Abstract
Granuphilin/Slp-4 is a member of the synaptotagmin-like protein family expressed in pancreatic β-cells and in the pituitary gland. We show by confocal microscopy that both granuphilin-a and -b colocalize with insulin-containing secretory granules positioned at the periphery of pancreatic β-cells. Overexpression of granuphilins in insulin-secreting cell lines caused a profound inhibition of stimulus-induced exocytosis. Granuphilins were found to bind to two components of the secretory machinery of pancreatic β-cells, the small GTP-binding protein Rab3 and the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)–binding protein Munc-18. The interaction with Rab3 occurred only with the GTP-bound form of the protein and was prevented by a point mutation in the effector domain of the GTPase. Structure-function studies using granuphilin-b mutants revealed that complete loss of Rab3 binding is associated with a reduction in the capacity to inhibit exocytosis. However, the granuphilin/Rab3 complex alone is not sufficient to mediate the decrease of exocytosis, suggesting the existence of additional binding partners. Taken together, our observations indicate that granuphilins play an important role in pancreatic β-cell exocytosis. In view of the postulated role of Munc-18 in secretory vesicle docking, our data suggest that granuphilins may also be involved in this process.
INTRODUCTION
Insulin release from pancreatic β-cells plays an essential role in blood glucose homeostasis. The moment-to-moment regulation of insulin secretion is obtained through tight control of the exocytotic process of pancreatic β-cells. Several components of the molecular machinery governing exocytosis in eukaryotic cells have been identified. It is now well established that the targeting or fusion of secretory vesicles with the plasma membrane necessitates the assembly of a complex between proteins associated with the plasma membrane, called t-SNAREs, and proteins anchored on the membrane of secretory vesicles, called v-SNAREs (Rothman, 1994; Jahn and Südhof, 1999). In pancreatic β-cells, the exocytotic process relies on the t-SNAREs syntaxin-1 and SNAP-25 and on the v-SNAREs VAMP-2 and cellubrevin (Lang, 1999; Easom, 2000). The formation of the soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) receptor (SNARE) complex is modulated by several regulatory components. These include the SNARE-binding protein N-Sec-1/Munc-18 and the members of the Rab3 GTPase family. Munc-18 binds to the inactive conformation of syntaxin-1 and participates in the docking of secretory vesicles at the plasma membrane (Dulubova et al., 1999; Voets et al., 2001). Munc-18 is then released from syntaxin-1 to enable the assembly of the SNARE complex (Dulubova et al., 1999; Voets et al., 2001). Rab3 acts at multiple levels in regulated secretion. In PC12 cells, Rab3 proteins control the total granule number and the number of granules docked at the plasma membrane (Martelli et al., 2000). The molecular mechanism regulating these phenomena is still unclear. In view of the results obtained with other Rab GTPases (Lupashin and Waters, 1997), Rab3 has been suggested to favor the dissociation of the complex between syntaxin-1 and Munc-18. Thus far, however, no direct interaction of Munc-18 with Rab3 or with its effectors has been reported. Rab3 GTPases also appear to modulate postdocking events. Thus, the number of synaptic vesicles that fuse with the plasma membrane in response to stimuli is increased in Rab3A-deficient mice (Geppert et al., 1997). In addition, consistent with an inhibitory function of Rab3 in regulated secretion, overexpression of dominant active mutants of Rab3 decreases exocytosis in many cell systems (Holz et al., 1994; Johannes et al., 1994; Roa et al., 1997; Gevrey et al., 2001), including pancreatic β-cells (Regazzi et al., 1996; Iezzi et al., 1999). The different functions of Rab3 are likely to be mediated through multiple effectors. Rabphilin-3A was the first Rab3 target to be isolated (Shirataki et al., 1992, 1993). The molecular determinants of rabphilin-3A that are responsible for the association with Rab3 have been identified. The Rab3 binding domain of rabphilin-3A consists of an amphipathic α-helix and a specific SGAWFF sequence separated by a Zn2+ finger motif (Joberty et al., 1999, Ostermeier and Brunger, 1999). Homologous domains are present in two other Rab3 effectors, RIM and Noc2, and point mutations in the α-helix of these proteins abolish the binding of Rab3 (Haynes et al., 2001; Sun et al., 2001; Wang et al., 2001). In the case of RIM, however, the Zn2+ finger and the SGAWFF motif do not contribute significantly to the interaction with GTPase (Sun et al., 2001; Wang et al., 2001). All the Rab3 targets have been shown to be involved in the secretory process (Chung et al., 1995; Wang et al., 1997; Iezzi et al., 2000). However, rabphilin-3A is not detectable in pancreatic β-cells and is unlikely to participate in the control of insulin release (Regazzi et al., 1996).
