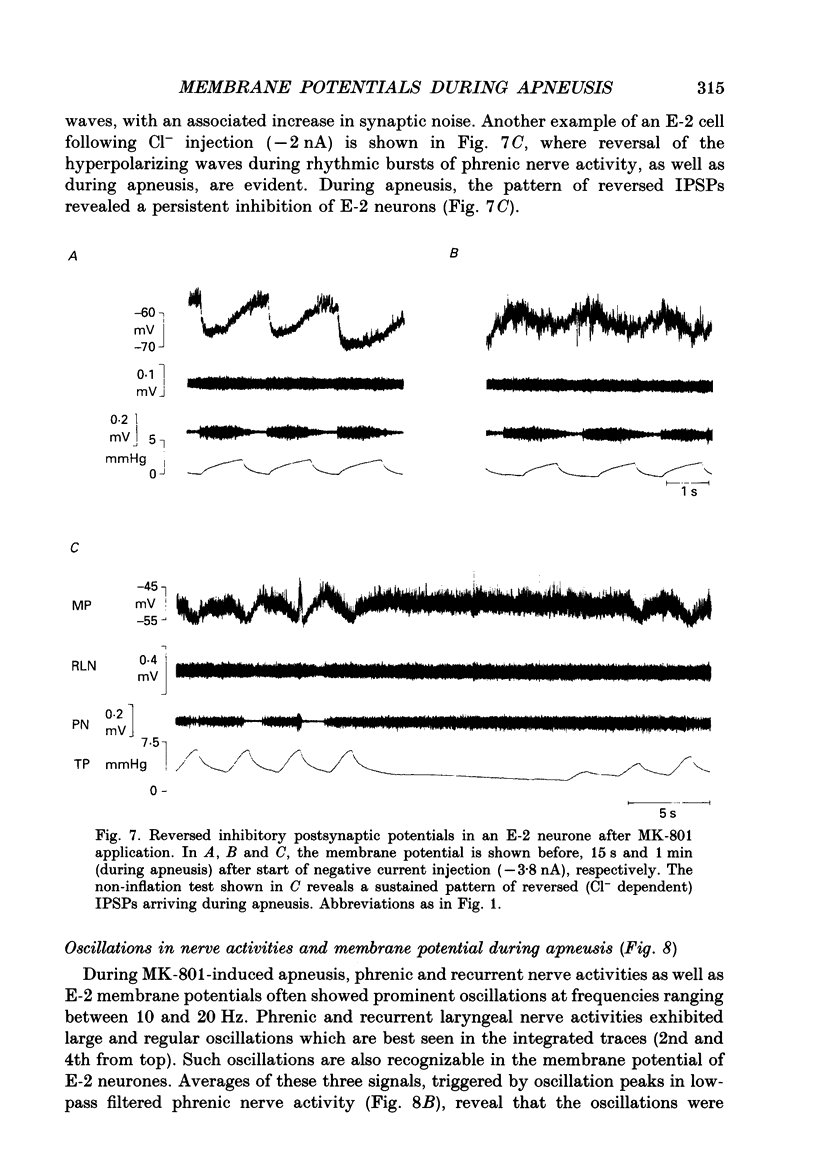
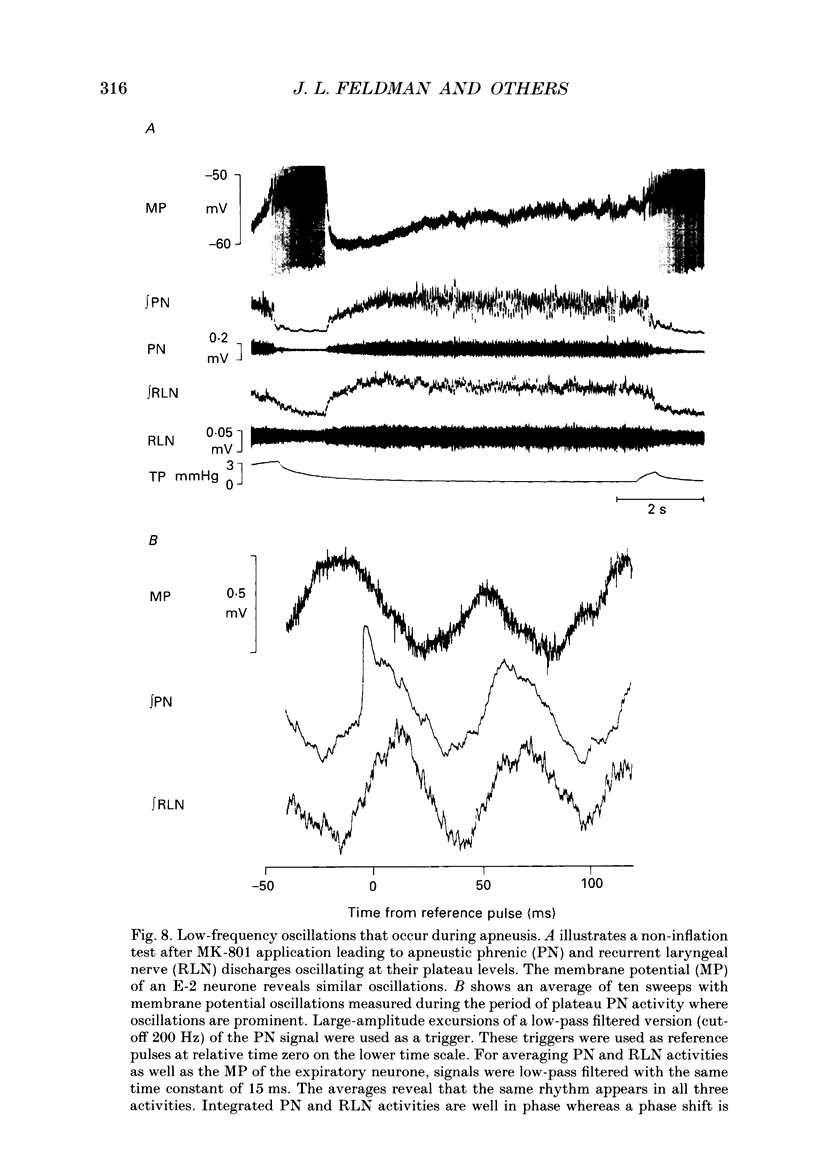
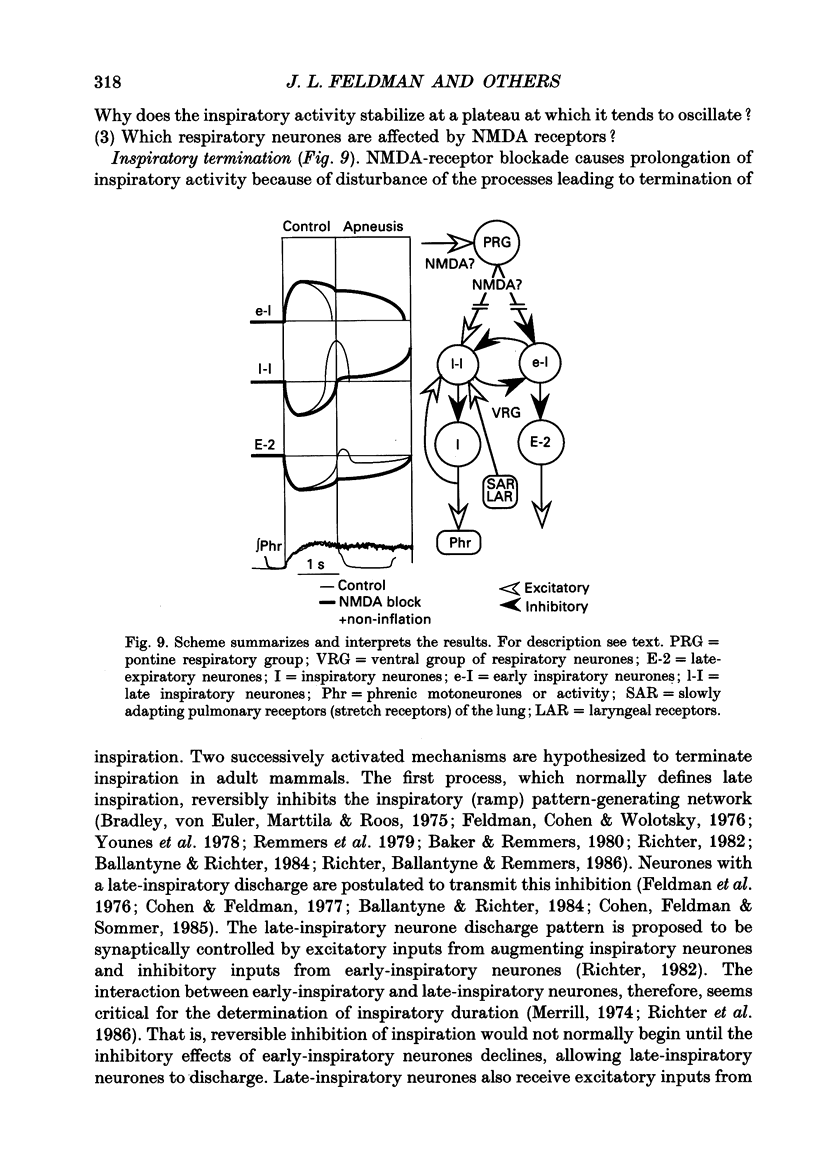
Abstract
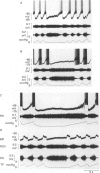
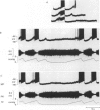
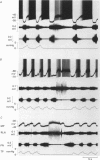
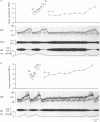
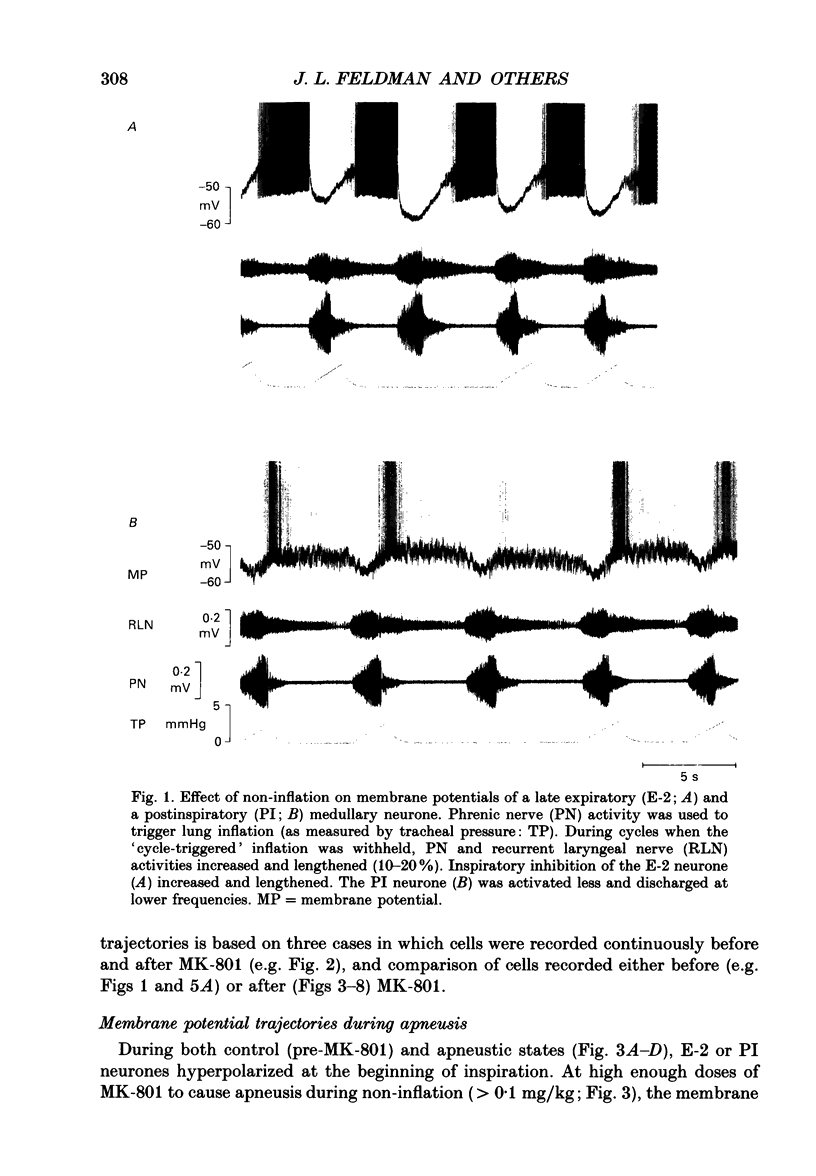
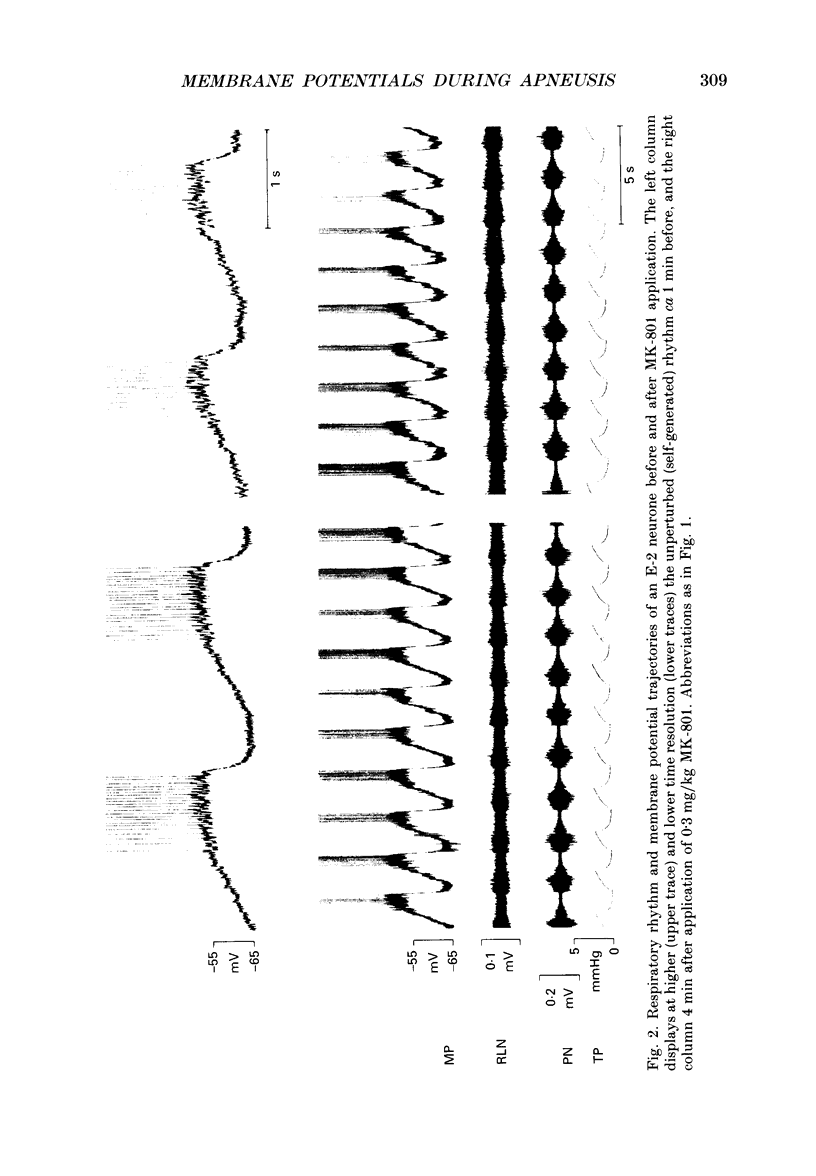
1. Termination of inspiration is an essential component of respiratory rhythm generation and its perturbation can result in apneusis, i.e. significant prolongation of mechanisms, we studied the postsynaptic events in respiratory neurones during apneustic respiratory periods, and compared them to normal respiratory cycles. 2. Experiments were performed in pentobarbitone-anaesthetized, paralysed, thoracotomized cats ventilated with a constant volume or a cycle-triggered constant pressure pump. Apneusis, separated by normal cycles, was induced as follows: the animal was ventilated by a cycle-triggered pump that normally inflated the lungs during the inspiratory burst of phrenic nerve discharge. The NMDA-receptor blocker MK-801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5, 10-iminemaleate] (0.3-0.7 mg/kg) was administered intravenously, and, for designated breaths, inflation of the lungs was withheld during neural inspiration. 