Abstract
Background
Patients who developed immune-related adverse events (irAEs) could benefit more from treatment with immune checkpoint inhibitors (ICIs) than those who did not develop irAEs. This study was designed to assess whether the occurrence of irAEs or their characteristics are correlated with survival in advanced patients treated with ICIs.
Methods
This retrospective cohort study enrolled a panel of cancer patients who received ICIs at a single institute. Kaplan‒Meier curves were generated to describe progression-free survival (PFS) and overall survival (OS) in patients with irAEs or specific irAE characteristics.
Results
A total of 238 patients were enrolled, 83 (34.9%) of whom developed at least one irAE. Overall, irAE development was associated with prolonged OS (not reached vs. 17.8 months, P < 0.001), PFS (8.7 vs. 4.8 months, P = 0.003), and an improved objective response rate (24.1% vs. 10.3%, P = 0.005). Furthermore, only skin or endocrine toxicities were associated with improved OS and PFS. On the basis of the results from organ-specific irAEs, the first development of skin or endocrine toxicities as protective irAEs rather than other irAEs was an independent indicator for predicting OS (P < 0.001) and PFS (P < 0.001). A protective irAE burden score based on organ-specific irAEs was further developed to show the significant protective effect of total irAEs on patient outcomes.
Conclusions
Not all irAEs are associated with prolonged survival. The identification of organ-specific irAEs is useful for stratifying patients who actually respond to and benefit from ICIs across different cancer types.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-13566-6.
Keywords: Immune-related adverse events, Immune checkpoint inhibitor, Organ specific, Prognosis, Advanced cancer
Background
Treatment with immune checkpoint inhibitors (ICIs), including cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1)/programmed cell death ligand-1 (PD-L1) inhibitors, has achieved extraordinary response rates and revolutionized the systematic management of various malignancies at early or advanced stages. Unlike conventional cytotoxic agents and targeted therapies that act directly to impair or destroy cancer cells, ICIs selectively restore and normalize the body’s antitumor immune responses by disrupting the immunoinhibitory signals mediated by the PD-1/PD-L1 and CTLA-4 axes [1]. Biomarkers reflecting the tumor immune microenvironment and intrinsic tumor cell features, such as PD-L1 expression, density of tumor-infiltrating lymphocytes, tumor mutational burden, and mismatch repair deficiency (dMMR)/microsatellite instability-high (MSI-H) status, are associated with the treatment effect of anti-PD-1/anti-PD-L1 therapy [2]. However, these reported biomarkers are not definitive predictors, and patients with positive PD-L1 expression may not always respond well to ICI therapy [3]. Thus, the identification of other indicators is needed to precisely predict the response of patients to ICIs and optimize clinical treatment decisions.
At present, several predictive and prognostic indicators for immunotherapy are under clinical investigation. Patient characteristics, including Eastern Cooperative Oncology Group (ECOG) performance status (PS), nutritional status, pretreatment neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammation index (SII), and endogenous glucocorticoid levels, have been reported to be correlated with treatment efficacy and survival in cancer patients treated with ICIs [4–9]. Recent studies have shown that certain metastatic sites, including the liver, bone, and pleura, are associated with poor immunotherapy outcomes [10, 11]. Additionally, the occurrence of immune-related adverse events (irAEs) presenting as autoimmune-like or inflammatory conditions following immunotherapy is a potentially useful on-treatment clinical predictive factor of patient outcomes. Patients who develop irAEs can benefit from treatment with ICIs for non-small cell lung cancer (NSCLC), hepatocellular carcinoma, and other tumor types [12–19]. Although the mechanisms of irAEs remain unclear, cumulative evidence reveals that irAEs represent an immune response to shared antigens between tumor cells and specific healthy organs [20]. Different characteristics of irAEs, such as severity, the number of systems or organs affected [21], the timing of onset [22, 23], and the specific organs affected, may affect the clinical benefit [24]. Notably, most of the available data revealing the correlation between the occurrence of irAEs and treatment efficacy and patient prognosis have focused only on specific irAE characteristics or tumor types. Here, we aimed to comprehensively dissect irAEs that occurred in a real-world cohort of solid tumor patients treated with immunotherapy and to determine the associations of specific irAE characteristics with patient outcomes.
Methods
Study population
This retrospective, single-institute, cohort study enrolled Chinese patients with advanced solid cancers who presented to the First Affiliated Hospital of Shandong First Medical University and were treated with ICIs from December 2019 to August 2023. The inclusion criteria were as follows: ≥18 years of age; histologically or cytologically confirmed locally advanced or metastatic solid cancer; receiving at least two cycles of PD-1/PD-L1 inhibitor (nivolumab, pembrolizumab, camrelizumab, sintilimab, tislelizumab, durvalumab, or atezolizumab) monotherapy or combination with chemotherapy or targeted therapy as a first, second or later line of treatment according to the National Comprehensive Cancer Network guidelines and guidelines of the Chinese Society of Clinical Oncology regarding a wide range of cancer types; experiencing at least one radiographic evaluation and follow-up visit; and having an ECOG PS of 0–2. Patients whose baseline lesions were not measurable via CT or MRI, whose response to therapy was not evaluated after the initiation of immunotherapy, who were suffering from multiple cancer types or autoimmune diseases, or whose history of immunotherapy with non-ICIs were excluded. The reporting of this study conforms to the REMARK statement (Method S1) [25].
Clinical annotation
The data were gathered through electronic medical records. The clinical characteristics of the patients included age, sex, ECOG PS, smoking status, body mass index, histology, complete blood analysis before and after immunotherapy initiation, and tumor node metastasis (TNM) stage at the start of treatment. The NLR and PLR were calculated by dividing the absolute number of peripheral blood neutrophils and the platelet count, respectively, by the lymphocyte count. The SII was calculated as the platelet × neutrophil count/lymphocyte count. Multiorgan irAEs were defined as 2 or more systems or organs that subsequently developed toxicities after ICI initiation. We used 3 months as a cutoff to define early-onset (≤ 3 months from ICI start) and late-onset (> 3 months from ICI start) irAEs according to previous methods [23]. The protective (associated with improved survival) or nonprotective irAEs (not associated with improved survival) referred to organ-specific irAEs that occurred in an individual patient for the first time after ICI initiation. We calculated the irAE duration as the interval between the first ICI dose and the first occurrence of any irAEs. Progression-free survival (PFS) was defined as the date of immunotherapy initiation to the date of disease progression or death from any cause, whichever occurred first. Patients who were alive without disease progression were censored on the date of their last disease assessment. Overall survival (OS) was defined as the time from immunotherapy initiation to death from any cause. Patients who were still alive were censored at the date of last contact. The objective response according to the radiographic findings was assessed as CR, PR, SD, or PD according to RECIST 1.1. The objective response rate (ORR) was defined as the proportion of patients who achieved a CR or PR after immunotherapy. The disease control rate (DCR) was defined as the percentage of patients who achieved CR, PR, or SD after immunotherapy.