Granuphilin is a member of the synaptotagmin-like protein (Slp) family (Fukuda and Mikoshiba, 2001; Fukuda et al., 2001) that was identified in a screening for genes differentially expressed between α- and β-cells of the islets of Langerhans (Wang et al., 1999). Granuphilin (also referred to as Slp-4) occurs in two alternatively spliced forms. Granuphilin-a is an 80-kDa protein with a structural organization resembling that of rabphilin-3A and RIM. The N-terminus of the protein includes an amphipathic α-helix, a Zn2+ finger motif, and a partially conserved SGAWFF sequence (TGDWFY). The C-terminus contains two C2-domains. Granuphilin-a is expressed almost exclusively in pancreatic β-cells and is associated with insulin-containing granules (Wang et al., 1999). The second isoform, granuphilin-b, is shorter than granuphilin-a and lacks one of the C2 domains. The tissue distribution and the subcellular localization of granuphilin-b were not investigated.
In this study, we determined the functional role of the two granuphilin isoforms in pancreatic β-cells and identified two of their binding partners. We found that granuphilins interact with Rab3 and Munc-18 and profoundly inhibit stimulus-induced secretion. In view of the known functions of Rab3 and Munc-18, these properties suggest that granuphilins can participate in the regulation of multiple steps in the secretory pathway.
MATERIALS AND METHODS
Material
Plasmids encoding wild-type Rab4, Rab6, and Rab13 were provided by Drs. M. Cormont (University of Nice, France), B. Goud (Curie Institute, Paris, France), and A. Zahraoui (Curie Institute, Paris, France), respectively. Full-length rat N-Sec-1/Munc-18–1 and syntaxin-1 were obtained from R. Scheller, Stanford University, Stanford, CA. The coding sequence of Munc-18 was amplified by PCR and subcloned in myc-pcDNA3, a modified pcDNA3 vector that provides a myc epitope tag (9E10) at the N-terminus of the protein (Regazzi et al., 1996). GDP and GTPγS were obtained from Roche (Rotkreuz, Switzerland). The antibody against insulin was purchased from Linco Research (St. Charles, MO). The antibody against Rab3A (clone 42.2) was obtained from Synaptic System (Göttingen, Germany). The secondary antibodies labeled with Cy3 and Oregon green were from Jackson ImmunoResearch Laboratories (West Grove, PA).
Cloning of Rat Granuphilin-a and -b and Generation of Granuphilin Constructs
The open reading frame of granuphilin-a and -b were cloned by PCR from an INS-1 cDNA library in pBK vector (Stratagene) kindly provided by Dr. C. Bonny, University of Lausanne, Switzerland. The PCR reaction was performed with the forward primer 5′-CGCGGATCCATGTCGGAGATACTAGACCTC-3′ and the reverse primer 5′-GCTCTAGATCATACACCCAGCTTCTGCTT-3′. Both primers were designed according to the sequence of mouse granuphilin-a (accession number AB025258). The PCR reaction was performed under the following conditions: 94°C for 2 min, 30 cycles at 94°C for 30 s, 52°C for 30 s, and 72°C for 2 min. For sequencing and expression experiments, the PCR products were inserted in the BamHI and XbaI cloning sites of myc-pcDNA3. Sequence analysis of the inserts was performed by MWG Biotech Company (Ebersberg, Germany). Granuphilin mutants were generated by site-directed mutagenesis using the QuickChange kit (Stratagene, La Jolla, CA). The plasmid used to produce the fusion protein GST-granuphilin (1–300) was generated by subcloning into the BamHI and XbaI cloning sites of pGEX-KG (Guan and Dixon, 1991) the DNA sequence coding for the first 300 amino acids of rat granuphilins.
