Abstract
Background
Behçet’s disease (BD) during pregnancy is a relatively rare condition, and there are currently no established guidelines for its management. The effects of BD on both mothers and children remain unclear. In this paper, we present the diagnostic and treatment processes for a patient with BD during pregnancy.
Case presentation
A 20-year-old woman, gravida 2, para 1, presented to our inpatient department with recurrent oral and genital ulcers at 28 weeks and 3 days of gestation. Given the complexity of the patient’s condition, we initiated the first multidisciplinary consultation to establish appropriate treatment regimens. However, the initial treatment was ineffective, and the patient experienced a fever for four days. Consequently, we conducted a second multidisciplinary consultation. The final treatment regimen included azithromycin, hydroxychloroquine, prednisone, and low-molecular-weight heparin calcium. The clinical symptoms resolved, and the female neonate was delivered successfully. No relapse was observed during the four-month follow-up.
Conclusions
The critical issues in treatment involve ensuring medication safety for pregnant women and preventing adverse pregnancy outcomes. Notably, one potential cause of adverse pregnancy outcomes in individuals with BD is vasculopathy, which necessitates prompt treatment with anticoagulant therapy. Obstetricians should closely monitor disease progression and remain vigilant for complications in order to minimize adverse outcomes during pregnancy.
Keywords: Behçet’s disease, Pregnancy, Anticoagulant therapy
Background
Behçet’s disease (BD) is a chronic multisystem vasculitis characterized by recurrent oral and genital ulcers, uveitis and skin lesions, and it may also involve the heart, gastrointestinal system, nervous system, kidneys, and joints. Epidemiological studies indicate significant geographical differences in the prevalence of BD, which is highest in countries along the ancient Silk Road, stretching from the Mediterranean basin to East Asia [1]. A comprehensive global review reported a prevalence of 10.3 per 100,000 individuals, while the incidence rate in China was estimated at 14 per 100,000 individuals [2].
BD often manifests in young women of childbearing age, with the primary symptoms during pregnancy being oral and genital ulcers, reported at rates of 17.8% and 8.9%, respectively. Other manifestations, though less common, include arthritis (4.4%), uveitis (2.2%), and neurologic symptoms (6.7%) [3]. The existing literature on BD in pregnancy remains limited and presents conflicting findings. Some studies indicate that BD does not increase the risk of adverse pregnancy outcomes [4, 5], while others report a higher incidence of preterm labor, cesarean sections, and gestational diabetes mellitus [3, 6]. Tânia Barros et al. propose that these adverse outcomes may be associated with vascular lesions [3].
In this article, we present a case of BD in pregnancy characterized by recurrent oral and genital ulcers. We also discuss the risk factors for BD that may contribute to adverse pregnancy outcomes and outline management strategies for patients with BD during pregnancy by reviewing the relevant literature.
Case presentation
A 20-year-old woman, gravida 2, para 1, has experienced recurrent oral ulcers since the first trimester without receiving treatment. Due to the presence of genital ulcers accompanied by swelling and pain, she underwent treatment with potassium permanganate (1:5000) in water during a sitz bath, as well as topical anti-inflammatory medications, three days prior at another hospital; however, there was no significant improvement. Consequently, she presented to our inpatient department with recurrent oral and genital ulcers at 28 weeks and 3 days of gestation. She reported no history of rheumatic, genetic, infectious, or psychiatric disorders. Additionally, there was no history of mental or genetic disorders.
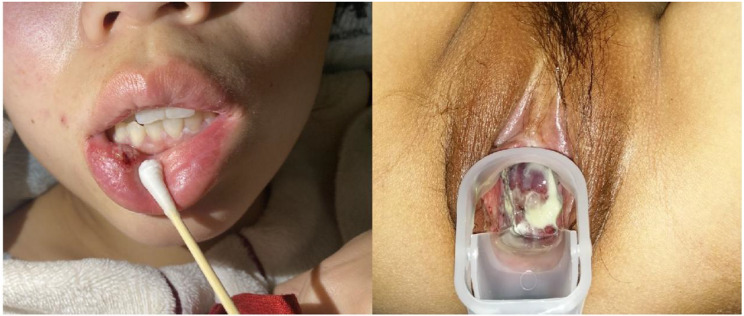
Upon admission, physical examinations revealed scattered, round ulcers of varying sizes and depths on the mucosa of the lower lip and the margin of the tongue, with a yellowish coating covering the base. There was congestion of the uvula, and round-like ulcers were also visible on the uvula (Fig. 1). The vulvar clitoris was swollen, and scattered ulcers of varying depths were observed on both the labia majora and labia minora, with notable adhesions on the right labium and a significant amount of yellowish-white discharge present, which was associated with evident tenderness (Fig. 1).
