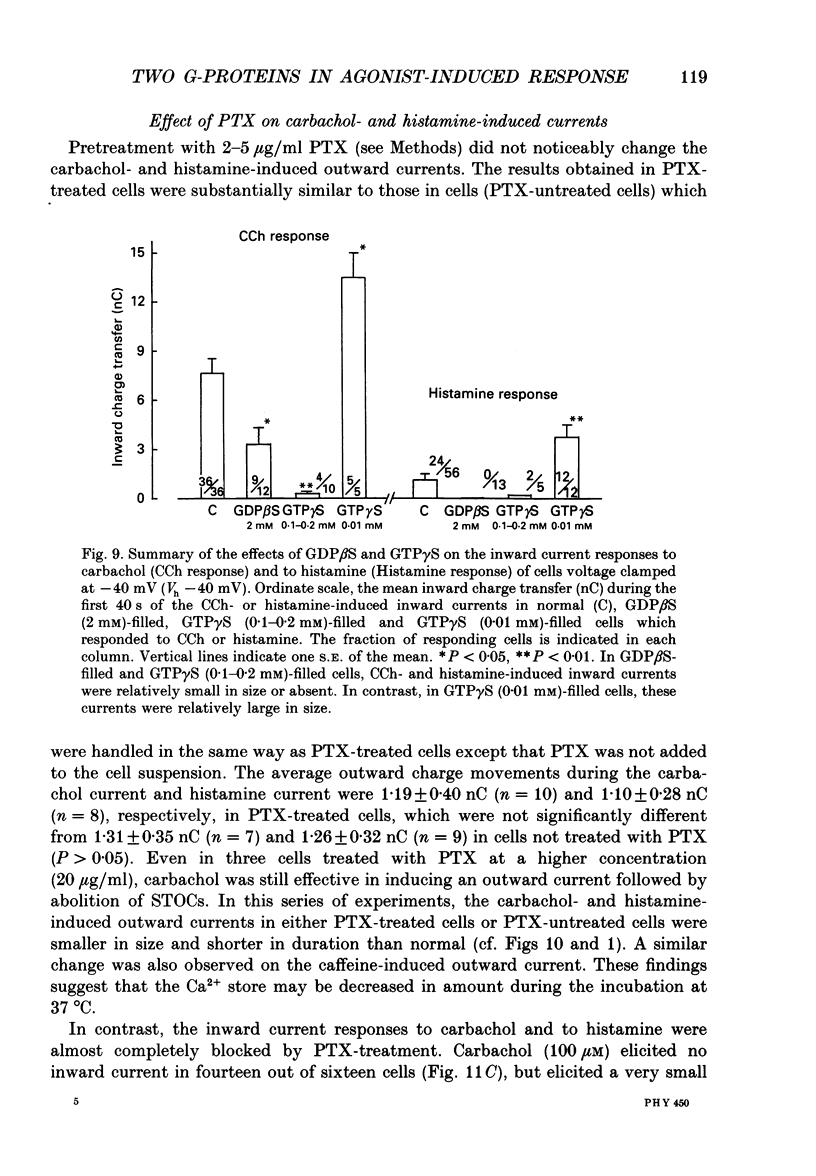
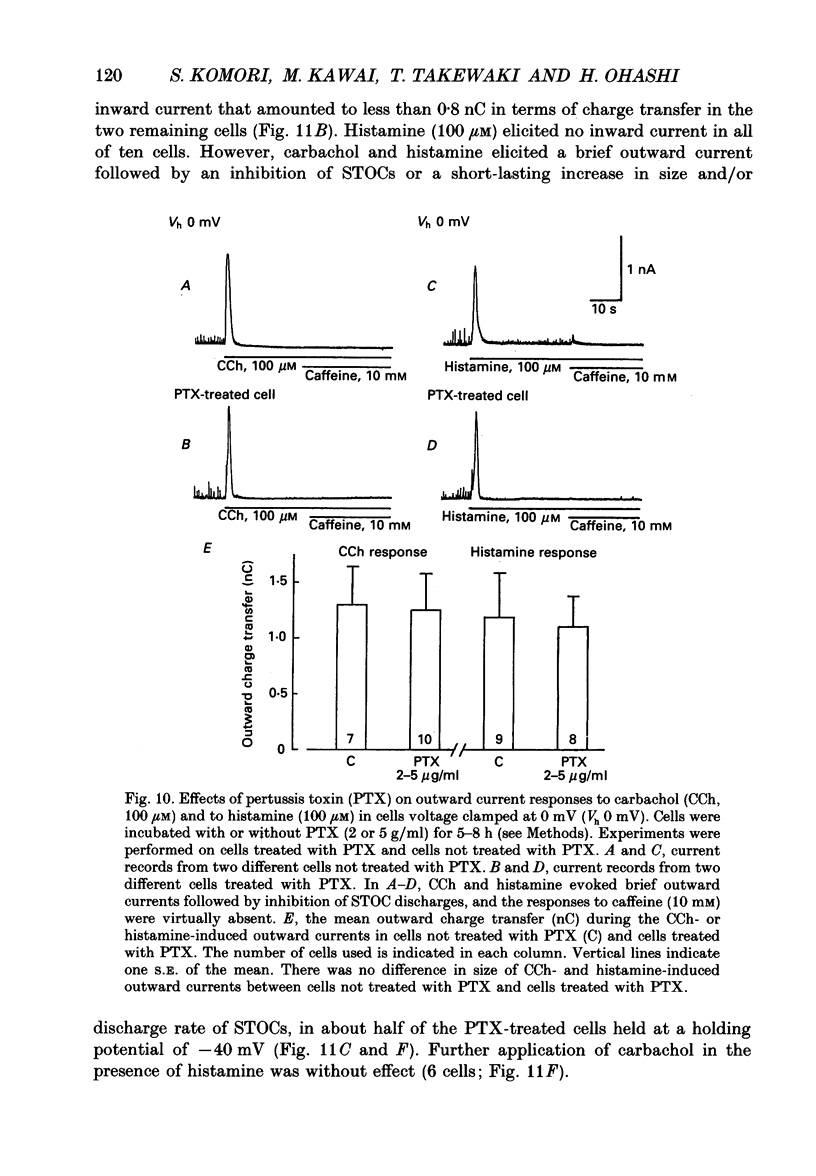
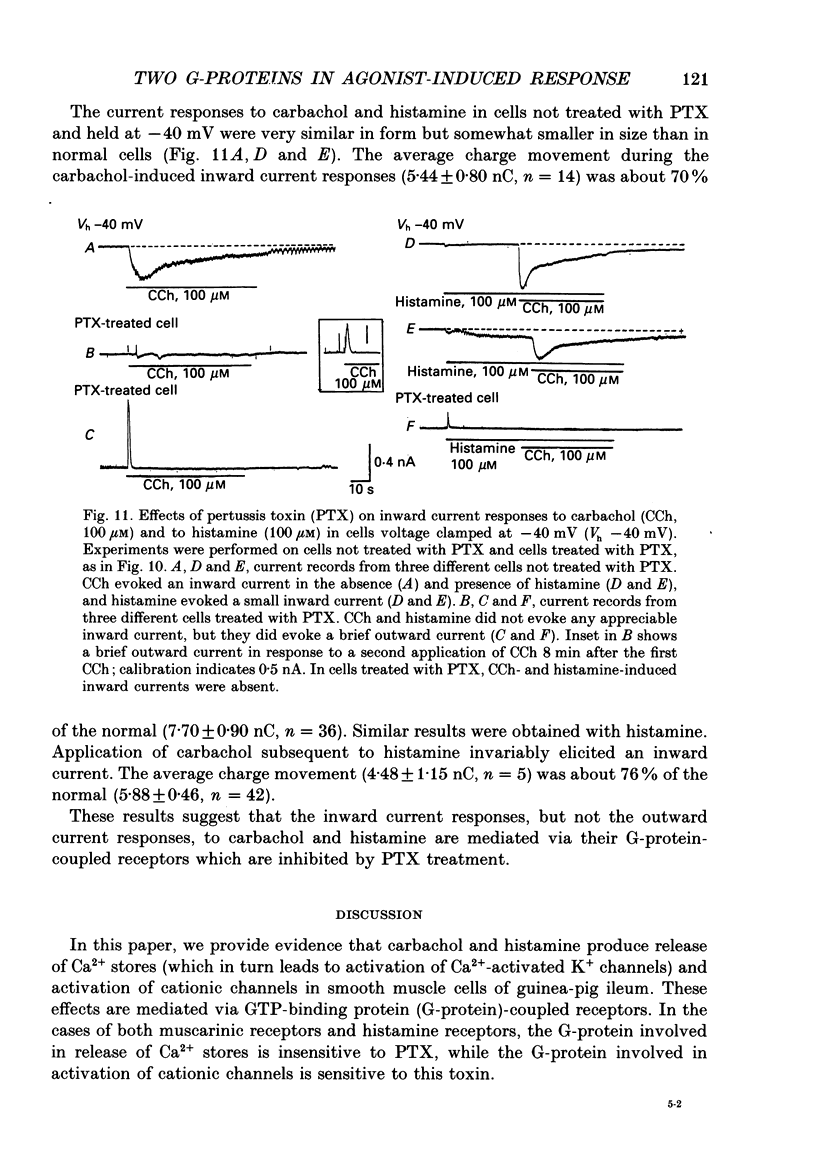
1. Single smooth muscle cells obtained by enzymic dispersion of the longitudinal muscle layer of guinea-pig ileum were used for recording membrane currents under whole-cell voltage clamp in response to carbachol (100 microM, unless otherwise stated) or histamine (100 microM) applied extracellularly. 2. At a holding potential of 0 mV, a transient outward current was evoked by carbachol and histamine. Responses to the two agonists were very similar in size and time course to the current response to caffeine (10 mM). The response to carbachol was virtually absent in the presence of histamine, and vice versa. Caffeine was without effect in the presence of either of these agonists. Inclusion of EGTA (10 or 20 mM) in the pipette abolished the responses to carbachol, histamine and caffeine. Thus, the outward current responses were considered to represent opening of Ca(2+)-activated K+ channels in response to a massive release of Ca2+ from the same stores by these three agents. 3. An inward current was evoked by carbachol and histamine, but not by caffeine at a holding potential of -40 mV, which was considered to represent opening of cationic channels. The carbachol-induced inward current was much longer in duration and larger in size than the histamine-induced inward current. 4. Inclusion of GDP beta S (2 mM) in the pipette abolished the inward and outward current responses to histamine, but inhibited only part of those to carbachol. 5. When the holding potential was held at 0 mV with inclusion of GTP gamma S (0.1-1 mM) in the pipette, spontaneous transient outward currents appeared immediately after break-through but disappeared a few minutes later. Under these conditions, caffeine (10 mM) was almost without effect, suggesting that GTP gamma S had released Ca2+ stores. When the holding potential was held at -40 mV and GTP gamma S (0.1 or 0.2 mM) was present in the pipette, an inward current developed a few minutes after break-through. During the GTP gamma S-induced inward current, application of carbachol or histamine produced no further inward current. However, when 0.01 mM-GTP gamma S was included in the pipette solution, carbachol- and histamine-induced inward currents were potentiated. 