Statistical analysis
Categorical variables, such as patient demographics, disease characteristics, and medical history, are reported as frequencies and percentages. Quantitative variables are presented as medians and ranges. For categorical variables, we used chi-square tests or Fisher’s exact tests (for nonnormally distributed groups of data) to compare patient characteristics among different groups. For continuous variables, independent sample t tests or Mann‒Whitney U tests (for nonnormally distributed groups of data) were used to compare patient groups. PFS and OS were compared via the Kaplan–Meier method and the log-rank test. To confirm that differences in Kaplan–Meier survival curves between patients with and without irAEs could not be attributed to immortal time bias, we used a 3-month landmark analysis. In the 3-month landmark analysis, patients who experienced an event within 3 months of ICI initiation and those who experienced irAEs after 3 months of ICI initiation were excluded. Univariate and multivariate Cox proportional hazards regression models were fitted to evaluate the associations between the endpoints and the organ-specific irAE prognosis, which were modeled as time-dependent variables. Several disease characteristics were considered as adjustment factors in the multivariable models: age, sex, BMI, smoking history, ECOG PS, clinical stage, distant metastasis, ICI type, treatment line, ICI treatment, radiation history, surgical history, and ANA status. To analyze the effect of total protective organ-specific irAEs on patient prognosis, a protective irAE burden score was obtained by summing the number of significant protective irAEs determined by the hazard ratio (HR) direction and P value according to a previous method [24]. This protective irAE burden score ranged from 0 to 2:1 for skin toxicity, 1 for endocrine toxicity, and 0 for other nonprotective toxicities. SPSS 26.0 software (SPSS, Inc., Chicago, IL, USA) was used for all the statistical tests. All the statistical tests were two-tailed, and P < 0.05 was used to indicate statistical significance.
Results
Patient characteristics
Among the cancer patients receiving immunotherapy, 24 were excluded because they did not have measurable lesions, because they received only one cycle of immunotherapy, or because they were not evaluable after immunotherapy. A total of 238 patients were ultimately enrolled. The most common malignancies were gastric cancer (29.8%), NSCLC (20.6%), esophageal cancer (6.3%), and hepatocellular carcinoma (HCC; 6.3%). The median follow-up time was 19.1 months. The median age was 61.0 years, and 27.3% were female. In the total cohort, most patients (92.9%) received combination immunotherapy, and seventeen patients (7.1%) received ICI monotherapy. Patients received a median of two previous lines of systematic therapy for advanced disease. For the entire population, the ORR and DCR were 15.1% and 80.3%, respectively. The median OS and PFS were 22.2 months (95% CI: 18.7–25.8) and 6.5 months (95% CI: 5.4–7.7), respectively. The patients’ baseline characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of included patients with advanced or metastatic cancer
| Characteristics | All | With irAEs | No irAEs | p |
|---|---|---|---|---|
| Age, years, median (IQR) | 61 (54–68) | 63 (54–68) | 61 (52–68) | 0.432 |
| Glucocorticoid, nmol/L, median (IQR) | 321.5 (210.7-414.1) | 299.2 (184.2-390.5) | 346.3 (239.1-455.2) | 0.018 |
| LYM, × 109/L, median (IQR) | 1.32 (1-1.75) | 1.3 (1.02–1.76) | 1.32 (0.99–1.71) | 0.969 |
| ALB, g/L, median (IQR) | 41 (36–44) | 41 (37–45) | 40 (36–44) | 0.651 |
| PAB, mg/L | 201 (144–262) | 219 (162–274) | 192 (137–251) | 0.202 |
| NLR, median (IQR) | 2.8 (1.8–4.5) | 2.7 (1.7–4.4) | 2.9 (1.9–4.5) | 0.515 |
| PLR, median (IQR) | 154.5 (105.9-230.2) | 143.4 (103.6-217.1) | 162 (107.1-244.5) | 0.324 |
| SII, median (IQR) | 564.8 (312.5-1037.7) | 556.2 (281.7-1016.7) | 565.6 (317.5-1095.1) | 0.324 |
| n (%) | n (%) | |||
| Gender | 0.154 | |||
| Male | 173 (72.7) | 65 (78.3) | 108 (69.7) | |
| Female | 65 (27.3) | 18 (21.7) | 47 (30.3) | |
| BMI, kg/m2 | 0.396 | |||
| < 25 | 177 (74.4) | 59 (71.1) | 118 (76.1) | |
| ≥ 25 | 61 (25.6) | 24 (28.9) | 37 (23.9) | |
| Smoking history | 0.773 | |||
| No | 126 (52.9) | 45 (54.2) | 81 (52.3) | |
| Yes | 112 (47.1) | 38 (45.8) | 74 (47.7) | |
| ECOG PS | 0.313 | |||
| 0–1 | 228 (95.8) | 81 (97.6) | 147 (94.8) | |
| 2 | 10 (4.2) | 2 (2.4) | 8 (5.2) | |
| Clinical stage | 0.894 | |||
| Stage III | 47 (19.7) | 16 (19.3) | 31 (20.0) | |
| Stage IV | 191 (80.3) | 67 (80.7) | 124 (80.0) | |
| Distant metastasis | 0.482 | |||
| No | 52 (21.8) | 16 (19.3) | 36 (23.2) | |
| Yes | 186 (78.2) | 67 (80.7) | 119 (76.8) | |
| ICI types | 0.536 | |||
| anti-PD-1 antibody | 225 (94.5) | 80 (96.4) | 145 (93.5) | |
| anti-PD-L1 antibody | 13 (5.5) | 3 (3.6) | 10 (6.5) | |
| Therapy line | 0.317 | |||
| 1 | 108 (45.4) | 34 (41.0) | 74 (47.7) | |
| ≥ 2 | 130 (54.6) | 49 (59.0) | 81 (52.3) | |
| ICI Treatment | 0.571 | |||
| Monotherapy | 17 (7.1) | 7 (8.4) | 10 (6.5) | |
| Combination | 221 (92.9) | 76 (91.6) | 145 (93.5) | |
| Radiation history | 0.135 | |||
| No | 193 (81.1) | 63 (75.9) | 130 (83.9) | |
| Yes | 45 (18.9) | 20 (24.1) | 25 (16.1) | |
| Surgical history | 0.097 | |||
| No | 115 (48.3) | 34 (41.0) | 81 (52.3) | |
| Yes | 123 (51.7) | 49 (59.0) | 74 (47.7) | |
| ANA | 0.580 | |||
| Negative | 54 (22.7) | 16 (24.5) | 38 (24.5) | |
| Positive | 84 (35.3) | 29 (14.8) | 55 (35.5) | |
| Unknown | 100 (42.0) | 38 (40.6) | 62 (40.0) | |
| CRP, mg/L | 0.043 | |||
| < 3.69 | 69 (29.0) | 32 (38.6) | 37 (23.9) | |
| ≥ 3.69 | 91 (38.2) | 25 (30.1) | 66 (42.6) | |
| Unknown | 78 (32.8) | 26 (31.3) | 52 (33.5) |
ALB, albumin; ANA, antinuclear antibody; BMI, body mass index; CRP, C-reactive protein; ICI, immune checkpoint inhibitor; irAEs, immune-related adverse events; NLR, neutrophil to lymphocyte ratio; PAB, Prealbumin; PD-1, programmed cell death-1; PD-L1, programmed death ligand 1; PLR, platelet to lymphocyte ratio; ECOG PS, Eastern Cooperative Oncology Group performance status; SII, systemic immune inflammation index; LYM: lymphocyte
The percentage of irAEs
Among the patients in this cohort, 83 (34.9%) developed one or more irAEs. Among patients with irAEs, 65 (78.3%) developed grade 1–2 toxicities, and 18 (21.7%) developed at least one grade 3–4 toxicity. Patients with irAEs were more likely to have reduced baseline serum glucocorticoid (P = 0.018) and C-reactive protein (CRP) (P = 0.043) levels (Table 1). The most common irAEs were skin (n = 23), pulmonary (n = 20), and endocrine (n = 15) toxicities (Table 2). The median time from ICI initiation to the occurrence of the first irAE was 3.4 months. A total of 22 (9.2%) and 48 (20.2%) patients developed multiorgan irAEs and late-onset irAEs, respectively. Among patients with multiorgan irAEs, a majority of patients (n = 17, 77.3%) developed 2-organ irAEs (Table S1).
