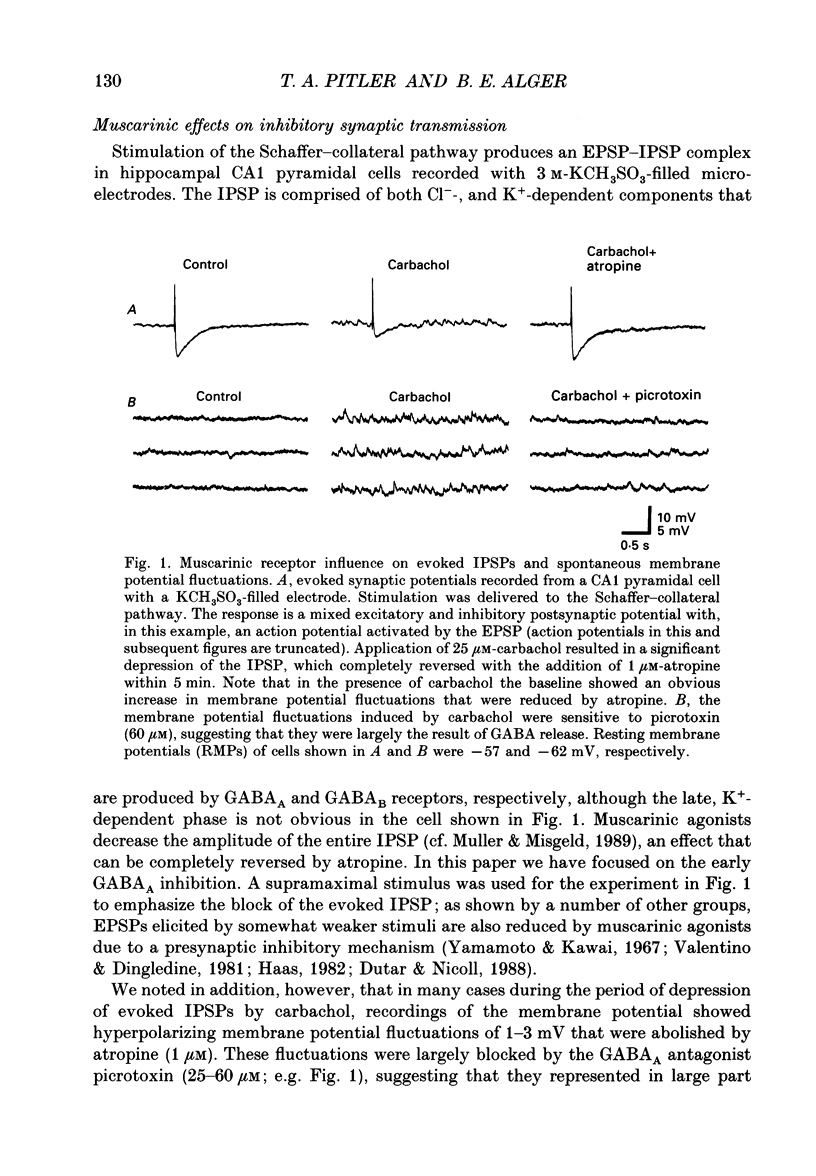
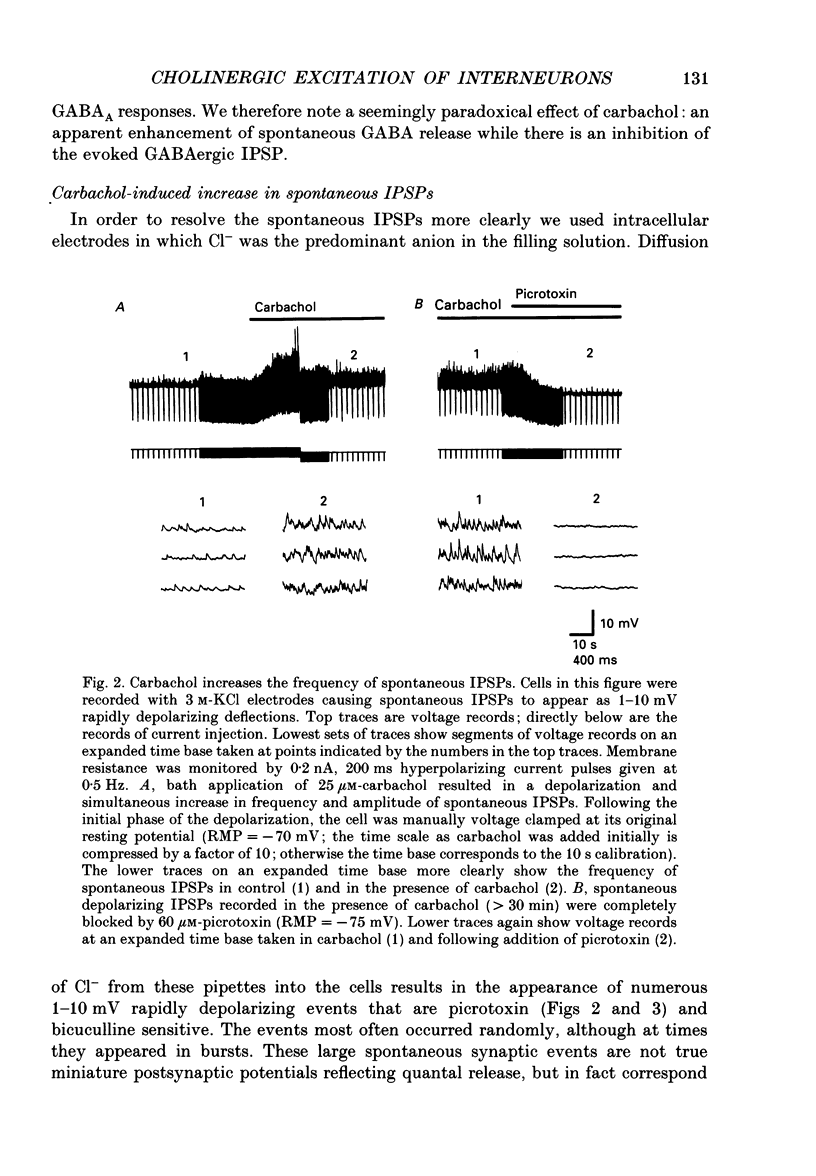
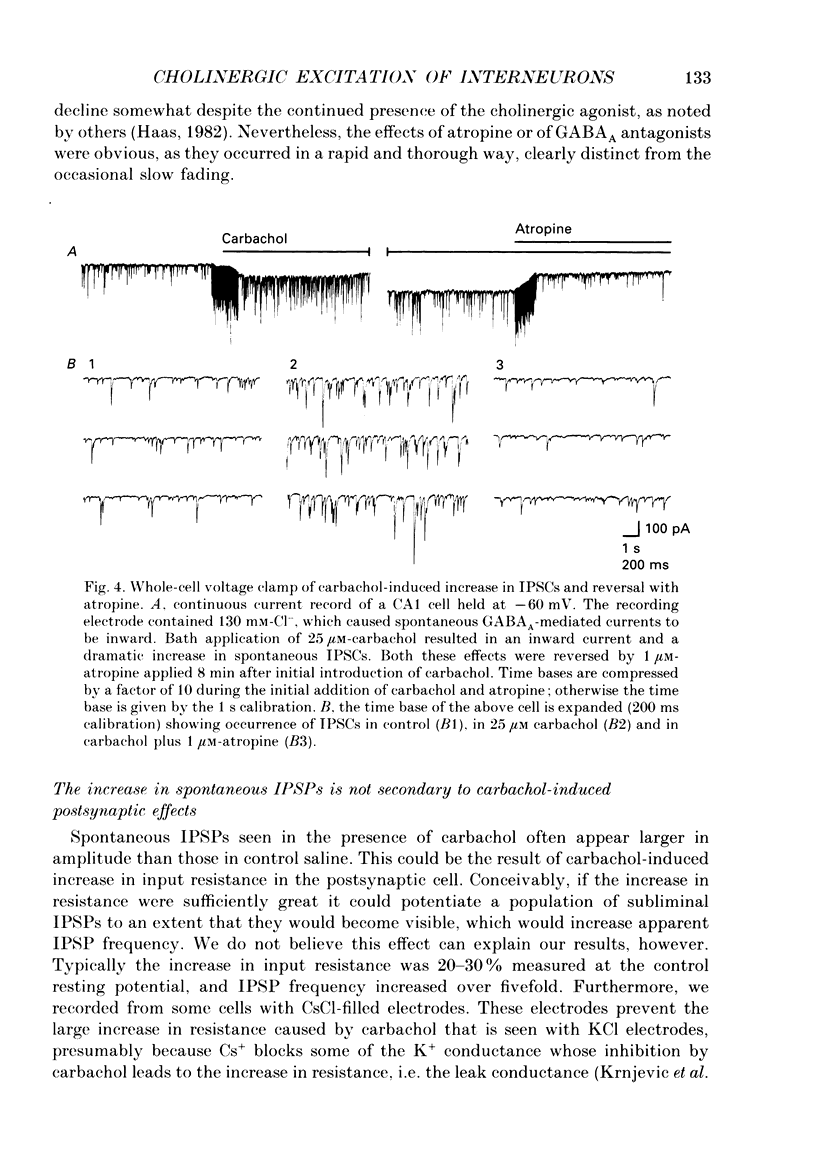
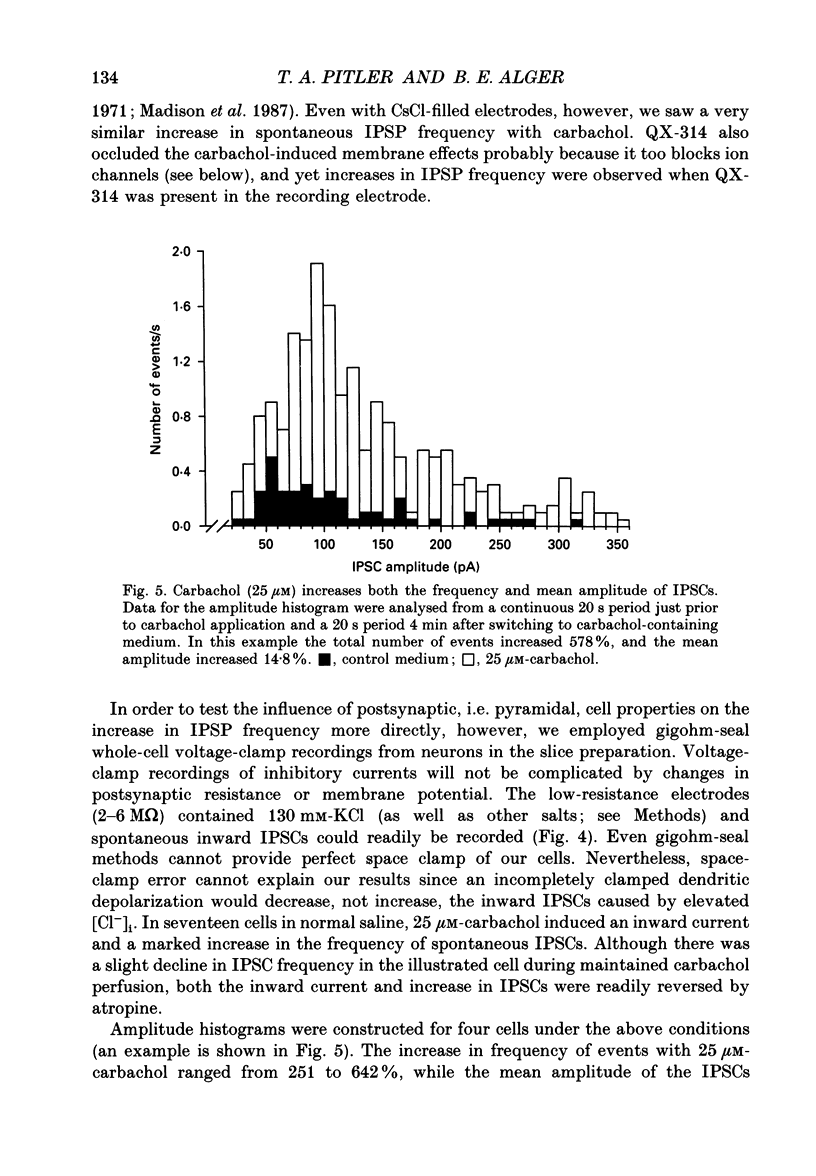
Abstract
1. Intracellular recordings were made from CA1 pyramidal cells in the rat hippocampal slice to study the cholinergic modulation of GABAergic inhibition. The cholinergic receptor agonist, carbamylcholine (carbachol), depressed evoked excitatory postsynaptic potentials (EPSPs) and evoked inhibitory postsynaptic potentials (IPSPs), but enhanced small spontaneously occurring membrane potential fluctuations