Fig. 1.
The Patient’s oral ulcers and genital ulcers
The blood and urine tests indicated the presence of inflammation, with hypersensitive C-reactive protein (hs-CRP) measured at 37.23 mg/L and an erythrocyte sedimentation rate (ESR) of 34 mm/H. Positive results were observed for the herpes simplex virus (HSV) type I antibody-IgG, anti-Streptolysin “O” test (ASO), anti-cytomegalovirus IgG, complement C3, and complement C4.
Based on the diagnostic criteria established by The International Criteria for Behcet’s Disease (ICBD) and in conjunction with clinical symptoms and laboratory findings, a diagnosis of BD was confirmed at 28 weeks and 3 days of gestation. Initially, treatment involved the administration of dexamethasone (6 mg i.m. every 12 h) to facilitate fetal lung maturation. Given the rarity of the disease, we initiated a multidisciplinary consultation. Following recommendations from dermatologists and clinical pharmacists, the treatment regimen was adjusted to include povidone iodine (200 ml ad us.ext daily) and polymyxin B ointment (100 mg ad us.ext daily) for the treatment of vulvar ulcerated surfaces, tin scatter (100 mg ad us.ext daily) for uvulopalatine ulcerated surfaces, along with aciclovir (450 mg i.m. every 8 h) and continued dexamethasone (6 mg i.m. every 12 h).
Five days after admission, the patient developed a fever that persisted for four days, with a maximum temperature of 38.6℃. Biopsies taken from the margins of the vulvar ulcer lesions revealed suppurative inflammation. A direct laryngoscopy indicated congestion of the mucosa in the palatoglossal arch, palatopharyngeal arch, and larynx, along with first-degree enlargement of the tonsils. Additionally, the surface of the uvula and the posterior pharyngeal wall exhibited white ulcers, while the middle and upper parts of the posterior pharyngeal wall, the root of the tongue, and the lower part of the posterior pharyngeal wall displayed hyperplastic lymphatic follicles. Based on the examination results and symptoms, we added cefoxitin sodium (2 g IV once) to our treatment regimen and replaced polymyxin B ointment with acyclovir ointment.
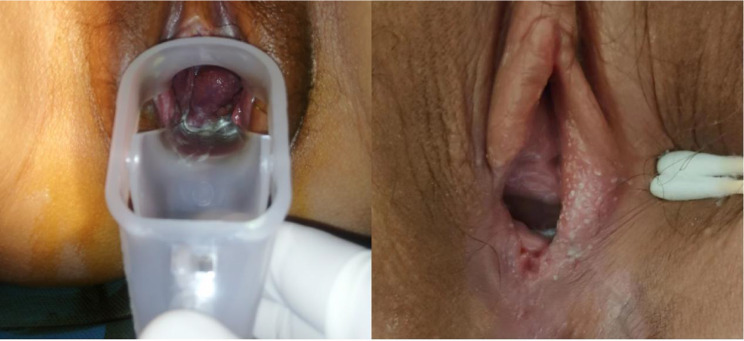
The second multidisciplinary consultation was initiated due to the patient’s lack of significant improvement. The treatment regimen was modified to include hydroxychloroquine (200 mg orally twice daily), prednisone (40 mg orally once daily), and low-molecular-weight heparin calcium (2050 IU subcutaneously once daily). Six days later, physical examinations revealed no obvious ulcers on the surface of the right labia majora, vaginal wall, and cervix, while the ulcers on the right labia minora showed improvement. A small amount of grayish-white purulent discharge was noted at the vaginal opening (Fig. 2).