Generation of an Antibody Against Granuphilin-a and -b
A polyclonal antibody recognizing granuphilin-a and -b was generated by injecting rabbits with purified GST-granuphilin (1–300) (Eurogentech, Seraing, Belgium). The immunoglobulin fraction of the antisera collected in the third bleed was purified on a protein G affinity column. The specificity of the antibody was verified by comparing the signal obtained by Western blotting or by immunofluorescence in COS cells transfected with an empty vector with that of granuphilin-a or with granuphilin-b.
Cell Culture and Transfection
The insulin-secreting cell lines HIT-T15 and INS-1 were cultured in RPMI 1640 supplemented with 5% fetal calf serum as described previously in detail (Regazzi et al., 1990; Asfari et al., 1992). Transient transfection experiments were performed by electroporating 3 × 106 cells in the presence of 30 μg of plasmid (Coppola et al., 1999). Immediately after electroporation, the cells were resuspended in culture medium and distributed in 24-well multiwell plates at a concentration of ∼3 × 105 cells/well. The expression levels of the different constructs were assessed by Western blotting using a mouse mAb directed against the myc epitope tag.
Subcellular Localization of Granuphilin-a and -b
Subcellular localization of granuphilins was determined by confocal microscopy. For this purpose, untransfected cells or cells expressing the granuphilin constructs were seeded on glass coverslips coated with 20 μg/ml laminin and 0.2 mg/ml poly-l-lysine. Two days later the cells were fixed in 4% paraformaldehyde and incubated for 2 hours at room temperature with the first antibody diluted in buffer A (PBS, pH 7.5, supplemented with 0.1% goat serum [vol/vol], 0.3% Triton-X-100 [vol/vol], and 20 mg/ml BSA). The coverslips were rinsed with PBS and incubated for 30 min at room temperature with the secondary antibodies diluted in buffer A. The coverslips were then washed and mounted for confocal microscopy (Leica, model TCS NT, Lasertechnik, Heidelberg, Germany).
Interaction of Granuphilin with Rab3 and Munc-18
The ability of Rab3 and Munc-18 to bind to granuphilins was tested in vitro with recombinant proteins and in vivo with a mammalian two-hybrid system (CheckMate, Promega, Madison, WI). In the first case, the GST-fusion protein containing the N-terminal domain of granuphilins (amino acids 1–300) was immobilized on glutathione-agarose beads and resuspended in buffer B: 20 mM HEPES pH 7.5, 150 mM KCl, 1 mM dithiothreitol, 5% glycerol, 0.05% Tween-20, and 1 mg/ml BSA. The beads were then incubated with 35S-labeled proteins produced by in vitro translation (Promega), with purified proteins or with cell extracts. His-tagged wild-type Rab3A was produced from a pQE30-based expression vector provided by F. Senic-Matuglia, Curie Institute, Paris, France. The protein was purified from bacterial extracts on Ni2+-NTA beads (Qiagen, Hilden, Germany) according to the manufacturer's protocols. When indicated, immediately before the binding experiment, the purified protein or the cell extracts were loaded for 15 min at 30°C with 1 mM GDP or 1 mM GTPγS. At the end of the incubation, the beads were washed in buffer B. The proteins remaining associated with the GST affinity columns were analyzed by SDS-PAGE and visualized by autoradiography or by Western blotting. To test the interaction with Rab3 or Munc-18 in living cells, the full-length cDNAs of granuphilin mutants were subcloned in frame with VP16 in the expression vector pACT (Promega). Rab3A mutants and Munc-18 were subcloned in frame with GAL4 in the expression vector pBIND (Promega). The GAL4 and VP16 fusion proteins were cotransfected in HIT-T15 cells along with a third plasmid (pG5luc, Promega) encoding five GAL4 binding sites upstream of the firefly luciferase gene. Two days after transfection, the cells were lysed and firefly and Renilla luciferase activity determined by use of the Dual-Luciferase reporter assay system (Promega). Renilla luciferase under the control of a constitutive promoter is encoded by pBIND and was used as an internal control to normalize the transfection efficiency.