Table 2.
Summary of incidence and severity of irAEs recorded in the study population
| Toxicity | All irAEs (n = 83), n (%) | G1-2 irAEs (n = 65), n (%) | G3-4 irAEs (n = 18), n (%) |
|---|---|---|---|
| Skin toxicity | 23 (27.7) | 21 (32.3) | 2 (11.1) |
| Rash | 11 (13.3) | 10 (15.4) | 2 (11.1) |
| RCCEP | 5 (6.0) | 5 (7.7) | 0 (0) |
| Psoriasis | 3 (3.6) | 3 (4.6) | 0 (0) |
| Others | 6 (7.2) | 5 (7.7) | 0 (0) |
| Pulmonary toxicity | 20 (24.1) | 14 (21.5) | 6 (33.3) |
| Endocrine toxicity | 15 (18.1) | 14 (21.5) | 1 (5.6) |
| Hypothyroidism | 6 (7.2) | 6 (9.2) | 0 (0) |
| Hypoadrenocorticism | 6 (7.2) | 6 (9.2) | 0 (0) |
| Diabetes | 1 (1.2) | 0 (0) | 1 (5.6) |
| Hyperthyroidism | 3 (3.6) | 2 (3.1) | 0 (0) |
| Cardiotoxicity | 8 (9.6) | 6 (9.2) | 2 (11.1) |
| Digestive system toxicity | 10 (12.0) | 5 (7.7) | 5 (27.8) |
| Gastrointestinal toxicity | 1 (1.2) | 0 (0) | 1 (5.6) |
| Pancreatic toxicity | 4 (4.8) | 2 (3.1) | 2 (11.1) |
| Hepatotoxicity | 5 (6.0) | 3 (4.6) | 2 (11.1) |
| Bone and joint and muscle toxicity | 4 (4.8) | 3 (4.6) | 1 (5.6) |
| Renal toxicity | 2 (2.4) | 2 (3.1) | 0 (0) |
| Other | 1 (1.2) | 0 (0) | 1 (5.6) |
irAEs: immune-related adverse events; RCCEP: reactive cutaneous capillary endothelial proliferation;
Associations between overall irAEs and survival
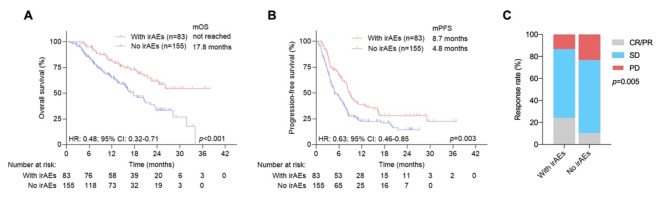
We first analyzed the associations between overall irAEs and patient outcomes. According to the univariate analysis, the occurrence of any irAEs (hazard ratio (HR): 0.48, P < 0.001) was associated with longer OS. The median OS for patients with and without irAEs was not reached and 17.8 months, respectively (Fig. 1A). Patients with irAEs had longer PFS than did those without irAEs (HR: 0.63, P = 0.003), with a median PFS of 8.7 vs. 4.8 months (Fig. 1B). Furthermore, the ORR was significantly greater in patients with irAEs (24.1%) than in patients without irAEs (10.3%) (P = 0.005) (Fig. 1C). The occurrence of irAEs was further confirmed to stratify patients with different prognoses in terms of PFS and OS in patients with different tumor types or different anticancer treatments (Fig. S1). In particular, patients with gastric cancer experiencing any type of toxicity had a significantly longer median OS (HR: 0.25, P = 0.009) and PFS (HR: 0.38, P = 0.01) than those with no toxicity.
Fig. 1.
Kaplan‒Meier survival curves for survival and objective response rates for advanced cancer patients with or without irAEs. (A) Kaplan‒Meier survival curves for OS in all populations. (B) Kaplan‒Meier survival curves for PFS in all populations. (C) Objective response rates for advanced cancer patients with or without irAEs
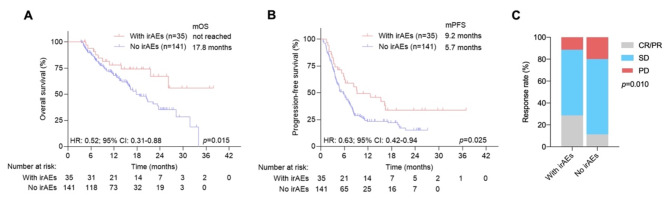
To alleviate the impact of immortal time bias, we used a 3-month landmark analysis in which patients who died prior to 3 months of ICI initiation or developed irAEs after 3 months of ICI initiation were excluded. There was a significant association with longer OS (HR: 0.52, P = 0.015) and PFS (HR: 0.63, P = 0.025) for patients with irAEs than for patients without irAEs (Fig. 2A and B). The ORR was significantly greater in patients with irAEs than in patients without irAEs according to the 3-month landmark analysis (Fig. 2C).
Fig. 2.
Kaplan‒Meier survival curves for survival and objective response rates for advanced cancer patients with or without irAEs according to the 3-month landmark analysis. (A) Kaplan‒Meier survival curves for OS in all populations. (B) Kaplan‒Meier survival curves for PFS in all populations. (C) Objective response rates for advanced cancer patients with or without irAEs
Associations between irAE characteristics and survival
Previous studies have indicated that several clinical characteristics of irAEs, including their severity, number of involved organs, and timing, significantly affect treatment efficacy and patient outcomes. We next determined whether these irAE features were associated with treatment efficacy and survival. Univariate analysis revealed that patients who developed grade 3–4 irAEs had similar OS (HR: 0.86, P = 0.732) but shorter PFS (HR: 0.48, P = 0.038) than did those who developed grade 1–2 irAEs (Fig. S2). The median OS and PFS were not significantly different between patients with single-organ and multiple-organ irAEs (for OS, hazard ratio (HR): 1.13, P = 0.783; for PFS, HR: 1.31, P = 0.355) or between patients with early-onset and late-onset irAEs (for OS, HR: 0.94, P = 0.883; for PFS, HR: 0.80, P = 0.402) (Figs. S3 and S4). The ORRs were also comparable in patients with grade 3–4 vs. grade 1–2 irAEs (16.7% vs. 26.2%, P = 0.602), single-organ vs. multiple-organ irAEs (23.0% vs. 27.3%, P = 0.684), and early-onset vs. late-onset irAEs (28.6% vs. 20.8%, P = 0.416) (Fig. S2-4).
