Abstract
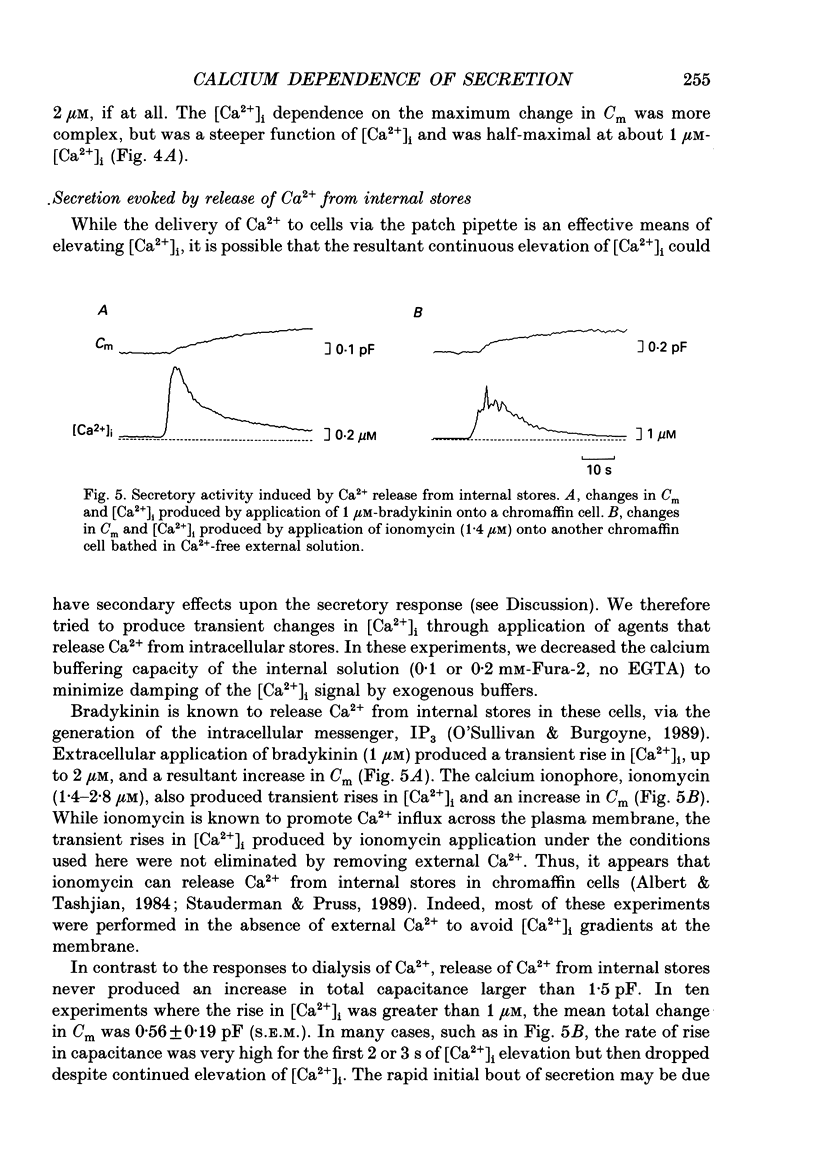
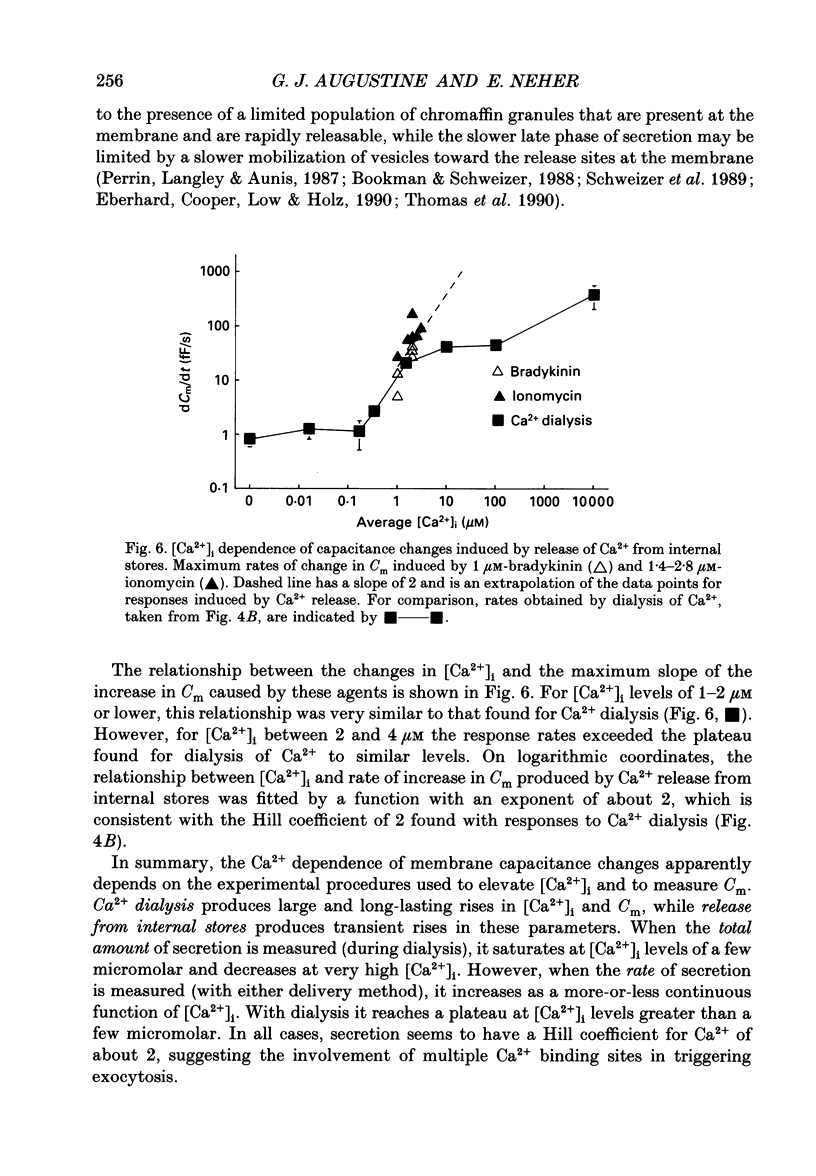
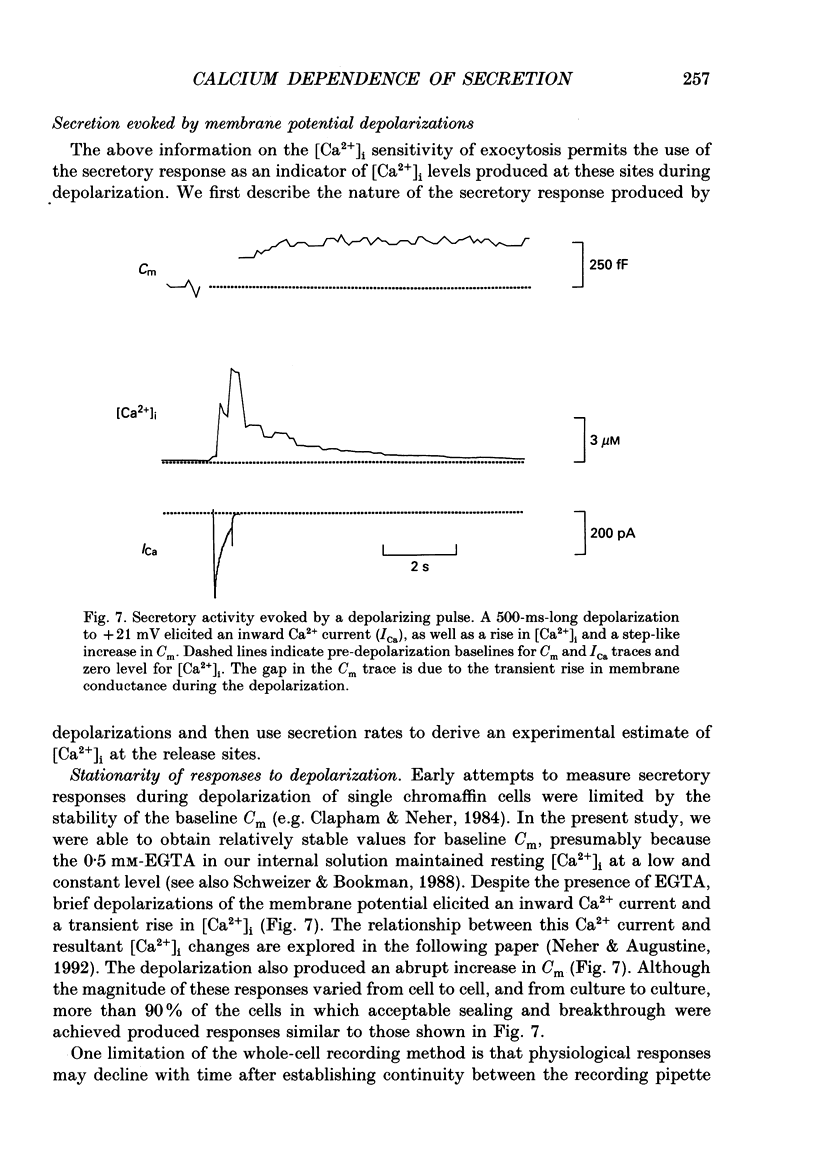
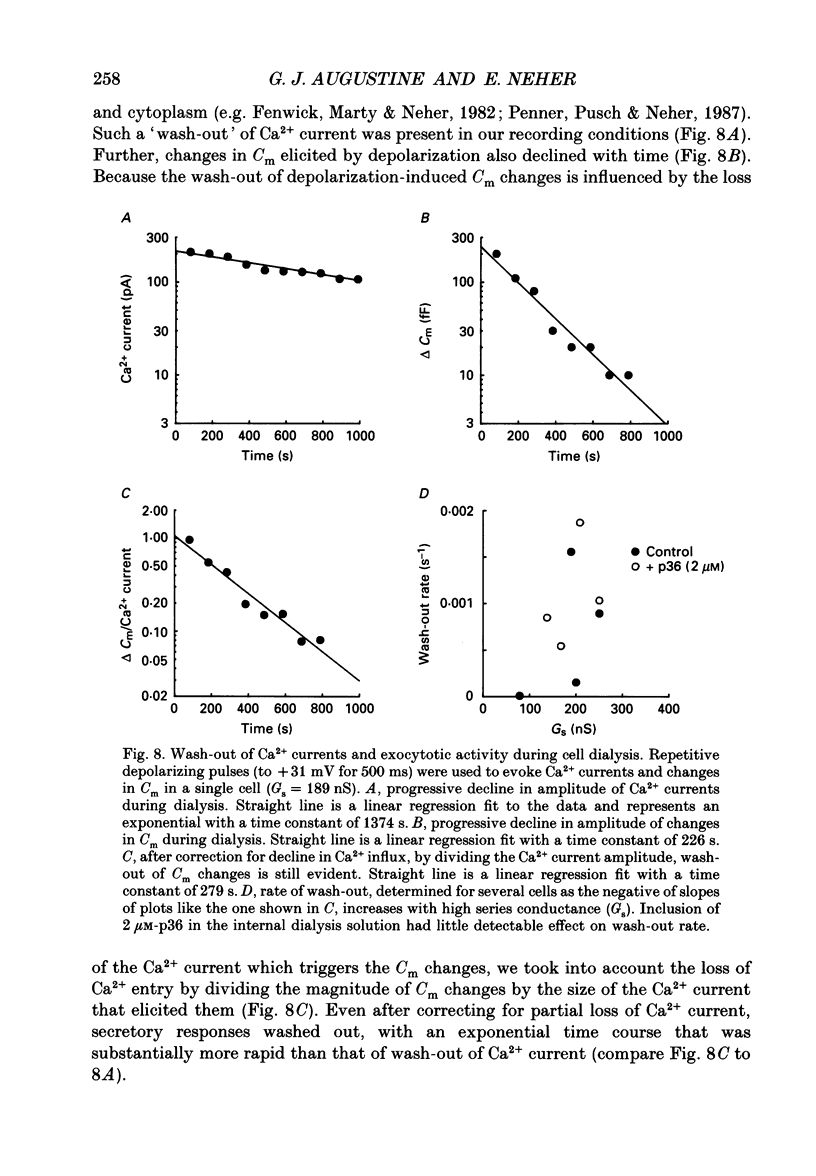
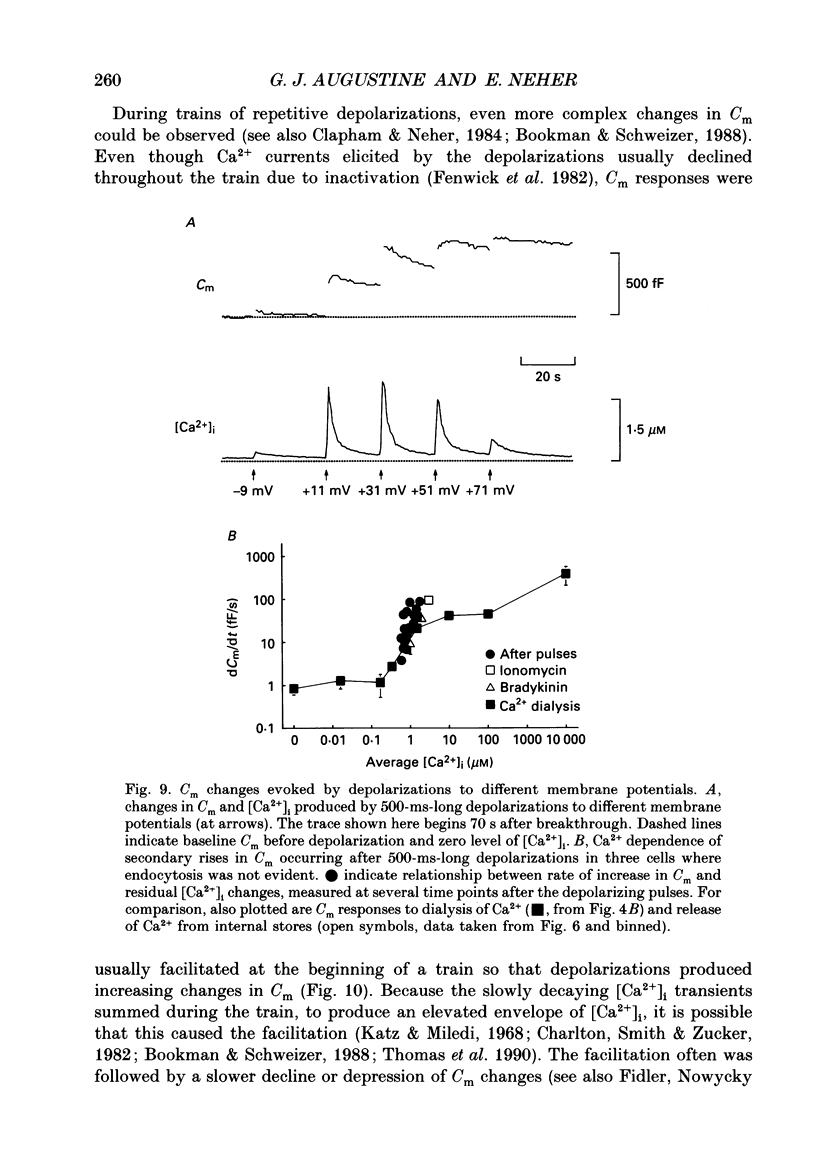
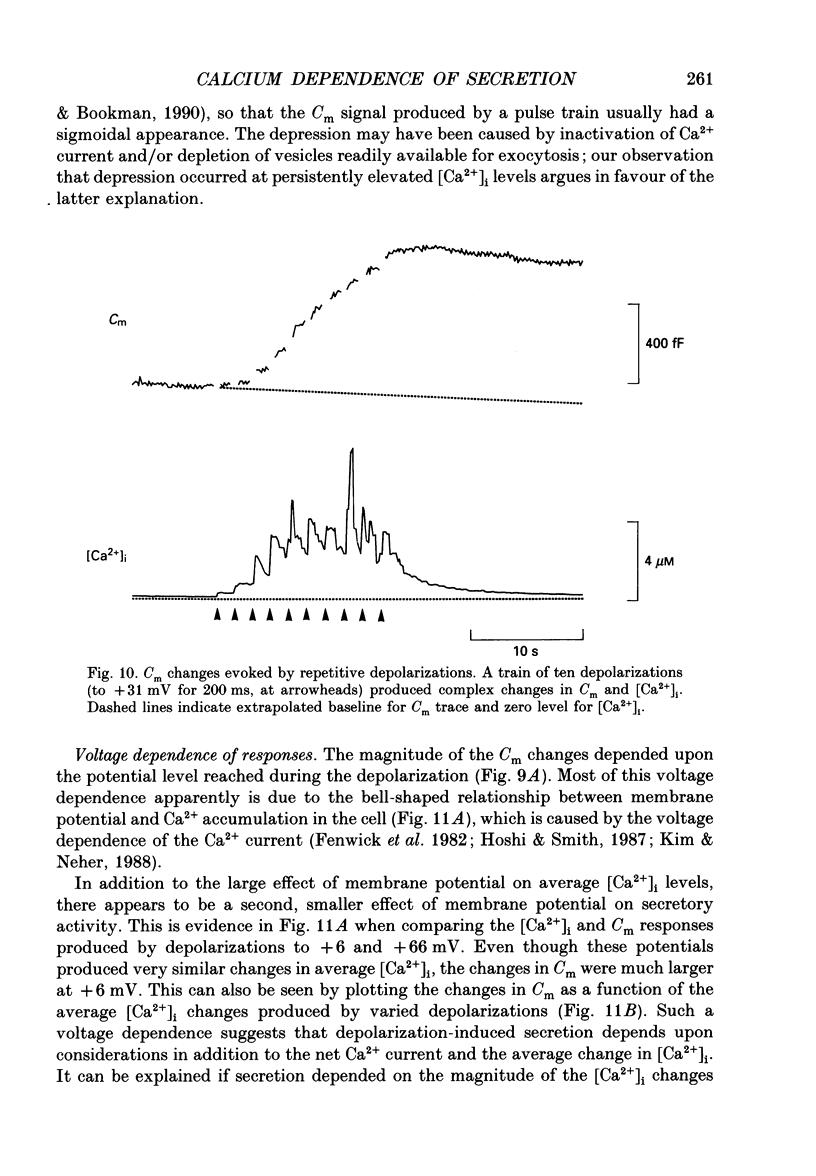
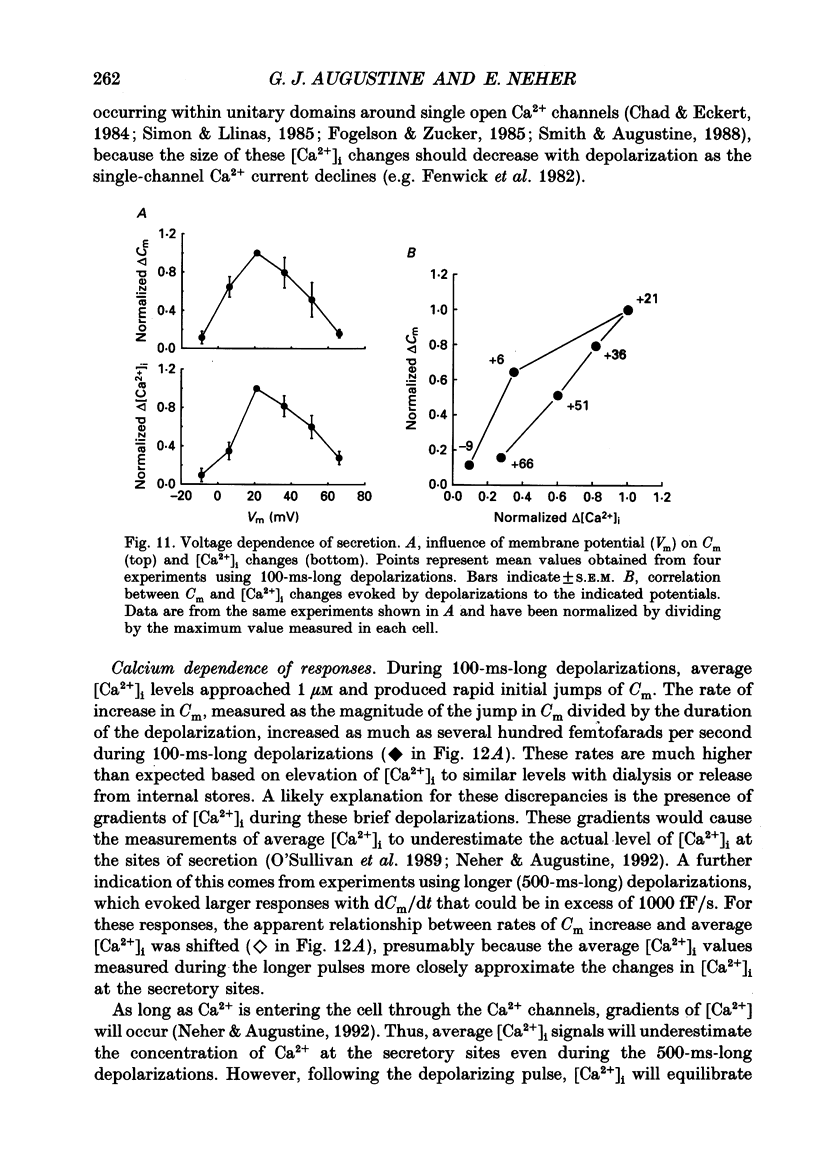
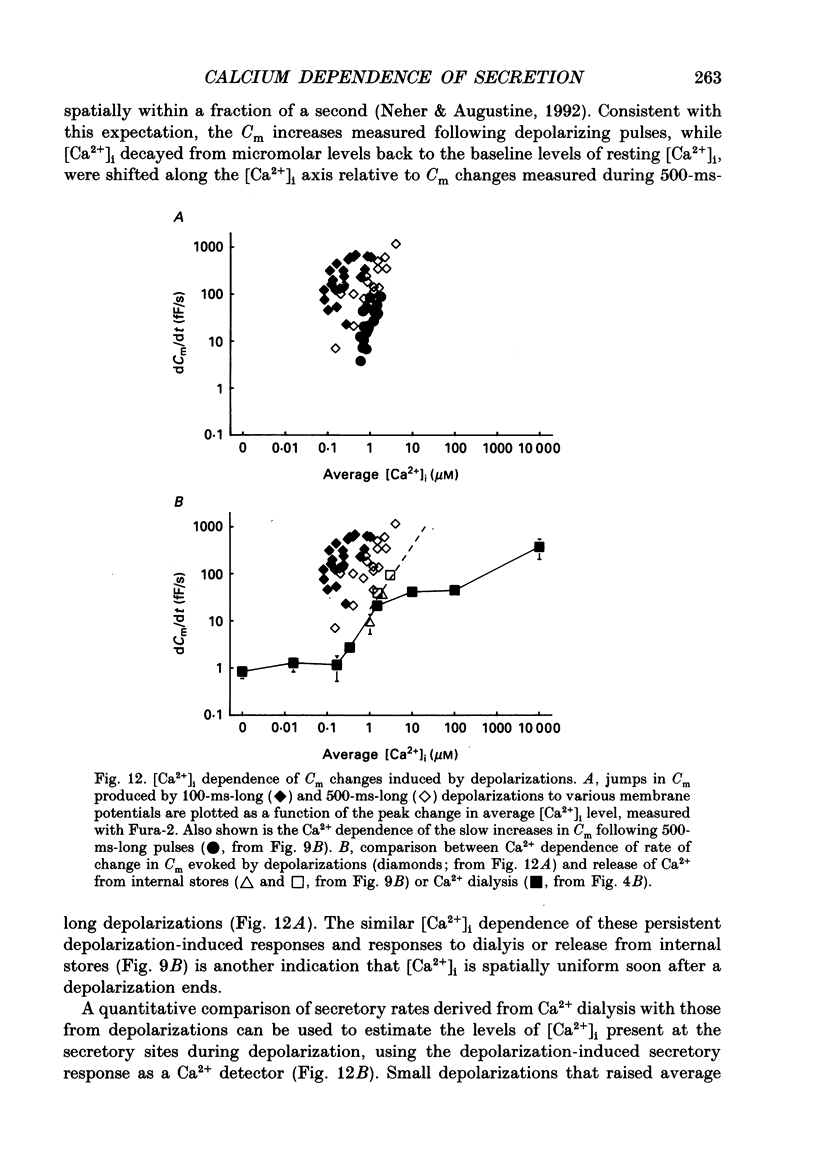
1. Measurements of membrane capacitance and intracellular Ca2+ concentration, [Ca2+]i, were used to examine the Ca2+ dependence of secretion in single adrenal chromaffin cells. 2. Intracellular dialysis of Ca2+, through a patch pipette, promoted secretion; the rate of secretion increased monotonically as [Ca2+]i was elevated, while the total amount of secretion reached a maximum at 1.5 microM-Ca2+ and declined at high [Ca2+]i. 3. Release of Ca2+ from internal stores, using bradykinin or ionomycin, transiently elevated [Ca2+]i and the rate of secretion. 4. Considering responses to both Ca2+ dialysis and release from internal stores, it appears that the rate of secretion increases over a range of [Ca2+]i levels above 0.2 microM and saturates at concentrations greater than 10 microM, if at all. Secretion appears to have a Hill coefficient for Ca2+ of about 2. At [Ca2+]i greater than 1-2 microM, prolonged elevation of [Ca2+]i, via dialysis, produced lower rates of secretion than transient elevation of [Ca2+]i caused by release from internal stores. This may have been caused by a depletion of readily releasable chromaffin granules during prolonged elevation of [Ca2+]i. 5. Brief depolarizing pulses produced transient rises in both [Ca2+]i and the rate of secretion. The ability of these pulses to evoke secretion 'washed out' during prolonged intracellular