Abstract
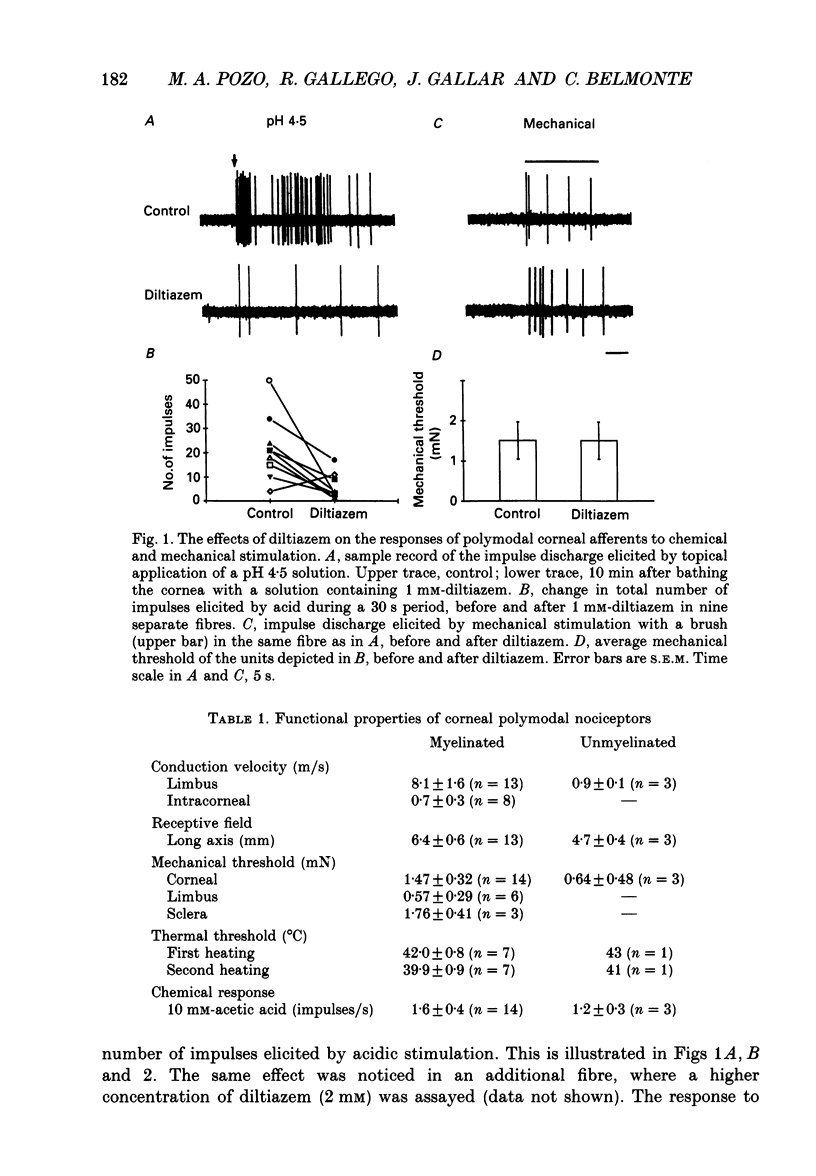
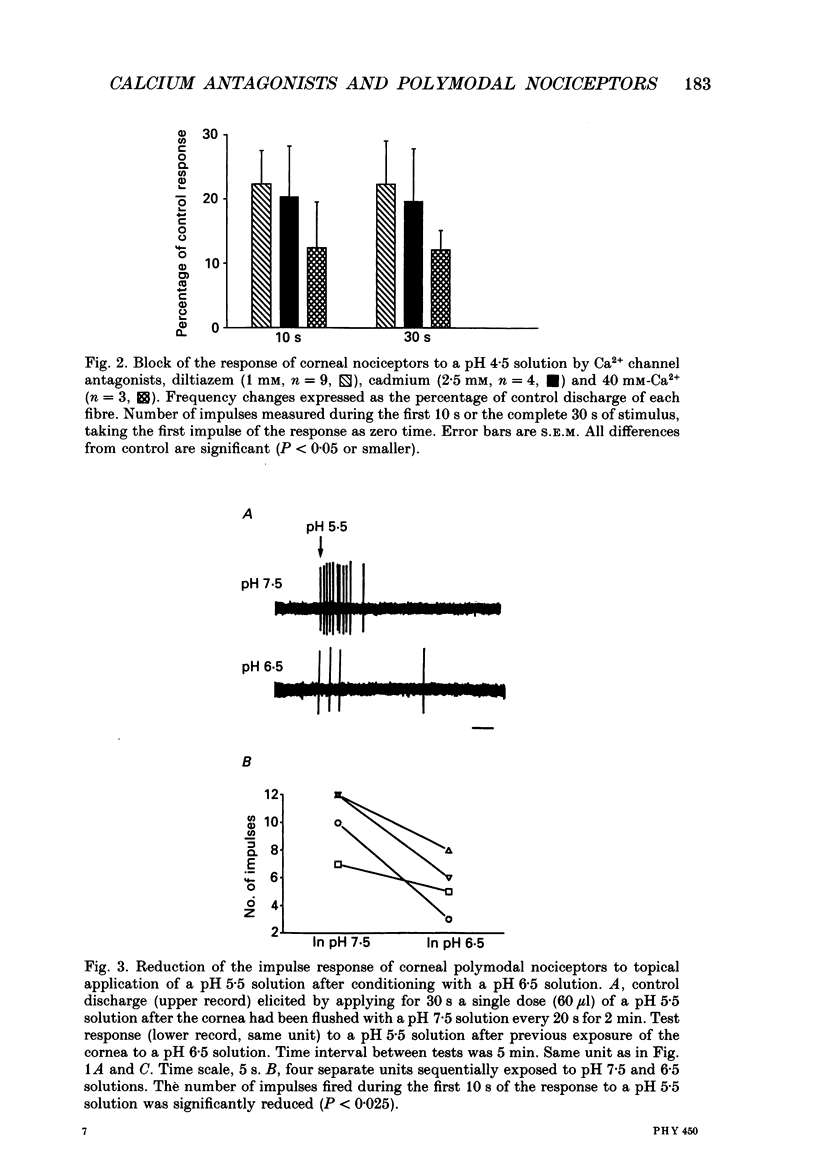
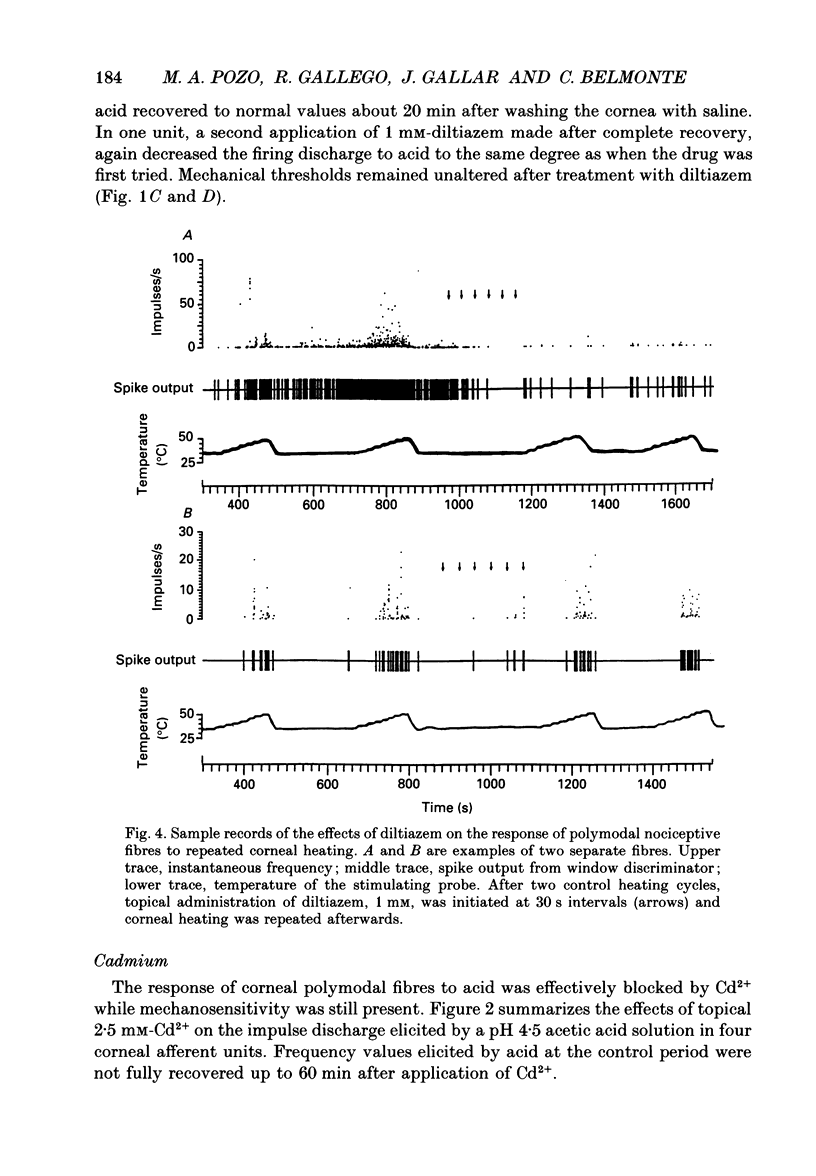
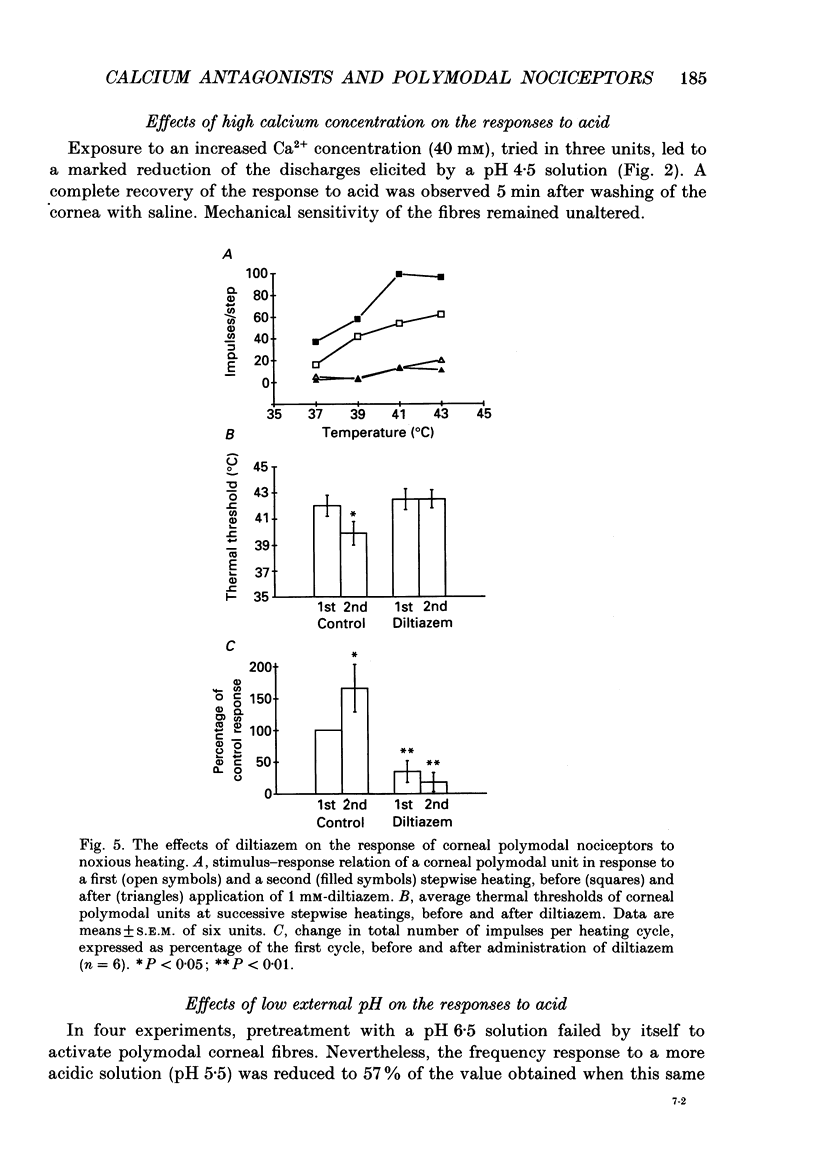
1. The possibility that a modified Ca2+ channel mediates chemical and thermal excitation of nociceptors was examined in single polymodal nociceptive fibres of the cat's cornea. 2. Ca2+ channel blockers cadmium (2.5 mM) and diltiazem (1 mM), and high external [Ca2+] (40 mM), markedly reduced nociceptive responses to topical acidic solutions (pH 4.5). 3. Decreasing the pH to 6.5 did not cause excitation, but reduced the subsequent response to pH 4.5 buffer. 4. Diltiazem (1 mM), applied after repeated stimulation with stepped heating pulses (35 to 47-49 degrees C in 2 degrees C steps) decreased the impulse responses elicited by heat. The decrease in threshold to thermal stimulation produced by repeated heating was blocked by diltiazem. 5. Mechanical threshold and mechanical responsiveness of corneal polymodal nociceptors was not modified by Ca2+ antagonists or by a high-Ca2+ solution. 6. These results offer indirect evidence that proton-activated Ca2+ channels mediate stimulation of nociceptors by acidic solutions. The same type of ionic channel appears to be involved in the response of nociceptors to heat and in sensitization, but not in their responsiveness to mechanical stimulation. The blockade of nociceptive responses by Ca2+ antagonists opens the possibility of using Ca2+ blockers as selective analgesic drugs.