3. Membrane potential trajectories of forty-one late expiratory (E-2) and eight postinspiratory (PI) neurones of the caudal ventral respiratory group were analysed before and/or after MK-801 administration, during normal and apneustic periods. 4. Before MK-801 administration, withholding lung inflation caused modest (10-20%) lengthening of the inspiratory period; after MK-801 administration, withholding inflation caused apneusis. Provided that the lungs were inflated during the inspiratory phase, the temporal pattern of phrenic nerve, recurrent laryngeal nerve and membrane potential trajectories of E-2 and PI neurones were not significantly altered by MK-801. Apneusis following NMDA-receptor blockade produced consistent changes in the synaptic activation patterns of E-2 neurones. In particular, the slow late inspiratory-related depolarization pattern of E-2 neurones was consistently retarded during apneustic inspiratory phases when compared to normal inspiratory phases. This was due to continuation of Cl(-)-mediated synaptic inhibition of E-2 neurones. Superior laryngeal nerve stimulation stopped apneusis and sustained membrane hyperpolarization of E-2 neurones similar to lung inflation. 5. During the plateau phase of apneusis, correlated 10-20 Hz oscillations could be observed in the integrated phrenic and recurrent laryngeal nerve activities as well as in the membrane potential of E-2 neurones. 6. We conclude that: (i) the prolonged inhibition of E-2 neurones during apneusis is indicative of the process responsible for the prolongation of the inspiratory phase.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




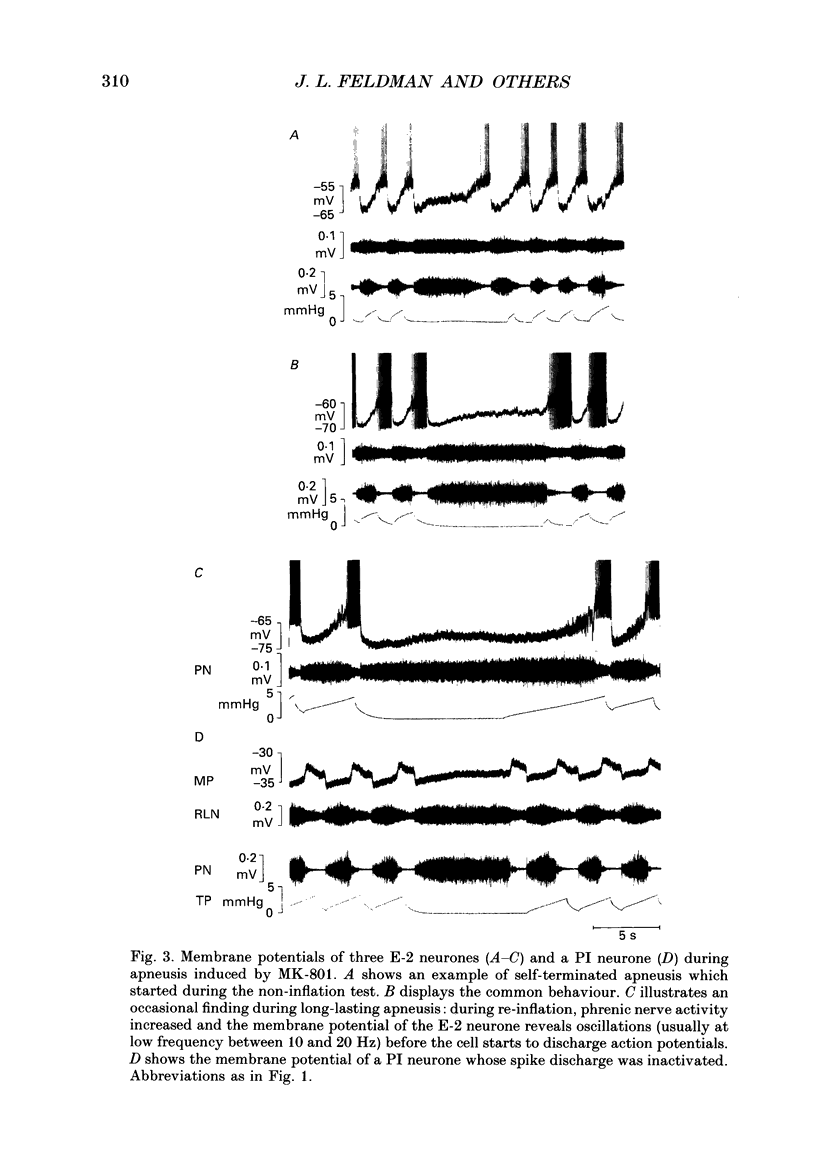
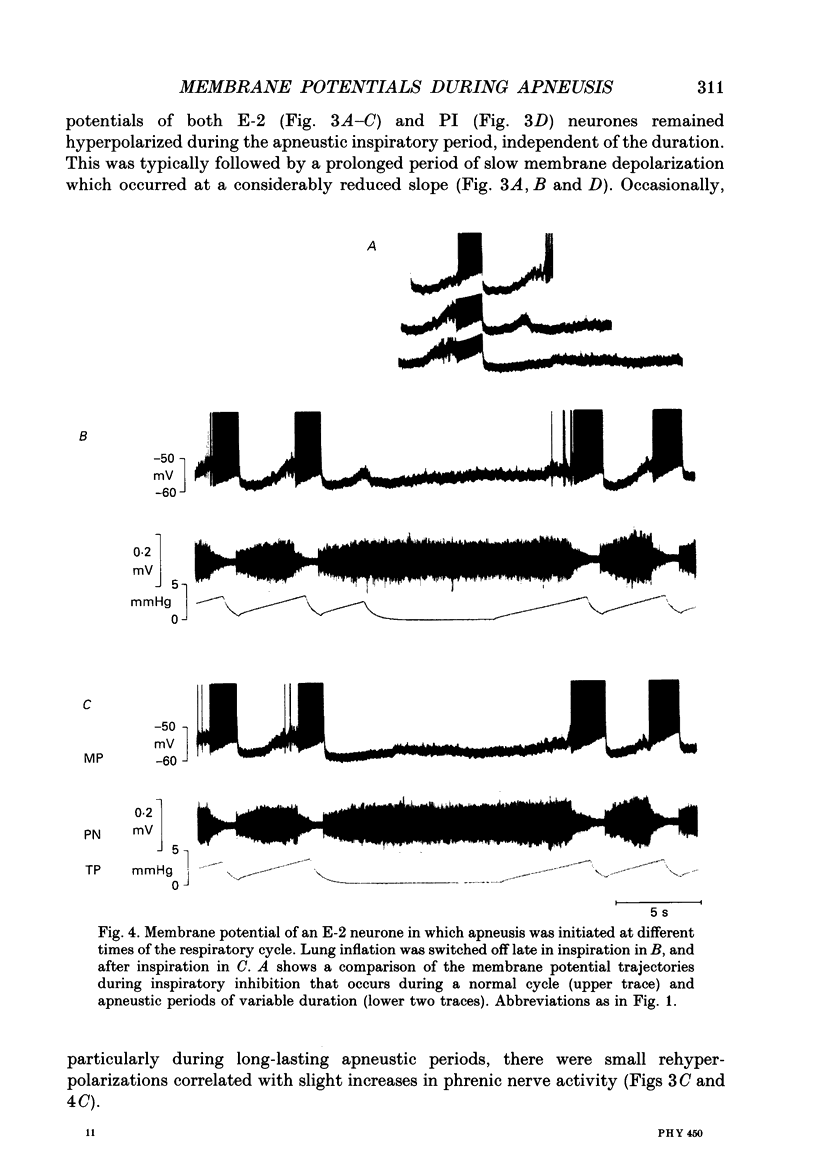
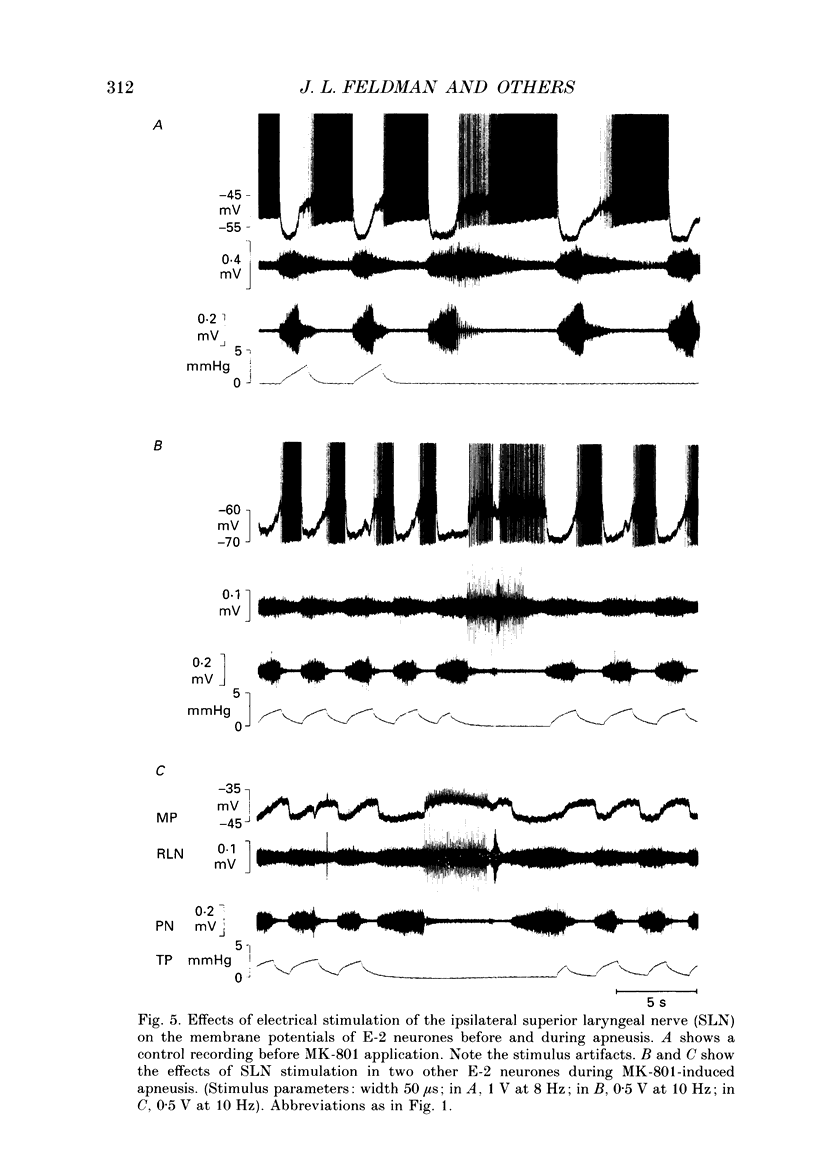

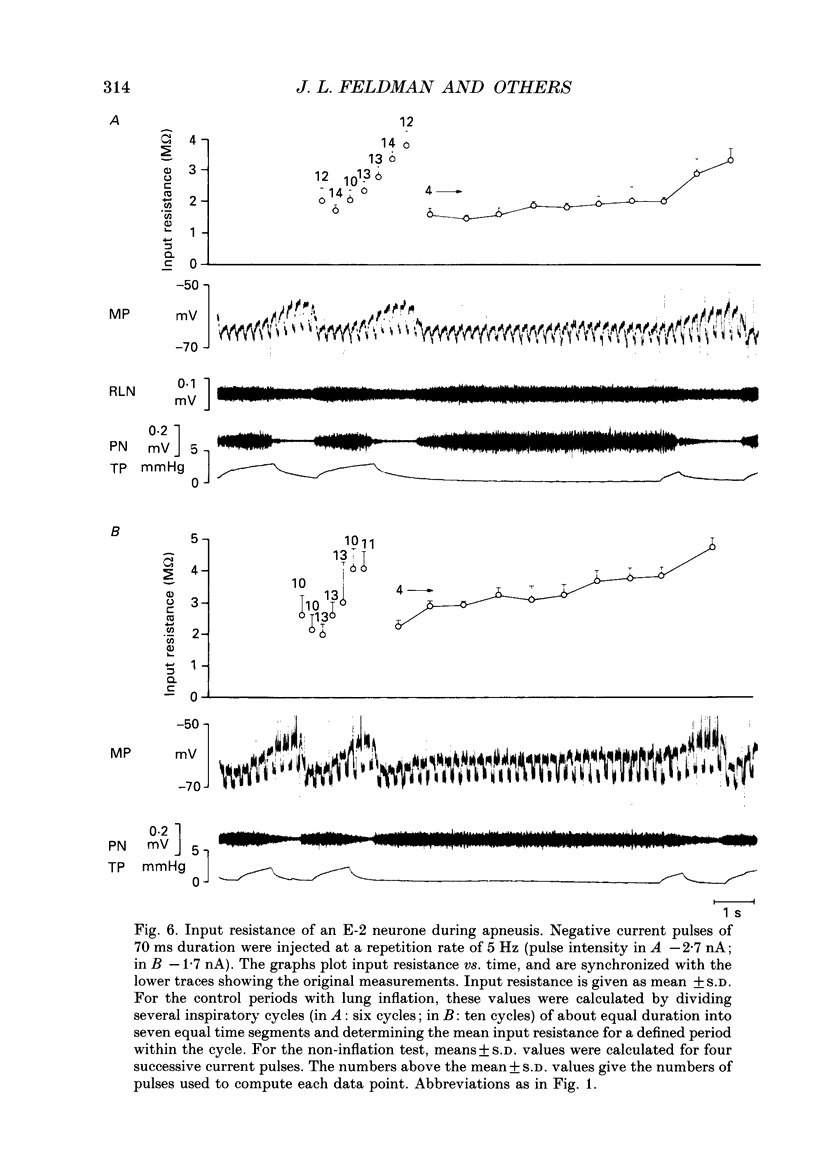






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders K., Ballantyne D., Bischoff A. M., Lalley P. M., Richter D. W. Inhibition of caudal medullary expiratory neurones by retrofacial inspiratory neurones in the cat. J Physiol. 1991 Jun;437:1–25. doi: 10.1113/jphysiol.1991.sp018580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. P., Jr, Remmers J. E. Temporal correlation of graded reversible inspiratory inhibition with discharge patterns of late inspiratory neurons located in the dorsal respiratory group in cats. Brain Res. 1980 Nov 3;200(2):331–340. doi: 10.1016/0006-8993(80)90924-5. [DOI] [PubMed] [Google Scholar]