Fig. 2.
The Patient’s situation at discharge
Subsequently, she was discharged from the hospital. Hydroxychloroquine (200 mg orally twice daily), prednisone (40 mg orally once daily), and low-molecular-weight heparin calcium (2050 IU subcutaneously once daily) were continued for two weeks, with no recurrence observed before delivery. The patient delivered a live-born female infant at 38 weeks and 5 days of gestation, with Apgar scores of 10 at both 1 and 5 min. The female neonate exhibited no abnormalities. At six weeks postpartum, the patient showed no signs of recurrence.
Discussion and literature review
BD is a rare disorder, and its etiology and pathogenesis remain unclear. However, growing evidence suggests that genetics, environmental factors, infections, and immune status all contribute to the development of BD [2]. In 1990, a collaboration of scientists from seven countries published the International Study Group (ISG) criteria for BD (Table 1) to establish a unified set of diagnostic criteria [4]. Despite this effort, the sensitivity of the ISG criteria for diagnosing BD was found to be low. To enhance sensitivity, the team developed a new set of criteria in 2014, known as ICBD (Table 2) [5]. The diagnostic point score system for BD requires a score of ≥ 4. In 2021, China introduced its own criteria based on the ICBD, and its diagnostic point score system is consistent with that of the ICBD [2].
Table 1.
International criteria for Behçet’s disease for the diagnosis
| Manifestation | Definition |
|---|---|
| Recurrent oral ulceration | Minor aphthous, major aphthous, or herpetiform ulcers observed by the physician or patient, which have recurred at least 3 times over a 12-month period |
| Plus any two of the following findings: | |
| Recurrent genital ulceration | Recurrent genital aphthous ulceration or scarring, observed by a physician or reported reliably by patient |
| Eye lesions | Anterior uveitis, posterior uveitis, or cells in vitreous on slit lamp examination; or retinal vasculitis observed by qualified physician (ophthalmologist) |
| Skin lesions |
Erythema nodosum-like lesions, observed by a physician or reported reliably by patient; Pseudofolliculitis or papulopustular lesions; or acneiform nodules observed by a physician in post adolescent patients not receiving glucocorticoids |
| Positive pathergy test | Test interpreted as positive by a physician at 24–48 h, performed with oblique insertion of a 20-gauge needle or smaller under sterile conditions |
Table 2.
International criteria for Behçet’s disease-point score system: scoring ≥ 4 indicates Behçet’s diagnosis
| Sign/symptom | Points |
|---|---|
| Ocular lesions | 2 |
| Genital aphthosis | 2 |
| Oral aphthosis | 2 |
| Skin lesions | 1 |
| Neurological manifestations | 1 |
| Vascular manifestations | 1 |
| Positive pathergy test* | 1 |
*Pathergy test is optional and the primary scoring system does not include pathergy testing. However, where pathergy testing is conducted one extra point may be assigned for a positive result
The primary symptoms of BD during pregnancy include oral ulcers, genital ulcers, skin lesions, and ocular inflammation [3]. Existing literature on the relationship between BD and pregnancy outcomes is limited and often contradictory [6–8]. A study conducted by Tien-Ming Chan et al. indicated that, aside from puerperal cerebrovascular disease and gestational diabetes mellitus, patients with BD do not face a significantly increased risk of complications, and neonatal outcomes appear unaffected by BD [4]. Conversely, research by Seohyuk Lee et al. reported that women with BD are at a higher risk for preterm labor and postpartum venous thromboembolism, with their newborns being more likely to be born prematurely [8]. Additionally, BD during pregnancy is associated with a greater likelihood of cesarean delivery, and this study also found that women with BD were more likely to require prenatal hospitalization compared to those without the condition [8].