6. Pretreated with 2-5 micrograms/ml pertussis toxin (PTX) did not change noticeably the outward current responses to carbachol and histamine, but abolished or markedly reduced the inward current responses.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Bolton T. B., Lang R. J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985 Jul 25;316(6026):345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielkiewicz-Vollrath B., Carpenter J. R., Schulz R., Cook D. A. Early production of 1,4,5-inositol trisphosphate and 1,3,4,5-inositol tetrakisphosphate by histamine and carbachol in ileal smooth muscle. Mol Pharmacol. 1987 May;31(5):513–522. [PubMed] [Google Scholar]
- Bolton T. B., Clark J. P., Kitamura K., Lang R. J. Evidence that histamine and carbachol may open the same ion channels in longitudinal smooth muscle of guinea-pig ileum. J Physiol. 1981 Nov;320:363–379. doi: 10.1113/jphysiol.1981.sp013955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Lim S. P. Properties of calcium stores and transient outward currents in single smooth muscle cells of rabbit intestine. J Physiol. 1989 Feb;409:385–401. doi: 10.1113/jphysiol.1989.sp017504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J., Hill S. J. Histamine-induced inositol phospholipid breakdown in the longitudinal smooth muscle of guinea-pig ileum. Br J Pharmacol. 1985 Jun;85(2):499–512. doi: 10.1111/j.1476-5381.1985.tb08887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hill S. J., Young J. M. Characterization of [3H]mepyramine binding to the longitudinal muscle of guinea pig small intestine. Mol Pharmacol. 1981 May;19(3):379–387. [PubMed] [Google Scholar]
- Hill S. J., Young J. M., Marrian D. H. Specific binding of 3H-mepyramine to histamine H1 receptors in intestinal smooth muscle. Nature. 1977 Nov 24;270(5635):361–363. doi: 10.1038/270361a0. [DOI] [PubMed] [Google Scholar]