that resembled IPSPs. Both atropine (1 microM) and picrotoxin (25-60 microM) abolished the small fluctuations. 2. Recording from cells with potassium or caesium chloride (KCl or CsCl)-filled microelectrodes enhanced and inverted spontaneous Cl(-)-dependent GABAA-mediated IPSPs. These events appeared to result from the spontaneous firing of GABAergic interneurons since they could be inhibited by picrotoxin or bicuculline and nearly eliminated by tetrodotoxin. 3. Muscarinic acetylcholine (ACh) receptor activation significantly increased the frequency of spontaneous-activity-dependent IPSPs from 1.7 +/- 0.4 s (mean +/- S.E.M.) in control saline to 7.0 +/- 1.1 s in carbachol (10-50 microM)-containing saline, although evoked IPSPs were inhibited. All effects of carbachol were completely reversed by atropine. 4. The increase in frequency of spontaneous IPSPs observed in carbachol was not secondary to changes in the postsynaptic cell and was not blocked by high doses of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 5-10 microM) and 2-amino-5-phosphonovaleric acid (APV, 10-20 microM), which abolished evoked excitatory transmission. Amplitude histograms showed an increase in mean size as well as of frequency of spontaneous IPSCs in carbachol. 5. Stimulation of cholinergic afferents in stratum oriens in the presence of the acetylcholinesterase inhibitor eserine (1 microM) also increased spontaneous IPSP frequency, and the time course of this response was similar to that of the muscarinic slow EPSP. Postsynaptic factors or the activation of glutamatergic excitatory pathways could not account for this effect. 6. Evoked monosynaptic IPSCs in CNQX and APV were diminished by carbachol. 