dialysis, due to both reduced Ca2+ influx and a diminished ability of the cell to secrete in response to a given Ca2+ load. 6. The kinetics of the secretory response depended upon the size of the depolarization-induced Ca2+ load; small rises in [Ca2+]i increased membrane capacitance only during the depolarization, while larger rises in [Ca2+]i produced increases both during and following the depolarization. The secretory responses that outlasted the depolarization appeared to be due to persistent elevation of [Ca2+]i. Secretory responses were sometimes followed by a slower decline in membrane capacitance, probably due to endocytosis of membrane. 7. Comparison of the rates of secretion measured during depolarization to those produced by Ca2+ dialysis or release from internal stores suggests that [Ca2+]i at secretory sites can exceed 10 microM during depolarization. The spatially averaged measurements of [Ca2+]i indicate much smaller levels of [Ca2+]i; thus, there must be pronounced spatial gradients of [Ca2+]i during depolarization.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler E. M., Augustine G. J., Duffy S. N., Charlton M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991 Jun;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert P. R., Tashjian A. H., Jr Relationship of thyrotropin-releasing hormone-induced spike and plateau phases in cytosolic free Ca2+ concentrations to hormone secretion. Selective blockade using ionomycin and nifedipine. J Biol Chem. 1984 Dec 25;259(24):15350–15363. [PubMed] [Google Scholar]