We conducted a review of cases of BD during pregnancy. Our literature review identified 18 reports of BD in this context [9–26]. Among these cases, 7 were diagnosed during pregnancy [9–15] (Table 3), while 11 were diagnosed prior to pregnancy [16–26] (Table 4). Two neonates exhibited symptoms of BD syndrome, as reported by O. Fain et al. [19] and A.G. Fam et al. [17]. In the case reported by O. Fain et al. [19], the infant presented with transient orogenital ulcerations and pustular cutaneous lesions. In contrast, the infant in the case by A.G. Fam et al. [17] exhibited fever, stomatitis, and ulcerated pustular lesions on the face, scalp, penis, and buttocks. Additionally, a neonate in another case presented by S. Jog et al. [20] was reported to be deceased. Basak Cakal et al. described a case in which the fetus was aborted, and the patient subsequently died due to extensive pyogenic suppuration throughout the abdominal cavity [23]. Furthermore, three cases reported by Zahra Mirfeizi et al. [26], G. Guzelian et al. [10], and Keita Fujikawa et al. [25] resulted in premature delivery. Fortunately, in the case we presented, BD during pregnancy did not appear to affect the neonate.
Table 3.
BD diagnosed during pregnancy
| References | Year | Gestational week | Organ involvement | Symptoms | The treatment | Outcome | Follow-up |
|---|---|---|---|---|---|---|---|
| J M Casanova et al. | 1987 | the third trimester | Skin and mucous membrane | genital ulcers | systemic steroids,steroid ointments | caesarean section | no recurrence |
| G Guzelian et al. | 1997 | the first trimester | Skin and mucous membrane,gastrointestinal tract | genital and oral ulcers ,abdominal pain | steroids | premature delivery | no recurrence |
| Kei Takayama et al. | 2013 | 12 weeks | Skin and mucous membrane,eye | blurred vision in right eye,oral and genital ulcers,erythema nodosum | steroid eyedrops,infliximab | viginal delivery | no recurrence |
| Maria Papadakiet al. | 2015 | 25 weeks | Skin and mucous membrane,eye | bilateral visual loss, oral ulcers | methylprednisolone | viginal delivery | no recurrence |
| Abiraj Kumar et al. | 2015 | 33 weeks | eye | diminution of vision in her right eye | methylprednisolone,prednisolone,steroids | caesarean section | recurrence |
| Saori Kiyoharaet al. | 2020 | 25 weeks | Skin and mucous membrane | oral and genital, ulcers, acneiform eruptions, erythema nodosum | prednisolone,methylprednisolone | viginal delivery | no recurrence |
| Ciara O’Gradyet al. | 2020 | the first trimester | Skin and mucous membrane,joint,eye | mucosal ulcerations, uveitis, and synovitis | etanercept,prednisolone | caesarean section | no recurrence |
Table 4.
BD diagnosed before pregnancy
| References | year | The history of BD | Organ involvement | Symptoms | The treatment | Outcome | Follow-up |
|---|---|---|---|---|---|---|---|
| E M Nóvak et al. | 1977 | 1 years | Neurological manifestations | cranial nerves palsies and pyramidal tract involvement | sistemic dexamethasone ,eye drops of atropine, fluorometolone | caesarean section | visual loss |
| A G Fam et al. | 1981 | 21 years | Skin and mucous membrane,joint,gastrointestinal tract,heart and great vessels | oral ulcers, inflammatory arthritis, superficial thrombophlebitis, colonic ulcers | corticosteroids, ACTH, azathioprine, methotrexate,levamisole,colchicine | Female neonate-healthy,Male neonate-affected | recurrence |
| R M Suchenwirth et al. | 1984 | 10 years | Skin and mucous membrane,neurological manifestations | oral and genital ulcers, acute encephalitis | corticosteroids | viginal delivery | recurrence |
| O Fain et al. | 1995 | 6 years | Skin and mucous membrane,heart and great vessels | superficial thrombophlebitis,pseudofolliculitis,oral and genital ulcers | colchicine | Neonate-affeted | no recurrence |
| S Jog et al. | 2001 | 7 years | Skin and mucous membrane, joint,gastrointestinal tract,heart and great vessels | oral and genital ulcers, arthritis, colitis, erythema nodosum,thrombophlebitis of lower limbs | steroids | neonatal death | no recurrence |
| P Fotaki et al. | 2002 | 11 years | Skin and mucous membrane, neurological manifestations | Oral and genital ulcers,erythema nodosum,headaches | analgetic therapy | viginal delivery | no recurrence |
| Ilseon Hwang et al. | 2009 | 4 years | Skin and mucous membrane, joint | oral ulcers, erythema nodosum, arthralgia | prednisolone | caesarean section | no recurrence |