7. We conclude that GABAergic inhibitory interneurons possess muscarinic receptors, that activation of these receptors increases the excitability of the interneurons and that synaptically released ACh increases interneuronal activity. Cholinergic reduction of the monosynaptic IPSC may point to additional complexity in cholinergic regulation of the GABA system.
Full text
PDF




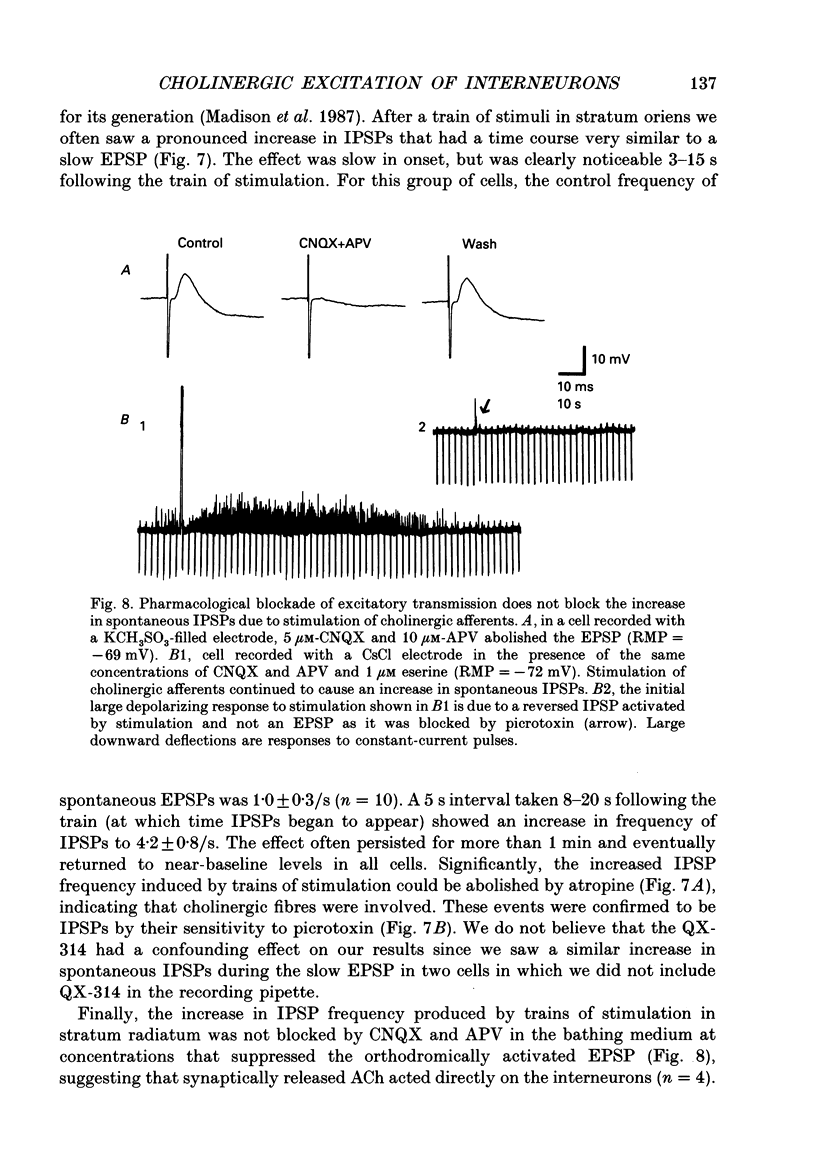



Selected References
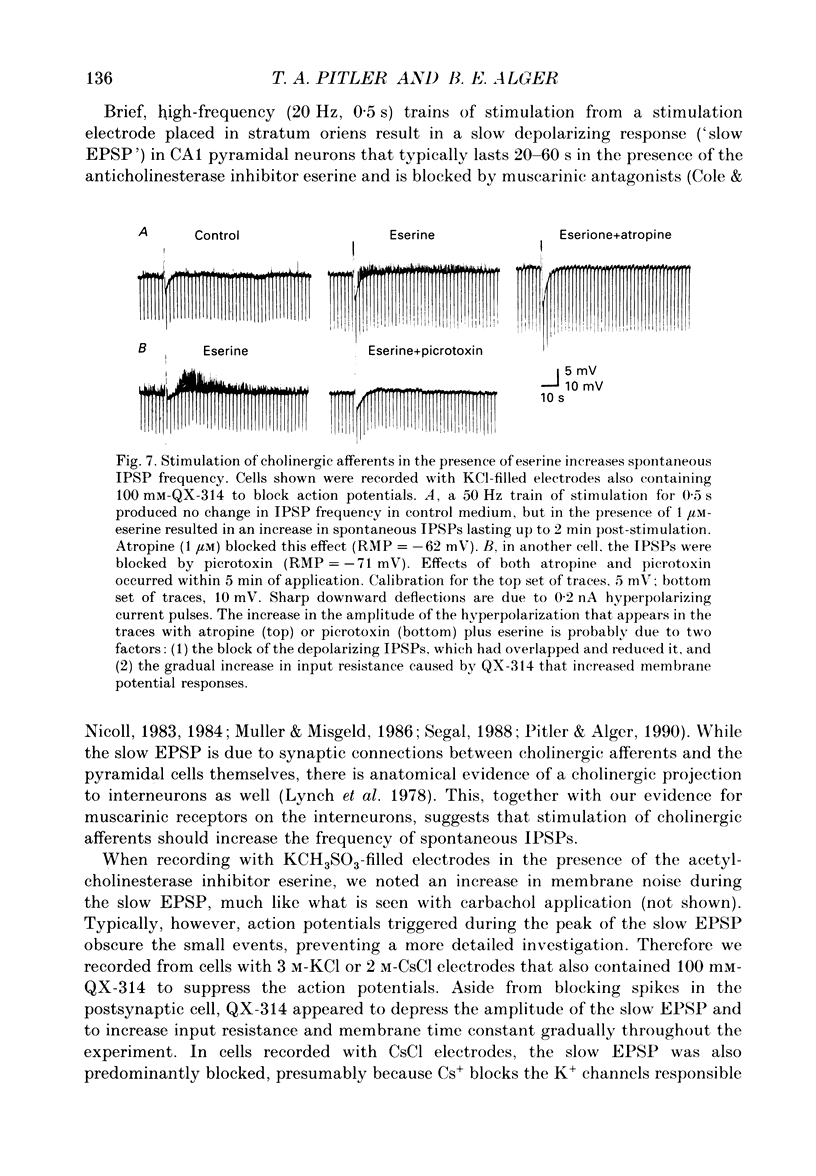
These references are in PubMed. This may not be the complete list of references from this article.