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989 Jul 27;340(6231):313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Almers W., Neher E. Gradual and stepwise changes in the membrane capacitance of rat peritoneal mast cells. J Physiol. 1987 May;386:205–217. doi: 10.1113/jphysiol.1987.sp016530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Buchanan J. A., Charlton M. P., Osses L. R., Smith S. J. Fingering the trigger for neurotransmitter secretion: studies on the calcium channels of squid giant presynaptic terminals. Soc Gen Physiol Ser. 1989;44:203–223. [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P. Calcium dependence of presynaptic calcium current and post-synaptic response at the squid giant synapse. J Physiol. 1986 Dec;381:619–640. doi: 10.1113/jphysiol.1986.sp016347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. doi: 10.1146/annurev.ne.10.030187.003221. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium entry into voltage-clamped presynaptic terminals of squid. J Physiol. 1985 Oct;367:143–162. doi: 10.1113/jphysiol.1985.sp015818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D. Secretory vesicle-associated proteins and their role in exocytosis. Annu Rev Physiol. 1990;52:647–659. doi: 10.1146/annurev.ph.52.030190.003243. [DOI] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984 May;45(5):993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek T. R., Jackson T. R., O'Sullivan A. J., Moreton R. B., Berridge M. J., Burgoyne R. D. Simultaneous measurements of cytosolic calcium and secretion in single bovine adrenal chromaffin cells by fluorescent imaging of fura-2 in cocultured cells. J Cell Biol. 1989 Sep;109(3):1219–1227. doi: 10.1083/jcb.109.3.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Trifluoperazine reduces inward ionic currents and secretion by separate mechanisms in bovine chromaffin cells. J Physiol. 