Effect of Granuphilin Constructs on Exocytosis
HIT-T15 or INS-1 cells (n = 3 × 106) were transiently cotransfected with 30 μg of the constructs under study and with 10 μg of a plasmid encoding human growth hormone (hGH). Three days later, the cells were washed and preincubated for 30 min in 20 mM HEPES, pH 7.4, 128 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 2.7 mM CaCl2. The medium was then removed, and the cells were incubated for 10 min in the same buffer (basal conditions) or in a buffer containing 20 mM HEPES, pH 7.4, 53 mM NaCl, 80 mM KCl, 1 mM MgCl2, 2.7 mM CaCl2, and 10 mM glucose (stimulatory conditions). In the case of INS-1 cells, the buffer used for the stimulatory conditions also included 1 μM forskolin and 1 mM IBMX. Exocytosis from transfected cells was assessed by measurement by ELISA (Roche, Rotkreuz, Switzerland) of the amount of hGH released in the medium during the incubation period.
Prediction of the Three-Dimensional Structure of Granuphilin
Computer-assisted modeling of the N-terminus of granuphilin was performed with the Swiss-Model program (GlaxoSmithKline, Uxbridge, UK) (Guex et al., 1999)
RESULTS
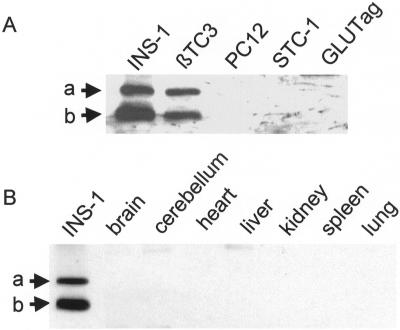
We cloned rat granuphilin-a and -b from a cDNA library derived from the insulin-secreting cell line INS-1. Sequencing of the open reading frames revealed that the two rat granuphilin isoforms are largely homologous with their mouse counterparts (Wang et al., 1999). The sequences of rat granuphilin-a and -b have been submitted to GenBank (GenBank accession numbers AF419341 and AF419342). To investigate the tissue distribution of granuphilins, we generated a polyclonal antibody directed against the N-terminal sequence that is common to the a and b isoforms. As shown in Figure 1A, the two insulin-secreting cell lines INS-1 and β-TC3 express similar levels of granuphilin-a and -b. In contrast, neither of the two granuphilin isoforms is detectable in the rat pheochromocytoma cell line PC12 or in the two mouse cholecystokinin-secreting enteroendocrine cell lines STC-1 and GLUTag. Granuphilin-a and -b were also not expressed in the main organs, including the brain (Figure 1B). These results confirm the distribution of granuphilin-a (Wang et al., 1999) and indicate that granuphilin-b expression is also essentially restricted to pancreatic β-cells.
Figure 1.
Tissue distribution of rat granuphilin-a and -b. (A) Aliquots (30 μg of protein) of homogenates of the indicated cell lines were separated on SDS-PAGE and blotted on nitrocellulose membranes. The proteins were analyzed by Western blotting using a polyclonal antibody directed against the common N-terminal fragment of granuphilins. The arrows indicate the position of granuphilin-a (a) and -b (b). The apparent Mr of bands a and b were 80 and 60 kDa, respectively. (B) Aliquots (30 μg of protein) of homogenates of the indicated rat organs were analyzed by Western blotting using the anti-granuphilin antibody. An INS-1 extract was run in parallel as a positive control.