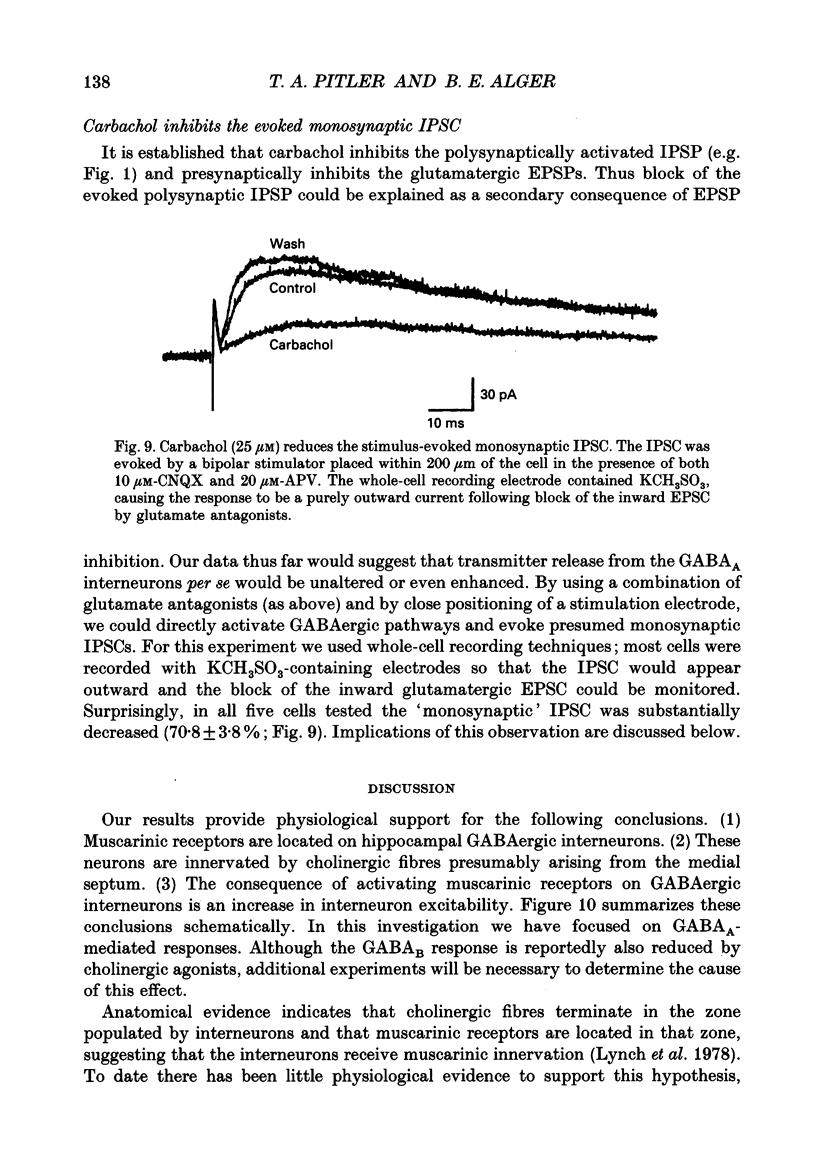
- Alger B. E., Nicoll R. A. Spontaneous inhibitory post-synaptic potentials in hippocampus: mechanism for tonic inhibition. Brain Res. 1980 Oct 27;200(1):195–200. doi: 10.1016/0006-8993(80)91108-7. [DOI] [PubMed] [Google Scholar]
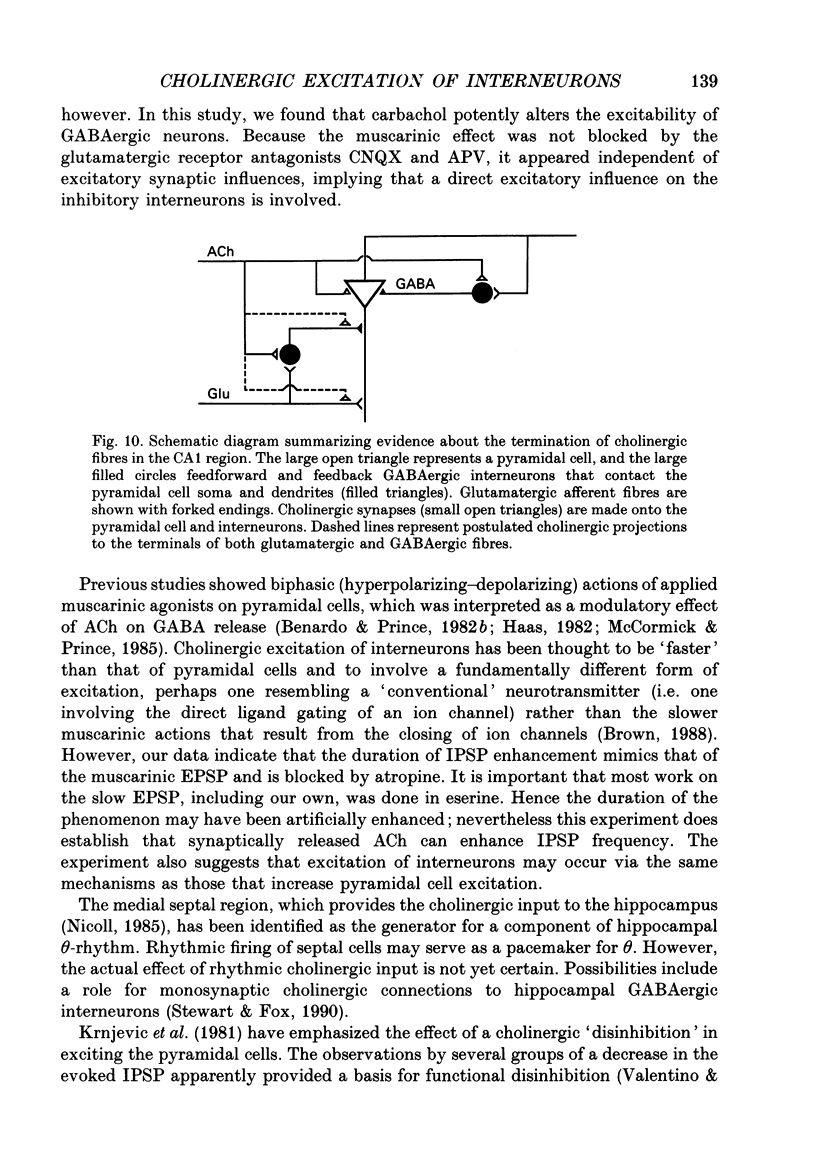
- Alger B. E., Pitler T. A., Williamson A. A prolonged post-tetanic hyperpolarization in rat hippocampal pyramidal cells in vitro. Brain Res. 1990 Jun 25;521(1-2):118–124. doi: 10.1016/0006-8993(90)91531-k. [DOI] [PubMed] [Google Scholar]
- Benardo L. S., Prince D. A. Cholinergic excitation of mammalian hippocampal pyramidal cells. Brain Res. 1982 Oct 14;249(2):315–331. doi: 10.1016/0006-8993(82)90066-x. [DOI] [PubMed] [Google Scholar]
- Benardo L. S., Prince D. A. Ionic mechanisms of cholinergic excitation in mammalian hippocampal pyramidal cells. Brain Res. 1982 Oct 14;249(2):333–344. doi: 10.1016/0006-8993(82)90067-1. [DOI] [PubMed] [Google Scholar]
- Blanton M. G., Lo Turco J. J., Kriegstein A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989 Dec;30(3):203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Brown D. A. M currents. Ion Channels. 1988;1:55–94. doi: 10.1007/978-1-4615-7302-9_2. [DOI] [PubMed] [Google Scholar]
- Cole A. E., Nicoll R. A. Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells. Science. 1983 Sep 23;221(4617):1299–1301. doi: 10.1126/science.6612345. [DOI] [PubMed] [Google Scholar]
- Cole A. E., Nicoll R. A. Characterization of a slow cholinergic post-synaptic potential recorded in vitro from rat hippocampal pyramidal cells. J Physiol. 1984 Jul;352:173–188. doi: 10.1113/jphysiol.1984.sp015285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Lester R. A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989 Jun;41(2):143–210. [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. Muscarine affects calcium-currents in rat hippocampal pyramidal cells in vitro. Neurosci Lett. 1987 May 19;76(3):301–306. doi: 10.1016/0304-3940(87)90419-8. [DOI] [PubMed] [Google Scholar]