- Ballantyne D., Richter D. W. Post-synaptic inhibition of bulbar inspiratory neurones in the cat. J Physiol. 1984 Mar;348:67–87. doi: 10.1113/jphysiol.1984.sp015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne D., Richter D. W. The non-uniform character of expiratory synaptic activity in expiratory bulbospinal neurones of the cat. J Physiol. 1986 Jan;370:433–456. doi: 10.1113/jphysiol.1986.sp015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham A. C., McCrimmon D. R. Neurones in a discrete region of the nucleus tractus solitarius are required for the Breuer-Hering reflex in rat. J Physiol. 1990 Aug;427:261–280. doi: 10.1113/jphysiol.1990.sp018171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley G. W., von Euler C., Marttila I., Roos B. A model of the central and reflex inhibition of inspiration in the cat. Biol Cybern. 1975 Aug 8;19(2):105–116. doi: 10.1007/BF00364107. [DOI] [PubMed] [Google Scholar]
- Champagnat J., Jacquin T., Richter D. W. Voltage-dependent currents in neurones of the nuclei of the solitary tract of rat brainstem slices. Pflugers Arch. 1986 Apr;406(4):372–379. doi: 10.1007/BF00590939. [DOI] [PubMed] [Google Scholar]
- Clark F. J., von Euler C. On the regulation of depth and rate of breathing. J Physiol. 1972 Apr;222(2):267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. I., Feldman J. L. Models of respiratory phase-switching. Fed Proc. 1977 Sep;36(10):2367–2374. [PubMed] [Google Scholar]
- Cohen M. I., Feldman J. L., Sommer D. Caudal medullary expiratory neurone and internal intercostal nerve discharges in the cat: effects of lung inflation. J Physiol. 1985 Nov;368:147–178. doi: 10.1113/jphysiol.1985.sp015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog Neurobiol. 1990;35(6):429–450. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Cohen M. I., Wolotsky P. Powerful inhibition of pontine respiratory neurons by pulmonary afferent activity. Brain Res. 1976 Mar 12;104(2):341–346. doi: 10.1016/0006-8993(76)90629-6. [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Gautier H. Interaction of pulmonary afferents and pneumotaxic center in control of respiratory pattern in cats. J Neurophysiol. 