| Basak Cakal et al. | 2011 | 4 years | Gastrointestinal tract | extensive pyogenic suppuration in the whole abdominal cavity | subtotal colectomy and end ileostomy | abortion | dead |
| Yuko Higashi et al. | 2013 | 8 years | Skin and mucous membrane,eye | oral and genital ulcers, uveitis and folliculitis | Granulocyte and monocyte adsorption apheresis | viginal delivery | no recurrence |
| Keita Fujikawa et al. | 2016 | 6 years | Skin and mucous membrane,gastrointestinal tract | fever, oral and genital ulcers,abdominal pain,hemorrhagic stool | Prednisolone,mesalazine | premature delivery | Recurrence |
| Zahra Mirfeiziet al. | 2018 | 6 months | Skin and mucous membrane,heart and great vessels | oral ulcers, erythema nodosum,right heart failure progresse | Prednisolone,ceftriaxone ,vancomycin,heparin | premature delivery | no recurrence |
The primary objective of treating BD during pregnancy is to swiftly suppress inflammatory exacerbations, minimize adverse pregnancy outcomes, and prevent recurrences that could lead to irreversible organ damage. Given the absence of specific criteria for BD in pregnancy, treatment regimens must be formulated based on existing guidelines for BD. Glucocorticoids serve as the cornerstone of treatment for BD, while additional medications are utilized depending on the site of involvement and the severity of the condition [5]. Medications such as methotrexate, mycophenolate mofetil, cyclophosphamide, and thalidomide, which have been associated with fetal malformations, are contraindicated during treatment [27]. Moreover, the use of other immunotherapeutic agents, such as colchicine, should be carefully regulated in terms of dosage.
It is estimated that 10–37% of patients with BD experience arterial or venous thrombosis [28]. Notably, venous involvement is more prevalent than arterial involvement; however, the hypercoagulable state during pregnancy heightens the risk of adverse obstetric outcomes. The literature reports three cases of phlebitis: two cases of superficial phlebitis [17, 19] and one case of thrombophlebitis in the lower extremities [20]. Additionally, three neonates have been reported with BD syndrome, including one case that resulted in death, as presented by S. Jog et al. It is hypothesized that vasculitis contributes to adverse pregnancy outcomes in patients with BD, making neonatal outcomes particularly vulnerable. This aligns with the findings of Jadaon J et al., who propose that vascular hypercoagulability during pregnancy is a significant factor in miscarriage among their patients with BD [28].
Anticoagulation or thromboprophylaxis is a suitable strategy for preventing adverse obstetric outcomes. Some studies indicate that the risk of venous thromboembolism in the first three months postpartum is 50 times higher than that in non-pregnant women [8]. In our case, we utilized low molecular weight heparin, which may have played a role in preventing relapse and deterioration of the patient’s condition during pregnancy and after delivery. Additionally, cesarean section is recognized as a risk factor for postpartum venous thrombosis [29]. Therefore, meticulous postpartum follow-up is essential for pregnant women with BD, particularly those who have undergone cesarean delivery. Obstetricians must remain vigilant regarding this coexisting complication and provide prompt treatment to avert adverse outcomes.
Conclusion
Obstetricians should be aware of this type of pregnancy and focus on vasculitis as a complication that can increase adverse pregnancy outcomes. Early anticoagulation is indicated to prevent such outcomes. Prompt initiation of anticoagulation and management of thrombosis are essential. After delivery, careful follow-up of both patients and infants is necessary to prevent recurrences.
Acknowledgements
Written informed consent for publication of their clinical images was obtained from the patient.
Abbreviations
- BD
Behçet’s disease
- hs-CRP
Hypersensitive C-reactive protein
- ESR
Erythrocyte sedimentation rate
- HSV
Herpes simplex virus
- ASO
Anti-Streptolysin “O” test
- UU
Ureaplasma urealyticum
- ANCA
Anti-neutrophil cytoplasmic antibodies
- ANA
Antinuclear antibody
- GBS
Group B streptococcus
- HPV
Human papilloma virus
- TCT
Thinprep cytologic test
- ICBD
International Criteria for Behçet’s disease
- WBC
White blood cells
- ISG
International Study Group
- MDT
Multidisciplinary treatment
Author contributions
X.Y. provided the patient’s information and pictures. X.L. wrote the manuscript. All authors have reviewed and approved the final manuscript.