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Krishtal O. A., Maruyama T. Proton-induced sodium current in frog isolated dorsal root ganglion cells. J Neurophysiol. 1990 Apr;63(4):805–813. doi: 10.1152/jn.1990.63.4.805. [DOI] [PubMed] [Google Scholar]
- Beck P. W., Handwerker H. O. Bradykinin and serotonin effects on various types of cutaneous nerve fibers. Pflugers Arch. 1974 Mar 11;347(3):209–222. doi: 10.1007/BF00592598. [DOI] [PubMed] [Google Scholar]
- Beck P. W., Handwerker H. O., Zimmermann M. Nervous outflow from the cat's foot during noxious radiant heat stimulation. Brain Res. 1974 Mar 8;67(3):373–386. doi: 10.1016/0006-8993(74)90488-0. [DOI] [PubMed] [Google Scholar]
- Belmonte C., Gallar J., Pozo M. A., Rebollo I. Excitation by irritant chemical substances of sensory afferent units in the cat's cornea. J Physiol. 1991 Jun;437:709–725. doi: 10.1113/jphysiol.1991.sp018621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C., Giraldez F. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. J Physiol. 1981 Dec;321:355–368. doi: 10.1113/jphysiol.1981.sp013989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P., Perl E. R. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969 Nov;32(6):1025–1043. doi: 10.1152/jn.1969.32.6.1025. [DOI] [PubMed] [Google Scholar]