1976 Jan;39(1):31–44. doi: 10.1152/jn.1976.39.1.31. [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Smith J. C. Cellular mechanisms underlying modulation of breathing pattern in mammals. Ann N Y Acad Sci. 1989;563:114–130. doi: 10.1111/j.1749-6632.1989.tb42194.x. [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Smith J. C., Ellenberger H. H., Connelly C. A., Liu G. S., Greer J. J., Lindsay A. D., Otto M. R. Neurogenesis of respiratory rhythm and pattern: emerging concepts. Am J Physiol. 1990 Nov;259(5 Pt 2):R879–R886. doi: 10.1152/ajpregu.1990.259.5.R879. [DOI] [PubMed] [Google Scholar]
- Foutz A. S., Champagnat J., Denavit-Saubié M. N-methyl-D-aspartate (NMDA) receptors control respiratory off-switch in cat. Neurosci Lett. 1988 May 3;87(3):221–226. doi: 10.1016/0304-3940(88)90452-1. [DOI] [PubMed] [Google Scholar]
- Gautier H., Bonora M., Gaudy J. H. Breuer-Hering inflation reflex and breathing pattern in anesthetized humans and cats. J Appl Physiol Respir Environ Exerc Physiol. 1981 Nov;51(5):1162–1168. doi: 10.1152/jappl.1981.51.5.1162. [DOI] [PubMed] [Google Scholar]
- Iscoe S., Feldman J. L., Cohen M. I. Properties of inspiratory termination by superior laryngeal and vagal stimulation. Respir Physiol. 1979 Apr;36(3):353–366. doi: 10.1016/0034-5687(79)90047-1. [DOI] [PubMed] [Google Scholar]