Associations between organ-specific irAEs and survival
To determine which patients with irAEs had a good prognosis, we analyzed the associations between system-specific or organ-specific irAEs and survival. According to the univariate analysis, the occurrence of skin (HR: 0.33, P = 0.016) or endocrine toxicities (HR: 0.20, P = 0.022) was associated with longer OS. Patients with skin toxicity (HR: 0.43, P = 0.008) or endocrine toxicity (HR: 0.52, P = 0.073) had longer PFS than those without skin or endocrine toxicity. Additionally, multivariate analysis revealed that only skin toxicity (for OS, hazard ratio (HR): 0.39, P = 0.04; for PFS, HR: 0.43, P = 0.008) and endocrine toxicity (for OS, HR: 0.18, P = 0.018; for PFS, HR: 0.48, P = 0.046) were significantly associated with improved OS and PFS (Table 3).
Table 3.
Cox proportional hazards regression models for OS and PFS in the entire study population with organ-specific irAEs
| Toxicity | OS, Univariable | OS, Multivariable | PFS, Univariable | PFS, Multivariable | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||||||
| Skin toxicity | 0.33 (0.13–0.81) | 0.016 | 0.39 (0.16–0.96) | 0.040 | 0.43 (0.24–0.80) | 0.008 | 0.43 (0.23–0.80) | 0.008 | ||||||
| Endocrine toxicity | 0.20 (0.05–0.79) | 0.022 | 0.18 (0.04–0.74) | 0.018 | 0.52 (0.26–1.06) | 0.073 | 0.48 (0.24–0.99) | 0.046 | ||||||
| Bone and joint and muscle toxicity | 1.89 (0.60–5.96) | 0.279 | 2.40 (0.74–7.80) | 0.147 | 1.59 (0.59–4.30) | 0.359 | 1.92 (0.70–5.22) | 0.204 | ||||||
| Pulmonary toxicity | 0.89 (0.45–1.77) | 0.738 | 0.65 (0.30–1.41) | 0.279 | 1.10 (0.67–1.82) | 0.706 | 0.95 (0.56–1.62) | 0.841 | ||||||
| Cardiotoxicity | 0.78 (0.25–2.47) | 0.677 | 0.72 (0.22–2.31) | 0.575 | 0.76 (0.34–1.72) | 0.506 | 0.67 (0.29–1.55) | 0.351 | ||||||
| Digestive system toxicity | 0.65 (0.21–2.05) | 0.461 | 0.68 (0.21–2.16) | 0.511 | 0.83 (0.39–1.76) | 0.618 | 0.84 (0.39–1.79) | 0.649 | ||||||
| Renal toxicity | 1.88 (0.46–7.63) | 0.379 | 2.50 (0.60-10.42) | 0.208 | 0.83 (0.21–3.34) | 0.790 | 0.99 (0.24–4.04) | 0.987 | ||||||
OS: overall survival; PFS: progression-free survival; HR: hazard ratio; irAEs: immune-related adverse events
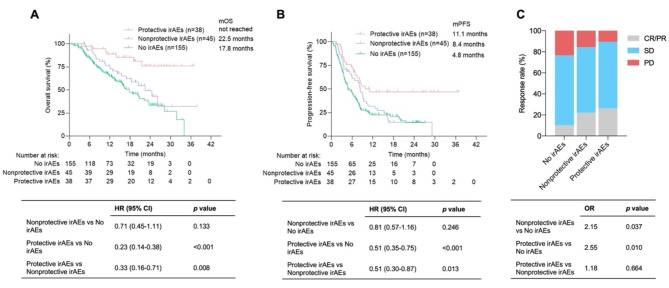
Considering that only the occurrence of skin or endocrine toxicities but not other organ toxicities was associated with improved survival according to the multivariate analysis, we defined skin or endocrine toxicities as protective organ-specific irAEs (n = 38) and others (n = 45) as nonprotective organ-specific irAEs. According to the univariate analysis, patients whose first irAE was protective had longer OS (mOS: not reached vs. 22.5 months, HR: 0.33, P = 0.008) and PFS (mPFS: 11.1 vs. 8.4 months, HR: 0.51, P = 0.013) than those whose first irAE was nonprotective (Fig. 3A and B). Multivariate analysis revealed that the first occurrence of protective irAEs was an independent indicator for predicting improved OS (HR: 0.24, P < 0.001) and PFS (HR: 0.43, P < 0.001) (Table S2). The ORR was 26.3% for patients with protective irAEs and 22.2% for patients with nonprotective irAEs. The ORR was associated with protective (P = 0.01) and nonprotective (P = 0.037) irAEs compared with patients with no irAEs (Fig. 3C). Furthermore, nonprotective irAEs were more likely to be grade 3–4 than protective irAEs were (33.3% vs. 7.9%, P = 0.005). To analyze the effect of total organ-specific irAEs following immunotherapy on patient prognosis, a protective irAE burden score was established on the basis of the number of protective irAEs (with 1 point for skin or endocrine toxicity), ranging from 0 to 2 points (Fig. 4A). The classification of the irAE cohort according to the score revealed 49.4% with 0 points, 42.2% with 1 point, and 8.4% with 2 points (Fig. 4B). Patients with 2 (HR: 0.28, P = 0.028) or 1 (HR: 0.39, P = 0.015) points had longer OS than those with 0 points (Fig. 4C). A score of 2 points was marginally associated with improved PFS (HR: 0.45, P = 0.056) (Fig. 4D), and a score of 1 point was significantly associated with prolonged PFS (HR: 0.50, P = 0.014). However, there were significant differences between irAEs with scores of 0 and 1 in terms of OS (Fig. 4C) and PFS (Fig. 4D).
Fig. 3.
Kaplan‒Meier survival curves for survival and objective response rates for advanced cancer patients with protective and nonprotective organ-specific irAEs. (A) Kaplan‒Meier survival curves for OS. (B) Kaplan‒Meier survival curves for PFS. (C) Objective response rates for advanced cancer patients with protective and nonprotective organ-specific irAEs
Fig. 4.