- Bevan S., Yeats J. Protons activate a cation conductance in a sub-population of rat dorsal root ganglion neurones. J Physiol. 1991 Feb;433:145–161. doi: 10.1113/jphysiol.1991.sp018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahl L. A., Iggo A. The effects of bradykinin and prostaglandin E1 on rat cutaneous afferent nerve activity. Br J Pharmacol. 1977 Feb;59(2):343–347. doi: 10.1111/j.1476-5381.1977.tb07498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croze S., Duclaux R., Kenshalo D. R. The thermal sensitivity of the polymodal nociceptors in the monkey. J Physiol. 1976 Dec;263(3):539–562. doi: 10.1113/jphysiol.1976.sp011644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N. W., Lux H. D., Morad M. Site and mechanism of activation of proton-induced sodium current in chick dorsal root ganglion neurones. J Physiol. 1988 Jun;400:159–187. doi: 10.1113/jphysiol.1988.sp017116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FJALLBRANT N., IGGO A. The effect of histamine, 5-hydroxytryptamine and acetylcholine on cutaneous afferent fibres. J Physiol. 1961 May;156:578–590. doi: 10.1113/jphysiol.1961.sp006694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M., Lynn B. The sensitization of high threshold mechanoreceptors with myelinated axons by repeated heating. J Physiol. 1977 Feb;265(2):549–563. doi: 10.1113/jphysiol.1977.sp011730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos A. P. Functional properties of primary afferent units probably related to pain mechanisms in primate glabrous skin. J Neurophysiol. 1976 Jan;39(1):71–83. doi: 10.1152/jn.1976.39.1.71. [DOI] [PubMed] [Google Scholar]