Funding
Not applicable.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of clinical details and clinical images. A copy of the written consent is available for review by the editor of the journal.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saadoun D, Wechsler B. Behçet’s disease. Orphanet J Rare Dis. 2012;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinese Medical Association Rheumatology Branch. Guidelines for the diagnosis and treatment of Behçet’s Disease[J]. Chin J Rheumatol. 2011;15(5):345–7. [Google Scholar]
- 3.Barros T, Braga A, Marinho A, Braga J. Behçet’s Disease and Pregnancy: A Retrospective Case-control Study. Yale J Biol Med. 2021;94(4):585–92. [PMC free article] [PubMed] [Google Scholar]
- 4.Criteria for diagnosis of Behçet’s disease. International Study Group for Behçet’s Disease. Lancet. 1990;335(8697):1078–80. [PubMed] [Google Scholar]
- 5.International Team for the Revision of the International Criteria for Behçet’s Disease (ITR-ICBD). The International Criteria for Behçet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2014;28(3):338–47. [DOI] [PubMed] [Google Scholar]
- 6.Noel N, Wechsler B, Nizard J, Costedoat-Chalumeau N, Boutin du LT, Dommergues M, Vauthier-Brouzes D, Cacoub P, Saadoun D. Behçet’s disease and pregnancy. Arthritis Rheum. 2013;65(9):2450–6. [DOI] [PubMed] [Google Scholar]
- 7.Chan TM, Chiou MJ, Kuo CF. Adverse pregnancy outcomes in women with Behçet’s disease: population-based registry linkage study in Taiwan. Clin Rheumatol. 2021;40(10):4135–42. [DOI] [PubMed] [Google Scholar]
- 8.Lee S, Czuzoj-Shulman N, Abenhaim HA. Behcet’s disease and pregnancy: obstetrical and neonatal outcomes in a population-based cohort of 12 million births. J Perinat Med. 2019;47(4):381–7. [DOI] [PubMed] [Google Scholar]
- 9.Casanova JM, Gonzalez J, Muñoz M, Bravo JM, Ramos J. Enfermedad de Behçet y embarazo[Behçet’s disease and pregnancy]. Med Cutan Ibero Lat Am. 1987;15(5):387–91. [PubMed] [Google Scholar]
- 10.Guzelian G, Norton ME. Behçet’s syndrome associated with intrauterine growth restriction: a case report and review of the literature.J Perinatol. 1997 Jul-Aug;17(4):318–20. [PubMed]
- 11.Takayama K, Ishikawa S, Enoki T, Kojima T, Takeuchi M. Successful treatment with infliximab for Behçet disease during pregnancy. Ocul Immunol Inflamm. 2013;21(4):321–3. [DOI] [PubMed] [Google Scholar]
- 12.Papadaki M, Lefebvre P, Janssens S, Daguzan M, Postelmans L, Caspers L, Willermain F. Bilateral retinal ischemic vasculopathy in a pregnant patient. Retin Cases Brief Rep. 2015 Spring;9(2):185–9. [DOI] [PubMed]
- 13.Kumar A, Yangzes S, Singh R. Frosted branch angiitis in one eye and impending CRVO in the other:a diagnostic dilemma. BMJ Case Rep. 2015;2015:bcr2014209073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiyohara S, Kanemaru H, Suzuki T, Nakamura K, Honda N, Makino K, Kajihara I, Makino T, Ohba T. Ihn H.Successful prednisolone treatment of peripartum Behçet disease associated with HLA-A26.Australas. J Dermatol. 2020;61(4):e441–2. [DOI] [PubMed] [Google Scholar]
- 15.O’Grady C, Quinn G, Keogh A, O’Keane C. Murad A.Certolizumab-induced facial eruption in a pregnant woman with severe Behcet’s disease. Dermatol Ther. 2020;33(6):e14162. [DOI] [PubMed] [Google Scholar]