- Haas H. L. Cholinergic disinhibition in hippocampal slices of the rat. Brain Res. 1982 Feb 4;233(1):200–204. doi: 10.1016/0006-8993(82)90942-8. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J. Presynaptic inhibitory action of acetylcholine in area CA1 of the hippocampus. Exp Neurol. 1978 Dec;62(3):787–797. doi: 10.1016/0014-4886(78)90284-4. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Pumain R., Renaud L. The mechanism of excitation by acetylcholine in the cerebral cortex. J Physiol. 1971 May;215(1):247–268. doi: 10.1113/jphysiol.1971.sp009467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Reiffenstein R. J., Ropert N. Disinhibitory action of acetylcholine in the rat's hippocampus: extracellular observations. Neuroscience. 1981;6(12):2465–2474. doi: 10.1016/0306-4522(81)90092-0. [DOI] [PubMed] [Google Scholar]
- Lacaille J. C., Schwartzkroin P. A. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. II. Intrasomatic and intradendritic recordings of local circuit synaptic interactions. J Neurosci. 1988 Apr;8(4):1411–1424. doi: 10.1523/JNEUROSCI.08-04-01411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Lancaster B., Nicoll R. A. Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci. 1987 Mar;7(3):733–741. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Two types of muscarinic response to acetylcholine in mammalian cortical neurons. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6344–6348. doi: 10.1073/pnas.82.18.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Misgeld U. Carbachol reduces IK,baclofen, but not IK,GABA in guinea pig hippocampal slices. Neurosci Lett. 1989 Jul 31;102(2-3):229–234. doi: 10.1016/0304-3940(89)90083-9. [DOI] [PubMed] [Google Scholar]