Kaplan‒Meier survival curves for survival and objective response rates in advanced cancer patients according to the protective irAE score. (A) Components of the protective irAE score for OS and PFS. (B) Distribution of patients according to the prognostic protective irAE score. (C) Kaplan‒Meier survival curves for OS. (D) Kaplan‒Meier survival curves for PFS
Discussion
This cohort study was designed to comprehensively evaluate the relationship between irAEs and outcomes in a panel of patients with advanced cancer who were treated with ICIs. Our results indicated that the development of irAEs was associated with prolonged survival, particularly organ-specific irAEs, but not other irAE characteristics, including severity, the number of systems or organs affected, and the timing of onset. The appearance of cutaneous and endocrine toxicities as protective irAEs was associated with improved PFS and OS. The present study provides some data supporting the use of on-treatment toxicity characteristics as prognostic and predictive indicators for patients treated with ICIs.
Our results revealed that patients with irAEs were more likely to have a reduced baseline CRP level, which is consistent with previous reports showing this positive association in patients with different tumor entities [26] but not with NSCLC or HCC [27–29]. The reduced baseline glucocorticoid level has been found to be related to increased efficacy and survival in patients receiving immunotherapy [9], but no reports have shown that it could affect ICI-related hypophysitis or other irAEs [30]. A number of studies investigating the association between irAE occurrence and survival have focused only on specific tumor types, such as NSCLC, hepatocellular carcinoma, and other tumors, for which positive findings have been reported [12–19]. Even a real-life multicenter cohort study revealed that immunotherapy with extended interval dosing after switching from canonical interval dosing and immunotherapy with upfront extended interval dosing did not increase the incidence or severity of irAEs. Among patients who switched to extended interval dosing or started upfront with extended interval dosing, there was also a significant association between irAE onset and improved survival [31]. Our results are in line with previously reported data regarding the positive association between overall irAE occurrence and ICI efficacy for a wide range of cancer types. Subgroup analysis showing the occurrence of irAEs was confirmed to stratify patients with different prognoses in terms of PFS and OS in patients with different tumor types or different anticancer treatments. In particular, patients with gastric cancer who experienced any type of toxicity had significantly longer median OS and PFS than did those with no toxicity, which was consistent with previous findings [32]. However, the number of cases with every cancer type is extremely limited, and it is difficult to analyze the associations between organ-specific irAEs and the prognoses of major cancer types. Some studies do not support this relationship between improved treatment efficacy and specific irAEs, such as immune-related pneumonitis and hepatitis [33–36]. In fact, the prognostic significance of irAEs may depend on some specific irAE characteristics, including the severity, number of systems or organs affected, timing of onset, and ICI drugs used [37, 38]. In contrast, the associations of grade 1–2, multiorgan, and late-onset irAEs with a positive or negative effect on OS were not expected in our study. Thus, we further investigated whether organ-specific irAEs affect patient prognosis. In a previous multicenter cohort study including more than 300 patients with dMMR/MSI-H metastatic colorectal cancer, a higher burden of endocrine and musculoskeletal irAEs determined by summing the grade of organ-specific irAEs had a protective effect on survival, whereas a higher burden of gastrointestinal, pneumonitis, neurological, liver, renal, and other irAEs had a detrimental effect on survival by summing the grade of organ-specific irAEs, suggesting the diverse influence of organ-specific irAEs on patient outcomes [24].
Here, we observed that only cutaneous and endocrine toxicities were associated with improved PFS and OS, which was in line with previous findings [34, 39–43]. The positive association of rheumatological toxicity with improved ICI efficacy and prognosis was not verified in our study, although this was reported in a previous study [24, 44]. One explanation could be that rheumatological toxicities such as arthralgia and arthritis were uncommonly observed in our cohort. We also did not observe that pneumonitis or gastrointestinal toxicity had a harmful effect on survival on the basis of the calculated hazard ratio (HR) direction and P value through multivariate analysis according to a previous report [24]. Therefore, we stratified irAEs into two groups (protective and nonprotective) according to the specific systems or organs affected. The results revealed that patients who experienced protective organ-specific irAEs had longer OS and PFS than did those who experienced nonprotective organ-specific irAEs. Thus, identification of the initial appearance of protective organ-specific irAEs could help screen patients who can derive durable clinical benefit from ICI therapy. Furthermore, we investigated the association between survival and the total protective irAE score, which represents a surrogate for overall protective irAE burden following immunotherapy. The results revealed a consistent positive relationship between these scores and patient outcomes. Patients with 2 or 1 points had longer OS and PFS than those with 0 points. However, there were significant differences between irAEs with scores of 0 and 1 in terms of OS and PFS. Therefore, the development of protective irAEs at any point after the initiation of immunotherapy can indicate clinical benefit. Patients with more protective irAEs seem to be more likely to benefit from immunotherapy than those with fewer protective irAEs.
Additionally, the immortal time bias may have affected the results. Patients with longer survival may have a greater chance of experiencing irAEs, whereas patients who die quickly following ICI treatment do not have sufficient time to develop irAEs. On the basis of these premises, landmark analyses and time-dependent regression models are usually applied to reduce immortal time bias [23, 37, 45]. In landmark analyses, patients who died prior to the present time point or developed irAEs after that time were excluded. Our results supported the positive associations of toxicity development with greater response rates and prolonged survival according to 3-month landmark time analyses and time-dependent regression models. In our studies, we also evaluated the occurrence of irAEs in terms of the system affected and the organ of initial origin, which further minimized the potential risk of survivor bias. Additionally, in our study, the ORRs were significantly different among patients with protective irAEs, nonprotective irAEs, and no irAEs. The immortal time bias was unlikely to explain the association between irAEs and tumor response because the time to response was similar among patients with protective irAEs, nonprotective irAEs, and no irAEs.
The reasons why different organ-specific irAEs are linked to diverse prognoses remain unknown. It is possible that the generation of some irAEs is tissue-specific and cross-reactive to antigens shared between cancer cells and normal tissues and that other irAEs may be unrelated to antitumor immune responses [20, 46, 47]. Compared with those in healthy controls, CD4+ and CD8+ tissue-resident memory T cells and Th1/Tc1 cytokines are preferentially expanded in patients with dermatitis and colitis [48]. The associations between specific irAEs and patient prognosis vary by tumor type. For example, the association between thyroiditis and OS was found to be strongest in lung cancer patients, which may be related to the shared developmental origin of the thyroid and lung epithelia [44]. Why do patients with protective irAEs, including cutaneous and endocrine toxicities, survive longer than those with nonprotective irAEs? Our study revealed that nonprotective irAEs were more likely to be grade 3–4 than protective irAEs were (33.3% vs. 7.9%). In addition, patients with protective irAEs are routinely advised to receive topical corticosteroids, systemic low-dose corticosteroids, or hormone replacement therapy rather than high-dose corticosteroids according to the present guidelines for the toxicity management of ICIs [49]. For example, immune-related type 1 diabetes mellitus, with presentations varying from asymptomatic hyperglycemia to diabetic ketoacidosis, can be successfully treated by meticulous glycemic and electrolyte regulation along with tailored intravenous insulin therapy [50]. The baseline and on-treatment use of high-dose corticosteroids has been found to influence the efficacy of immunotherapy [51, 52]. Future studies are needed to investigate the biological basis of the contribution of organ-specific toxicity to the durable clinical benefits of ICIs and to develop pharmacological interventions to reduce the negative effects of nonprotective toxicity.