- Karczewski W. A., Nasłońska E., Romaniuk J. R. Inspiratory facilitatory and inhibitory vagal influences during apnoea in rabbits. Acta Neurobiol Exp (Wars) 1980;40(3):575–592. [PubMed] [Google Scholar]
- Lawson E. E., Richter D. W., Ballantyne D., Lalley P. M. Peripheral chemoreceptor inputs to medullary inspiratory and postinspiratory neurons of cats. Pflugers Arch. 1989 Sep;414(5):523–533. doi: 10.1007/BF00580987. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Mifflin S., Richter D. W. The effects of QX-314 on medullary respiratory neurones. Brain Res. 1987 Sep 8;420(1):22–31. doi: 10.1016/0006-8993(87)90235-6. [DOI] [PubMed] [Google Scholar]
- Remmers J. E., Richter D. W., Ballantyne D., Bainton C. R., Klein J. P. Reflex prolongation of stage I of expiration. Pflugers Arch. 1986 Aug;407(2):190–198. doi: 10.1007/BF00580675. [DOI] [PubMed] [Google Scholar]
- Richter D. W., Ballantyne D., Remmers J. E. The differential organization of medullary post-inspiratory activities. Pflugers Arch. 1987 Nov;410(4-5):420–427. doi: 10.1007/BF00586520. [DOI] [PubMed] [Google Scholar]
- Richter D. W. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982 Oct;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Ellenberger H. H., Ballanyi K., Richter D. W., Feldman J. L. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991 Nov 1;254(5032):726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Greer J. J., Liu G. S., Feldman J. L. Neural mechanisms generating respiratory pattern in mammalian brain stem-spinal cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. J Neurophysiol. 1990 Oct;64(4):1149–1169. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- Stella G. On the mechanism of production, and the physiological significance of "apneusis". J Physiol. 1938 Jun 14;93(1):10–23. doi: 10.1113/jphysiol.1938.sp003621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes M. K., Remmers J. E., Baker J. Characteristics of inspiratory inhibition by phasic volume feedback in cats. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):80–86. doi: 10.1152/jappl.1978.45.1.80. [DOI] [PubMed] [Google Scholar]
- von Euler C., Marttila I., Remmers J. E., Trippenbach T. Effects of lesions in the parabrachial nucleus on the mechanisms for central and reflex termination of inspiration in the cat. Acta Physiol Scand. 1976 Mar;96(3):324–337. doi: 10.1111/j.1748-1716.1976.tb10203.x. [DOI] [PubMed] [Google Scholar]