- Inoue R., Isenberg G. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. J Physiol. 1990 May;424:57–71. doi: 10.1113/jphysiol.1990.sp018055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Isenberg G. Intracellular calcium ions modulate acetylcholine-induced inward current in guinea-pig ileum. J Physiol. 1990 May;424:73–92. doi: 10.1113/jphysiol.1990.sp018056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Acetylcholine activates single sodium channels in smooth muscle cells. Pflugers Arch. 1987 Sep;410(1-2):69–74. doi: 10.1007/BF00581898. [DOI] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Actions of guanine nucleotides and cyclic nucleotides on calcium stores in single patch-clamped smooth muscle cells from rabbit portal vein. Br J Pharmacol. 1989 Jul;97(3):973–982. doi: 10.1111/j.1476-5381.1989.tb12039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Calcium release induced by inositol 1,4,5-trisphosphate in single rabbit intestinal smooth muscle cells. J Physiol. 1991 Feb;433:495–517. doi: 10.1113/jphysiol.1991.sp018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Role of G-proteins in muscarinic receptor inward and outward currents in rabbit jejunal smooth muscle. J Physiol. 1990 Aug;427:395–419. doi: 10.1113/jphysiol.1990.sp018178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Short-term desensitization of muscarinic K+ channel current in isolated atrial myocytes and possible role of GTP-binding proteins. Pflugers Arch. 1987 Oct;410(3):227–233. doi: 10.1007/BF00580270. [DOI] [PubMed] [Google Scholar]
- Leurs R., Brozius M. M., Smit M. J., Bast A., Timmerman H. Effects of histamine H1-, H2- and H3-receptor selective drugs on the mechanical activity of guinea-pig small and large intestine. Br J Pharmacol. 1991 Jan;102(1):179–185. doi: 10.1111/j.1476-5381.1991.tb12150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. P., Bolton T. B. A calcium-dependent rather than a G-protein mechanism is involved in the inward current evoked by muscarinic receptor stimulation in dialysed single smooth muscle cells of small intestine. Br J Pharmacol. 