This cohort study has a few limitations. The retrospective design, nature of the single-institute study, small sample size, multiple cancer types, and lack of inclusion of an ICI-sensitive population, such as patients with PD-L1 overexpression or dMMR/MSI-H, could bias the results. However, the effects of the occurrence of irAEs on patient prognosis have been identified in NSCLC patients with ≥ 50% PD-L1 expression and CRC patients with dMMR/MSI-H [24, 53, 54]. In addition, this cohort included very few patients with melanoma or lung cancer, and no patients were treated with combined PD-1 and CTLA-4 inhibitors because combination therapy with PD-1 and CTLA-4 inhibitors has not been extensively approved in China for treating advanced cancer. Severe or lethal irAEs can be life-threatening and worsen patient survival, but the development of grade ≥ 3 irAEs is significantly associated with longer OS in advanced melanoma patients [38]. Recent investigations have indicated that subsequent treatment with steroids plus second-line immunosuppressants for severe irAEs potentially influences treatment efficacy and patient prognosis in patients with advanced melanoma treated with first-line ipilimumab plus nivolumab [55], but these findings were not verified in our study. Finally, we did not develop aggregated burden scores to distinguish protective and harmful toxicities on the basis of organ-specific irAEs because significantly harmful irAEs were not observed in our population. However, total protective irAEs were also associated with patient prognosis according to the protective irAE burden score following immunotherapy.
Conclusions
In conclusion, this study increases the understanding of the associations between irAEs or irAE characteristics and prognosis and suggests that identifying organ-specific toxicities (protective or nonprotective) but not other irAE characteristics may be useful for stratifying patients who actually respond to and benefit from ICI therapy across different cancer types. Future investigations should focus on prospective or multiple-institute clinical trials to validate our findings revealing organ-specific toxicity at the site of origin of irAEs as surrogate markers for predicting patient prognosis for specific cancer types.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge all participating patients and their families. We are also thankful to the staff of all the institutions involved in this study.Preliminary findings of this study was partly presented by poster at the American Society of Clinical Oncology (ASCO) Annual Meeting 2024 in Chicago, America. Available form: https://meetings.asco.org/abstracts-presentations/231320.
Abbreviations
- CTLA-4
Cytotoxic T lymphocyte-associated antigen-4
- DCR
Disease control rate
- dMMR
Mismatch repair deficiency
- ECOG
Eastern Cooperative Oncology Group
- HR
Hazard ratio
- HCC
Hepatocellular carcinoma
- ICIs
Immune checkpoint inhibitors
- irAEs
Immune-related adverse events
- MSI-H
Microsatellite instability-high
- NLR
Neutrophil-to-lymphocyte ratio
- NSCLC
Non-small cell lung cancer
- ORR
Objective response rate
- OS
Overall survival
- PD-1
Programmed cell death protein-1
- PD-L1
Programmed cell death ligand-1
- PS
Performance status
- PFS
Progression-free survival
- SII
Systemic immune-inflammation index
- TNM
Tumor node metastasis
Author contributions
XH, YC, HX and JW made substantial contributions to the study conception and design, acquisition of data, and data analysis. JW drafted the manuscript. YC, YZ, YG and WN made substantial contributions to the study design and revision of the manuscript. XH, YG, QX, JL, BW, and BZ analyzed and interpreted the data and edited the manuscript. XH, YC, HX, and YG contributed to the acquisition of the data. All the authors have read and approved the final manuscript.
Funding
This work was partially supported by the National Natural Science Foundation of China (Grant No. 81572875), Shandong Provincial Natural Science Foundation (Grant No. ZR202102190539), Cultivating Fund of The First Affiliated Hospital of Shandong First Medical University (Grant No. QYPY2022NSFC0613), CSCO-MSD Cancer Research Foundation (Grant No. Y-MSD2020-0350), CSCO-PILOT Cancer Research Foundation (Grant No. Y-2019AZMS-0440), and Wu Jieping Medical Foundation for Clinical Scientific Research (Grant No. 320.6750.2020-12-16). The funders played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
The datasets generated during the current study are not publicly available owing to ethical restrictions but are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study involving human participants rigorously complied with the ethical guidelines established by the Declaration of Helsinki. Ethical approval was obtained from the Research Ethics Committee of the First Affiliated Hospital of Shandong First Medical University (No. YXLL-KY-2022–059). Additionally, due to the retrospective design of the study, the Research Ethics Committee of the First Affiliated Hospital of Shandong First Medical University granted a waiver of the requirement for the informed consent. We confirm that all methods were performed in accordance with the relevant guidelines.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xinyue Han, Yingcui Chen, and Hong Xie contributed equally to this work.
References
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70. [DOI] [PubMed] [Google Scholar]
- 2.Bai R, Lv Z, Xu D, Cui J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res. 2020;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, Wu K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gounant V, Duruisseaux M, Soussi G, Van Hulst S, Bylicki O, Cadranel J, Wislez M, Trédaniel J, Spano JP, Helissey C, et al. Does very poor performance Status systematically preclude single Agent Anti-PD-1 Immunotherapy? A Multicenter Study of 35 consecutive patients. Cancers (Basel). 2021;13(5):1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Jin J, Tang M, Li P, Zhou LN, Du YP, Chen MB. Prognostic Nutritional Index Predicts Outcome of PD-L1 Negative and MSS Advanced Cancer Treated with PD-1 Inhibitors. Biomed Res Int.2022, 2022:6743126. [DOI] [PMC free article] [PubMed]
- 6.Tang Y, Cui Y, Li LL, Guan YP, Feng DF, Yin BB, Liang XF, Yin J, Jiang R, Liang J, et al. Dynamics of early serum tumour markers and neutrophil-to-lymphocyte ratio Predict response to PD-1/PD-L1 inhibitors in Advanced Non-small Cell Lung Cancer. Cancer Manag Res. 2021;13:8241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, Templeton AJ, Früh M. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–81. [DOI] [PubMed] [Google Scholar]