1984 Aug;353:541–564. doi: 10.1113/jphysiol.1984.sp015350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshenko P., Penner R., Neher E. Novel chloride conductance in the membrane of bovine chromaffin cells activated by intracellular GTP gamma S. J Physiol. 1991 May;436:711–724. doi: 10.1113/jphysiol.1991.sp018575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L. A., Holz R. W. Catecholamine secretion from digitonin-treated adrenal medullary chromaffin cells. J Biol Chem. 1983 Apr 25;258(8):4989–4993. [PubMed] [Google Scholar]
- Eberhard D. A., Cooper C. L., Low M. G., Holz R. W. Evidence that the inositol phospholipids are necessary for exocytosis. Loss of inositol phospholipids and inhibition of secretion in permeabilized cells caused by a bacterial phospholipase C and removal of ATP. Biochem J. 1990 May 15;268(1):15–25. doi: 10.1042/bj2680015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Fogelson A. L., Zucker R. S. Presynaptic calcium diffusion from various arrays of single channels. Implications for transmitter release and synaptic facilitation. Biophys J. 1985 Dec;48(6):1003–1017. doi: 10.1016/S0006-3495(85)83863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V., Weber K. Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J. 1984 Jan;3(1):227–233. doi: 10.1002/j.1460-2075.1984.tb01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Landis D. M. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J Neurocytol. 1974 Mar;3(1):109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Smith S. J. Large depolarization induces long openings of voltage-dependent calcium channels in adrenal chromaffin cells. J Neurosci. 