1988 Oct;95(2):325–327. doi: 10.1111/j.1476-5381.1988.tb11649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loirand G., Pacaud P., Mironneau C., Mironneau J. GTP-binding proteins mediate noradrenaline effects on calcium and chloride currents in rat portal vein myocytes. J Physiol. 1990 Sep;428:517–529. doi: 10.1113/jphysiol.1990.sp018225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallows R. S., Bolton T. B. Relationship between stimulated phosphatidic acid production and inositol lipid hydrolysis in intestinal longitudinal smooth muscle from guinea pig. Biochem J. 1987 Jun 15;244(3):763–768. doi: 10.1042/bj2440763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel A. D., Whiting R. L. The binding of [3H]4-diphenylacetoxy-N-methylpiperidine methiodide to longitudinal ileal smooth muscle muscarinic receptors. Eur J Pharmacol. 1990 Feb 6;176(2):197–205. doi: 10.1016/0014-2999(90)90528-e. [DOI] [PubMed] [Google Scholar]
- Mihara S., North R. A., Surprenant A. Somatostatin increases an inwardly rectifying potassium conductance in guinea-pig submucous plexus neurones. J Physiol. 1987 Sep;390:335–355. doi: 10.1113/jphysiol.1987.sp016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Oashi H., Takewaki T., Okada T. Calcium and the contractile effect of carbachol in the depolarized guinea-pig taenia caecum. Jpn J Pharmacol. 1974 Aug;24(4):601–611. doi: 10.1254/jjp.24.601. [DOI] [PubMed] [Google Scholar]
- Prestwich S. A., Bolton T. B. Measurement of picomole amounts of any inositol phosphate isomer separable by h.p.l.c. by means of a bioluminescence assay. Biochem J. 1991 Mar 15;274(Pt 3):663–672. doi: 10.1042/bj2740663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaguri T., Hirata M., Itoh T., Koga T., Kuriyama H. Guanine nucleotide binding protein involved in muscarinic responses in the pig coronary artery is insensitive to islet-activating protein. Biochem J. 1986 Nov 1;239(3):567–574. doi: 10.1042/bj2390567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]