- 16.Nóvak EM, Werneck LC, Mora AH. Doença de Behçet com envolvimento neurologico. Relato de um caso em gestante [Behcet’s syndrome with neurologic involvement. Report of a case in a pregnant women]. Arq Neuropsiquiatr. 1977;35(2):146–50. Portuguese. [DOI] [PubMed] [Google Scholar]
- 17.Fam AG, Siminovitch KA, Carette S, From L. Neonatal Behçet’s syndrome in an infant of a mother with the disease. Ann Rheum Dis. 1981;40(5):509–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suchenwirth RM. Beitrag zum Problem Morbus Behçet und Nervensystem 10jährige Verlaufsbeobachtung mit Schwangerschafte/Literaturübersicht [Behcet’s disease and the nervous system–a 10-year follow-up with pregnancy. Rev literature] Fortschr Neurol Psychiatr. 1984;52(2):41–7. German. [DOI] [PubMed] [Google Scholar]
- 19.Fain O, Mathieu E, Lachassinne E, Buisson P, Bodemer C, Gaudelus J, Thomas M. Neonatal Behçet’s disease. Am J Med. 1995;98(3):310–1. [DOI] [PubMed] [Google Scholar]
- 20.Jog S, Patole S, Koh G, Whitehall J. Unusual presentation of neonatal Behcets disease. Am J Perinatol. 2001;18(5):287–92. [DOI] [PubMed] [Google Scholar]
- 21.Fotaki P, Deligeoroglou E, Michailidis E, Vitoratos N, Kokkalis D, Siristatidis X, Creatsas G. Sindrom na Adamantiadi-Bekhchet i bremennost predstaviane na edin sluchaĭ [Adamantiades-Behcet’s syndrome and pregnancy: a case report]. Akush Ginekol (Sofiia). 2002;42(1):38–9. Bulgarian. [PubMed] [Google Scholar]
- 22.Hwang I, Lee CK, Yoo B, Lee I. Necrotizing villitis and decidual vasculitis in the placentas of mothers with Behçet disease. Hum Pathol. 2009;40(1):135–8. [DOI] [PubMed] [Google Scholar]
- 23.Cakal B, Koklu S, Beyazit Y, Ozdemir A, Beyazit F, Ulker A. Fatal colonic perforation in a pregnant with Behçet’s disease. J Crohns Colitis. 2011;5(3):273–4. [DOI] [PubMed] [Google Scholar]
- 24.Higashi Y, Shimokawa M, Kawai K, Kanekura T. Granulocyte and monocyte adsorption apheresis for Behçet’s disease in a pregnant woman. J Dermatol. 2013;40(12):1042–4. [DOI] [PubMed] [Google Scholar]
- 25.Fujikawa K, Endo Y, Mizokami A, Takahashi K, Tabuchi M, Ohba K, Nakamura H, Kawakami A. Successful Treatment with Adalimumab for Intestinal Behcet’s Disease during Pregnancy. Intern Med. 2016;55(10):1375–8. [DOI] [PubMed] [Google Scholar]
- 26.Mirfeizi Z, Memar B, PourZand H, Molseghi MH, Rezaei Shahmirzadi A, Abdolahi N. Ventricular endomyocardial fibrosis in a pregnant female with Behçet’s disease. Asian Cardiovasc Thorac Ann. 2018;26(8):619–21. [DOI] [PubMed] [Google Scholar]
- 27.Machen L, Clowse ME. Vasculitis and Pregnancy. Rheum Dis Clin North Am. 2017;43(2):239–47. [DOI] [PubMed] [Google Scholar]
- 28.Jadaon J, Shushan A, Ezra Y, Sela HY, Ozcan C, Rojansky N. Behçet’s disease and pregnancy. Acta Obstet Gynecol Scand. 2005. Oct;84(10):939–44. [DOI] [PubMed]
- 29.Hiwarkar P, Stasi R, Sutherland G, Shannon M. Deep vein and intracardiac thrombosis during the post-partum period in Behcet’s disease. Int J Hematol. 2010;91:679–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript or supplementary information files.