1987 Feb;7(2):571–580. doi: 10.1523/JNEUROSCI.07-02-00571.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B. The cell biology of the nerve terminal. Neuron. 1988 Aug;1(6):431–438. doi: 10.1016/0896-6273(88)90174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. T., Westhead E. W. Cellular responses to Ca2+ from extracellular and intracellular sources are different as shown by simultaneous measurements of cytosolic Ca2+ and secretion from bovine chromaffin cells. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9881–9885. doi: 10.1073/pnas.86.24.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. I., Neher E. IgG from patients with Lambert-Eaton syndrome blocks voltage-dependent calcium channels. Science. 1988 Jan 22;239(4838):405–408. doi: 10.1126/science.2447652. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Kesteven N. T. Evoked transient intracellular free Ca2+ changes and secretion in isolated bovine adrenal medullary cells. Proc R Soc Lond B Biol Sci. 1983 May 23;218(1211):177–199. doi: 10.1098/rspb.1983.0033. [DOI] [PubMed] [Google Scholar]
- Konishi M., Olson A., Hollingworth S., Baylor S. M. Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys J. 1988 Dec;54(6):1089–1104. doi: 10.1016/S0006-3495(88)83045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim N. F., Nowycky M. C., Bookman R. J. Direct measurement of exocytosis and calcium currents in single vertebrate nerve terminals. Nature. 1990 Mar 29;344(6265):449–451. doi: 10.1038/344449a0. [DOI] [PubMed] [Google Scholar]