- 8.He M, Chen ZF, Zhang L, Gao X, Chong X, Li HS, Shen L, Ji J, Zhang X, Dong B, et al. Associations of subcutaneous fat area and systemic Immune-inflammation index with survival in patients with advanced gastric cancer receiving dual PD-1 and HER2 blockade. J Immunother Cancer. 2023;11(6):e007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui Y, Han X, Liu H, Xie Q, Guan Y, Yin B, Xiao J, Feng D, Wang X, Li J, et al. Impact of endogenous glucocorticoid on response to immune checkpoint blockade in patients with advanced cancer. Front Immunol. 2023;14:1081790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng JY, Gou Q, Yang L, Chen ZH, Yang MY, Yang XR, Yan HH, Wei XW, Liu JQ, Su J, et al. Immune suppressive microenvironment in liver metastases contributes to organ-specific response of immunotherapy in advanced non-small cell lung cancer. J Immunother Cancer. 2023;11(7):e007218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimizu T, Miyake M, Nishimura N, Inoue K, Fujii K, Iemura Y, Ichikawa K, Omori C, Tomizawa M, Maesaka F, et al. Organ-specific and mixed responses to Pembrolizumab in patients with unresectable or metastatic urothelial carcinoma: a Multicenter Retrospective Study. Cancers (Basel). 2022;14(7):1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Sbeih H, Ali FS, Qiao W, Lu Y, Patel S, Diab A, Wang Y. Immune checkpoint inhibitor-induced colitis as a predictor of survival in metastatic melanoma. Cancer Immunol Immunother. 2019;68(4):553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, et al. Association of Immune-related adverse events with Nivolumab Efficacy in Non-small-cell Lung Cancer. JAMA Oncol. 2018;4(3):374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussaini S, Chehade R, Boldt RG, Raphael J, Blanchette P, Maleki Vareki S, Fernandes R. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors - a systematic review and meta-analysis. Cancer Treat Rev. 2021;92:102134. [DOI] [PubMed] [Google Scholar]
- 15.Amoroso V, Gallo F, Alberti A, Paloschi D, Ferrari Bravo W, Esposito A, Cosentini D, Grisanti S, Pedersini R, Petrelli F, et al. Immune-related adverse events as potential surrogates of immune checkpoint inhibitors’ efficacy: a systematic review and meta-analysis of randomized studies. ESMO Open. 2023;8(2):100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukushima T, Morimoto M, Kobayashi S, Ueno M, Uojima H, Hidaka H, Kusano C, Chuma M, Numata K, Tsuruya K, et al. Association between Immune-related adverse events and survival in patients with Hepatocellular Carcinoma treated with Atezolizumab Plus Bevacizumab. Oncologist. 2023;28(7):e526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshikawa Y, Imamura M, Yamauchi M, Hayes CN, Aikata H, Okamoto W, Miyata Y, Okada M, Hattori N, Sugiyama K, et al. Prevalence of immune-related adverse events and anti-tumor efficacy following immune checkpoint inhibitor therapy in Japanese patients with various solid tumors. BMC Cancer. 2022;22(1):1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster CC, Couey MA, Kochanny SE, Khattri A, Acharya RK, Tan YC, Brisson RJ, Leidner RS, Seiwert TY. Immune-related adverse events are associated with improved response, progression-free survival, and overall survival for patients with head and neck cancer receiving immune checkpoint inhibitors. Cancer. 2021;127(24):4565–73. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda Y, Fujiwara R, Oguchi T, Komai Y, Numao N, Yamamoto S, Oki R, Urasaki T, Takahashi S, Yonese J, et al. Prognostic significance of Immune-related adverse events in metastatic renal cell carcinoma patients treated with Immune-checkpoint-inhibitors. Cancer Diagn Progn. 2023;3(3):327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cottrell T, Zhang J, Zhang B, Kaunitz GJ, Burman P, Chan HY, Verde F, Hooper JE, Hammers H, Allaf ME, et al. Evaluating T-cell cross-reactivity between tumors and immune-related adverse events with TCR sequencing: pitfalls in interpretations of functional relevance. J Immunother Cancer. 2021;9(7):e002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, Ettinger DS, Hann CL, Brahmer JR, Ricciuti B, et al. Multisystem Immune-related adverse events Associated with Immune Checkpoint inhibitors for treatment of Non-small Cell Lung Cancer. JAMA Oncol. 2020;6(12):1952–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosoya K, Fujimoto D, Morimoto T, Kumagai T, Tamiya A, Taniguchi Y, Yokoyama T, Ishida T, Hirano K, Matsumoto H, et al. Association between Early Immune-related adverse events and clinical outcomes in patients with Non-small Cell Lung Cancer treated with Immune Checkpoint inhibitors. Clin Lung Cancer. 2020;21(4):e315–28. [DOI] [PubMed] [Google Scholar]
- 23.Hsiehchen D, Naqash AR, Espinoza M, Von Itzstein MS, Cortellini A, Ricciuti B, Owen DH, Laharwal M, Toi Y, Burke M, et al. Association between immune-related adverse event timing and treatment outcomes. Oncoimmunology. 2022;11(1):2017162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasca V, Barretta F, Corti F, Lonardi S, Niger M, Elez ME, Fakih M, Jayachandran P, Shah AT, Salati M, et al. Association of immune-related adverse events with the outcomes of immune checkpoint inhibitors in patients with dMMR/MSI-H metastatic colorectal cancer. J Immunother Cancer. 2023;11(1):e005493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics: reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst. 2005;97(16):1180–4. [DOI] [PubMed] [Google Scholar]
- 26.Mehra T, Dongre K, Boesing M, Frei P, Suenderhauf C, Zippelius A, Leuppi JD, Wicki A, Leuppi-Taegtmeyer AB. PrePre-treatment comorbidities, C-reactive protein and eosinophil count, and immune-related adverse events as predictors of survival with checkpoint inhibition for multiple tumoour entities. Cancer Med. 2023;12(11):12253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi H, Nishio M, Akamatsu H, Goto Y, Miura S, Gemma A, Yoshino I, Misumi T, Kijima T, Takase N, et al. Association between Immune-related adverse events and Atezolizumab in previously treated patients with Unresectable Advanced or recurrent Non-small Cell Lung Cancer. Cancer Res Commun. 2024;4(11):2858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onodera R, Chiba S, Nihei S, Fujimura I, Akiyama M, Utsumi Y, Nagashima H, Kudo K, Maemondo M. High level of C-reactive protein as a predictive factor for immune-related adverse events of immune checkpoint inhibitors in non-small cell lung cancer: a retrospective study. J Thorac Dis. 2023;15(8):4237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Y, Wang S, Su N, Pan S, Tu B, Zhao J, Shen Y, Qiu Q, Liu X, Luan J, et al. Increased circulating levels of CRP and IL-6 and decreased frequencies of T and B lymphocyte subsets are Associated with Immune-related adverse events during combination therapy with PD-1 inhibitors for Liver Cancer. Front Oncol. 2022;12:906824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bando H, Yamamoto M, Urai S, Motomura Y, Sasaki Y, Ohmachi Y, Kobatake M, Tsujimoto Y, Oi-Yo Y, Suzuki M, et al. Fluctuations in plasma adrenocorticotropic hormone concentration may predict the onset of immune checkpoint inhibitor-related hypophysitis. J Immunother Cancer. 2024;12(2):e008634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cantini L, Paoloni F, Pecci F, Spagnolo F, Genova C, Tanda ET, Aerts S, Rebuzzi SE, Fornarini G, Zoratto F, et al. Safety of extended interval dosing immune checkpoint inhibitors: a multicenter cohort study. J Natl Cancer Inst. 2023;115(7):796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Xu S, Wang J, Lv Y, Wang N, Lai R, Sha Z, Zhao Q, Guo Z. Are anti-PD-1-associated immune related adverse events a harbinger of favorable clinical prognosis in patients with gastric cancer? BMC Cancer. 