- Kim D. K., Tateishi N., Akaike N. Proton-gated sodium current in parasympathetic ganglion cells of frog heart. J Neurophysiol. 1990 May;63(5):1060–1067. doi: 10.1152/jn.1990.63.5.1060. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Lux H. D., Morad M. Proton-induced transformation of calcium channel in chick dorsal root ganglion cells. J Physiol. 1987 May;386:603–633. doi: 10.1113/jphysiol.1987.sp016553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the membrane of sensory neurons may participate in nociception. Neuroscience. 1981;6(12):2599–2601. doi: 10.1016/0306-4522(81)90105-6. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Pidoplichko V. I. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Mizumura K. Chemical responses of polymodal receptors of the scrotal contents in dogs. J Physiol. 1980 Feb;299:219–231. doi: 10.1113/jphysiol.1980.sp013121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa T., Mizumura K. Mechanical and thermal responses of polymodal receptors recorded from the superior spermatic nerve of dogs. J Physiol. 1980 Feb;299:233–245. doi: 10.1113/jphysiol.1980.sp013122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDAHL O. Experimental skin pain induced by injection of water-soluble substances in humans. Acta Physiol Scand Suppl. 1961;179:1–89. [PubMed] [Google Scholar]
- Lang E., Novak A., Reeh P. W., Handwerker H. O. Chemosensitivity of fine afferents from rat skin in vitro. J Neurophysiol. 1990 Apr;63(4):887–901. doi: 10.1152/jn.1990.63.4.887. [DOI] [PubMed] [Google Scholar]