- Lindau M., Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988 Feb;411(2):137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man-Son-Hing H., Zoran M. J., Lukowiak K., Haydon P. G. A neuromodulator of synaptic transmission acts on the secretory apparatus as well as on ion channels. Nature. 1989 Sep 21;341(6239):237–239. doi: 10.1038/341237a0. [DOI] [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Zimmerberg J. Diffusion into the patch-clamp recording pipette of a factor necessary for muscarinic current response. Cell Signal. 1989;1(3):259–268. doi: 10.1016/0898-6568(89)90043-0. [DOI] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan A. J., Cheek T. R., Moreton R. B., Berridge M. J., Burgoyne R. D. Localization and heterogeneity of agonist-induced changes in cytosolic calcium concentration in single bovine adrenal chromaffin cells from video imaging of fura-2. EMBO J. 1989 Feb;8(2):401–411. doi: 10.1002/j.1460-2075.1989.tb03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva C., Cohen I. S., Mathias R. T. Calculation of time constants for intracellular diffusion in whole cell patch clamp configuration. Biophys J. 1988 Nov;54(5):791–799. doi: 10.1016/S0006-3495(88)83017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzak A., Böck G., Fischer-Colbrie R., Schauenstein K., Schmidt W., Lingg G., Winkler H. Exocytotic exposure and retrieval of membrane antigens of chromaffin granules: quantitative evaluation of immunofluorescence on the surface of chromaffin cells. J Cell Biol. 1984 May;98(5):1817–1824. doi: 10.1083/jcb.98.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R., Neher E., Dreyer F. Intracellularly injected tetanus toxin inhibits exocytosis in bovine adrenal chromaffin cells. Nature. 1986 Nov 6;324(6092):76–78. doi: 10.1038/324076a0. [DOI] [PubMed] [Google Scholar]