2022;22(1):1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe S, Ota T, Hayashi M, Ishikawa H, Otsubo A, Shoji S, Nozaki K, Ichikawa K, Kondo R, Miyabayashi T, et al. Prognostic significance of the radiologic features of pneumonitis induced by anti-PD-1 therapy. Cancer Med. 2020;9(9):3070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Shi Y, Ding H, Feng Y, Zhang T, Liang Y, Wang H, Song X, Chen B, Xia W, et al. Different associations between organ-specific immune-related adverse event and survival in non-small cell lung cancer patients treated with programmed death-1 inhibitors-based combination therapy. Ther Adv Med Oncol. 2023;15:17588359231210678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tiu BC, Zubiri L, Iheke J, Pahalyants V, Theodosakis N, Ugwu-Dike P, Seo J, Tang K, Sise ME, Sullivan R, et al. Real-world incidence and impact of pneumonitis in patients with lung cancer treated with immune checkpoint inhibitors: a multi-institutional cohort study. J Immunother Cancer. 2022;10(6):e004670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biewenga M, van der Kooij MK, Wouters MWJM, Aarts MJB, van den Berkmortel FWPJ, de Groot JWB, Boers-Sonderen MJ, Hospers GAP, Piersma D, van Rijn RS, et al. Checkpoint inhibitor induced hepatitis and the relation with liver metastasis and outcome in advanced melanoma patients. Hepatol Int. 2021;15(2):510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suo A, Chan Y, Beaulieu C, Kong S, Cheung WY, Monzon JG, Smylie M, Walker J, Morris D, Cheng T. Anti-PD1-Induced Immune-related adverse events and survival outcomes in Advanced Melanoma. Oncologist. 2020;25(5):438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu CE, Yang CK, Peng MT, Huang PW, Chang CF, Yeh KY, Chen CB, Wang CL, Hsu CW, Chen IW, et al. The association between immune-related adverse events and survival outcomes in Asian patients with advanced melanoma receiving anti-PD-1 antibodies. BMC Cancer. 2020;20(1):1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muir CA, Clifton-Bligh RJ, Long GV, Scolyer RA, Lo SN, Carlino MS, Tsang VHM, Menzies AM. Thyroid Immune-related adverse events following Immune checkpoint inhibitor treatment. J Clin Endocrinol Metab. 2021;106(9):e3704–13. [DOI] [PubMed] [Google Scholar]
- 41.Kotwal A, Kottschade L, Ryder M. PD-L1 inhibitor-Induced Thyroiditis is Associated with Better overall survival in Cancer patients. Thyroid. 2020;30(2):177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paderi A, Giorgione R, Giommoni E, Mela MM, Rossi V, Doni L, Minervini A, Carini M, Pillozzi S, Antonuzzo L. Association between Immune related adverse events and outcome in patients with metastatic renal cell carcinoma treated with Immune Checkpoint inhibitors. Cancers (Basel). 2021;13(4):860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, Lin K, Quaglino P, Rappersberger K, Ortiz-Urda S. Pembrolizumab Cutaneous adverse events and their Association with Disease Progression. JAMA Dermatol. 2015;151(11):1206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kostine M, Rouxel L, Barnetche T, Veillon R, Martin F, Dutriaux C, Dousset L, Pham-Ledard A, Prey S, Beylot-Barry M, et al. FHU ACRONIM. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer-clinical aspects and relationship with tumr response: a single- prospective cohort study. Ann Rheum Dis. 2018;77(3):393–8. [DOI] [PubMed] [Google Scholar]
- 45.Street S, Chute D, Strohbehn I, Zhao S, Rengarajan M, Faje A, Seethapathy H, Lee M, Seethapathy R, Drobni Z, et al. The positive effect of immune checkpoint inhibitor-induced thyroiditis on overall survival accounting for immortal time bias: a retrospective cohort study of 6596 patients. Ann Oncol. 2021;32(8):1050–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berner F, Bomze D, Diem S, Ali OH, Fässler M, Ring S, Niederer R, Ackermann CJ, Baumgaertner P, Pikor N, et al. Association of checkpoint inhibitor-Induced toxic effects with Shared Cancer and tissue antigens in Non-small Cell Lung Cancer. JAMA Oncol. 2019;5(7):1043–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blum SM, Rouhani SJ, Sullivan RJ. Effects of immune-related adverse events (irAEs) and their treatment on antitumor immune responses. Immunol Rev. 2023;318(1):167–78. [DOI] [PubMed] [Google Scholar]
- 48.Reschke R, Shapiro JW, Yu J, Rouhani SJ, Olson DJ, Zha Y, Gajewski TF. Checkpoint Blockade-Induced Dermatitis and Colitis are dominated by Tissue-Resident Memory T Cells and Th1/Tc1 cytokines. Cancer Immunol Res. 2022;10(10):1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, Atkins MB, Brassil KJ, Caterino JM, Chau I, et al. Management of Immune-related adverse events in patients treated with Immune checkpoint inhibitor therapy: ASCO Guideline Update. J Clin Oncol. 2021;39(36):4073–126. [DOI] [PubMed] [Google Scholar]
- 50.Kani ER, Karaviti E, Karaviti D, Gerontiti E, Paschou IA, Saltiki K, Stefanaki K, Psaltopoulou T, Paschou SA. Pathophysiology, diagnosis, and management of immune checkpoint inhibitor-induced diabetes mellitus. Endocrine. 2024. Epub ahead of print. [DOI] [PubMed]
- 51.Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, Martínez-Bernal G, Ferrara R, Lai WV, Hendriks LEL, et al. Impact of baseline steroids on efficacy of programmed cell Death-1 and programmed death-ligand 1 blockade in patients with non-small-cell Lung Cancer. J Clin Oncol. 2018;36(28):2872–8. [DOI] [PubMed] [Google Scholar]
- 52.Bai X, Hu J, Betof Warner A, Quach HT, Cann CG, Zhang MZ, Si L, Tang B, Cui C, Yang X, et al. Early Use of High-Dose glucocorticoid for the management of irAE is Associated with poorer survival in patients with Advanced Melanoma treated with Anti-PD-1 monotherapy. Clin Cancer Res. 2021;27(21):5993–6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raynes G, Stares M, Low S, Haron D, Sarwar H, Abhi D, Barrie C, Laird B, Collaborative CC, Phillips I, et al. Immune-related adverse events, biomarkers of systemic inflammation, and survival outcomes in patients receiving Pembrolizumab for Non-small-cell Lung Cancer. Cancers (Basel). 2023;15(23):5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faoro L, Brusegan A, Russi A, Calderone V, Martelli A, Marranconi E, Carpanese D, Berti E, Coppola M. Analysis of the relation between adverse events and overall survival in patients treated with pembrolizumab as a first-line treatment for metastatic NSCLC. BMC Pharmacol Toxicol. 2023;24(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Not OJ, Verheijden RJ, van den Eertwegh AJM, Haanen JBAG, Aarts MJB, van den Berkmortel FWPJ, Blank CU, Boers-Sonderen MJ, de Groot JB, Hospers GAP, et al. Association of Immune-related adverse event management with survival in patients with Advanced Melanoma. JAMA Oncol. 2022;8(12):1794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are not publicly available owing to ethical restrictions but are available from the corresponding author upon reasonable request.