- Martin H. A., Basbaum A. I., Kwiat G. C., Goetzl E. J., Levine J. D. Leukotriene and prostaglandin sensitization of cutaneous high-threshold C- and A-delta mechanonociceptors in the hairy skin of rat hindlimbs. Neuroscience. 1987 Aug;22(2):651–659. doi: 10.1016/0306-4522(87)90360-5. [DOI] [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977 May;267(1):75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S., Schmidt R. F. Activation of group IV afferent units from muscle by algesic agents. Brain Res. 1974 Jun 7;72(2):305–310. doi: 10.1016/0006-8993(74)90870-1. [DOI] [PubMed] [Google Scholar]
- Nobile M., Carbone E., Lux H. D., Zucker H. Temperature sensitivity of Ca currents in chick sensory neurones. Pflugers Arch. 1990 Mar;415(6):658–663. doi: 10.1007/BF02584002. [DOI] [PubMed] [Google Scholar]
- Sato J., Mizumura K., Kumazawa T. Effects of ionic calcium on the responses of canine testicular polymodal receptors to algesic substances. J Neurophysiol. 1989 Jul;62(1):119–125. doi: 10.1152/jn.1989.62.1.119. [DOI] [PubMed] [Google Scholar]
- Schaible H. G., Schmidt R. F. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. J Neurophysiol. 1985 Nov;54(5):1109–1122. doi: 10.1152/jn.1985.54.5.1109. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J. Selective responsiveness of polymodal nociceptors of the rabbit ear to capsaicin, bradykinin and ultra-violet irradiation. J Physiol. 1987 Jul;388:9–23. doi: 10.1113/jphysiol.1987.sp016598. [DOI] [PMC free article] [PubMed] [Google Scholar]