- Penner R., Neher E. The role of calcium in stimulus-secretion coupling in excitable and non-excitable cells. J Exp Biol. 1988 Sep;139:329–345. doi: 10.1242/jeb.139.1.329. [DOI] [PubMed] [Google Scholar]
- Penner R., Pusch M., Neher E. Washout phenomena in dialyzed mast cells allow discrimination of different steps in stimulus-secretion coupling. Biosci Rep. 1987 Apr;7(4):313–321. doi: 10.1007/BF01121453. [DOI] [PubMed] [Google Scholar]
- Perrin D., Langley O. K., Aunis D. Anti-alpha-fodrin inhibits secretion from permeabilized chromaffin cells. Nature. 1987 Apr 2;326(6112):498–501. doi: 10.1038/326498a0. [DOI] [PubMed] [Google Scholar]
- Pumplin D. W., Reese T. S., Llinás R. Are the presynaptic membrane particles the calcium channels? Proc Natl Acad Sci U S A. 1981 Nov;78(11):7210–7213. doi: 10.1073/pnas.78.11.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Raju B., Murphy E., Levy L. A., Hall R. D., London R. E. A fluorescent indicator for measuring cytosolic free magnesium. Am J Physiol. 1989 Mar;256(3 Pt 1):C540–C548. doi: 10.1152/ajpcell.1989.256.3.C540. [DOI] [PubMed] [Google Scholar]
- Reichardt L. F., Kelly R. B. A molecular description of nerve terminal function. Annu Rev Biochem. 1983;52:871–926. doi: 10.1146/annurev.bi.52.070183.004255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M., Jacobs R. A., Hudspeth A. J. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990 Nov;10(11):3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R., Adler E. M., Charlton M. P. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990 Dec;5(6):773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- Sala F., Hernández-Cruz A. Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J. 1990 Feb;57(2):313–324. doi: 10.1016/S0006-3495(90)82533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian T., Aunis D., Bader M. F. Loss of proteins from digitonin-permeabilized adrenal chromaffin cells essential for exocytosis. J Biol Chem. 1987 Dec 5;262(34):16671–16676. [PubMed] [Google Scholar]