- Müller W., Misgeld U. Slow cholinergic excitation of guinea pig hippocampal neurons is mediated by two muscarinic receptor subtypes. Neurosci Lett. 1986 Jun 18;67(2):107–112. doi: 10.1016/0304-3940(86)90381-2. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. A simple chamber for recording from submerged brain slices. J Neurosci Methods. 1981 Aug;4(2):153–156. doi: 10.1016/0165-0270(81)90049-2. [DOI] [PubMed] [Google Scholar]
- Pitler T. A., Alger B. E. Activation of the pharmacologically defined M3 muscarinic receptor depolarizes hippocampal pyramidal cells. Brain Res. 1990 Nov 26;534(1-2):257–262. doi: 10.1016/0006-8993(90)90137-z. [DOI] [PubMed] [Google Scholar]
- Segal M. Multiple action of acetylcholine at a muscarinic receptor studied in the rat hippocampal slice. Brain Res. 1982 Aug 19;246(1):77–87. doi: 10.1016/0006-8993(82)90144-5. [DOI] [PubMed] [Google Scholar]
- Stewart M., Fox S. E. Do septal neurons pace the hippocampal theta rhythm? Trends Neurosci. 1990 May;13(5):163–168. doi: 10.1016/0166-2236(90)90040-h. [DOI] [PubMed] [Google Scholar]
- Valentino R. J., Dingledine R. Presynaptic inhibitory effect of acetylcholine in the hippocampus. J Neurosci. 1981 Jul;1(7):784–792. doi: 10.1523/JNEUROSCI.01-07-00784.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S., Johnston D. Muscarinic depression of synaptic transmission at the hippocampal mossy fiber synapse. J Neurophysiol. 1990 Oct;64(4):1089–1097. doi: 10.1152/jn.1990.64.4.1089. [DOI] [PubMed] [Google Scholar]
- Yamamoto C., Kawai N. Presynaptic action of acetylcholine in thin sections from the guinea pig dentate gyrus in vitro. Exp Neurol. 1967 Oct;19(2):176–187. doi: 10.1016/0014-4886(67)90016-7. [DOI] [PubMed] [Google Scholar]