- Schweizer F. E., Schäfer T., Tapparelli C., Grob M., Karli U. O., Heumann R., Thoenen H., Bookman R. J., Burger M. M. Inhibition of exocytosis by intracellularly applied antibodies against a chromaffin granule-binding protein. Nature. 1989 Jun 29;339(6227):709–712. doi: 10.1038/339709a0. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Stauderman K. A., Pruss R. M. Dissociation of Ca2+ entry and Ca2+ mobilization responses to angiotensin II in bovine adrenal chromaffin cells. J Biol Chem. 1989 Nov 5;264(31):18349–18355. [PubMed] [Google Scholar]
- Thomas P., Surprenant A., Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990 Nov;5(5):723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- Wilson S. P., Kirshner N. Calcium-evoked secretion from digitonin-permeabilized adrenal medullary chromaffin cells. J Biol Chem. 1983 Apr 25;258(8):4994–5000. [PubMed] [Google Scholar]
- Zimmerberg J., Sardet C., Epel D. Exocytosis of sea urchin egg cortical vesicles in vitro is retarded by hyperosmotic sucrose: kinetics of fusion monitored by quantitative light-scattering microscopy. J Cell Biol. 1985 Dec;101(6):2398–2410. doi: 10.1083/jcb.101.6.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Grafenstein H., Roberts C. S., Baker P. F. Kinetic analysis of the triggered exocytosis/endocytosis secretory cycle in cultured bovine adrenal medullary cells. J Cell Biol. 1986 Dec;103(6 Pt 1):2343–2352. doi: 10.1083/jcb.103.6.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]