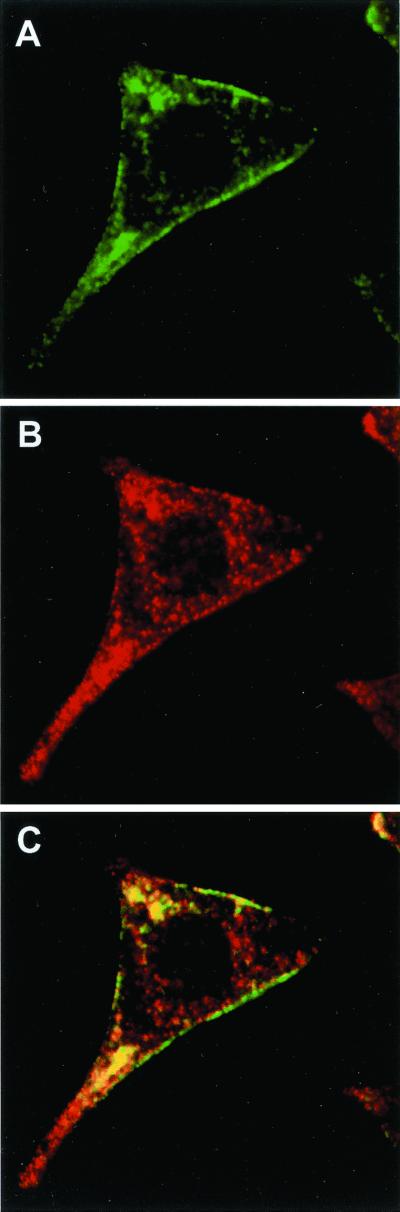
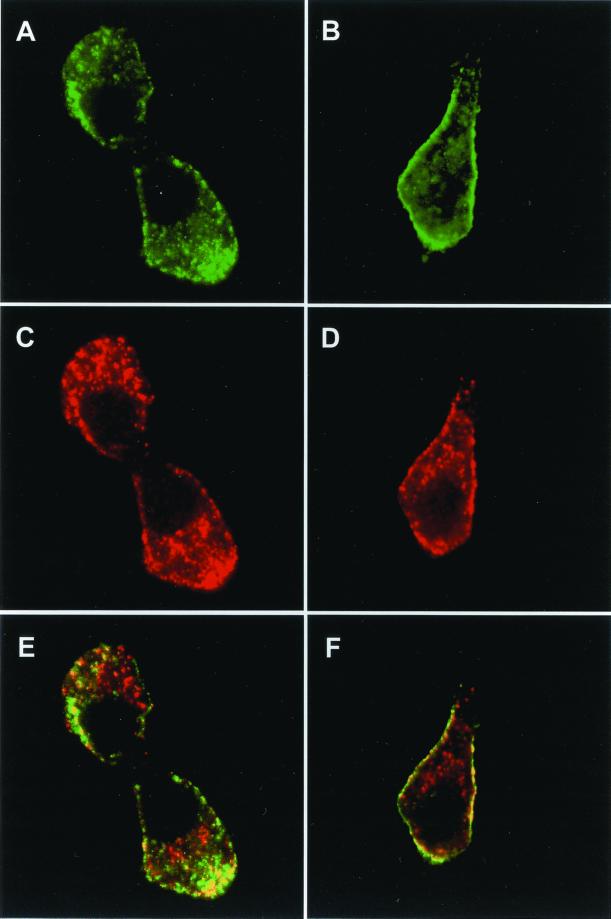
Next, we analyzed by confocal microscopy the subcellular localization of granuphilins in INS-1 cells. As shown in Figure 2, endogenous granuphilins colocalized with insulin-containing secretory granules. However, the secretory granules were not equally labeled with the anti-granuphilin antibody. In fact, granuphilin immunoreactivity was concentrated mainly at the periphery of the cells (Figure 2A), whereas the secretory granules located in the perinuclear region were not labeled (Figure 2B). This suggests that granuphilins may preferentially associate with mature granules. To obtain a detailed analysis of the distribution of each of the two granuphilin isoforms, INS-1 cells were transfected with myc-tagged granuphilin-a or -b. The cells were then analyzed by confocal microscopy using an anti-myc antibody (Figure 3). Using this approach, we found that transfected granuphilin-a and -b display a subcellular distribution similar to the endogenous proteins and colocalize mainly with insulin-containing secretory granules located at the periphery of the cells.
Figure 2.
Subcellular localization of endogenous granuphilins. INS-1 cells were grown on glass coverslips coated with laminin and poly-l-lysine. The cells were fixed with paraformaldehyde and the coverslips incubated with a rabbit polyclonal antibody directed against the N-terminus of granuphilins and an anti-insulin antibody raised in guinea pig. The subcellular distribution of granuphilins was analyzed by confocal microscopy using an Oregon-green–labeled anti-rabbit antibody. The position of secretory granules was visualized with an anti-guinea pig antibody coupled to Cy3. (A) Anti-granuphilins; (B) anti-insulin; (C) overlay between images A and B.
Figure 3.
Subcellular localization of granuphilin-a and -b. INS-1 cells transiently transfected with myc-tagged granuphilin-a (A, C, E) or -b (B, D, F) were grown for 2 days on glass coverslips coated with laminin and poly-l-lysine. The cells were fixed and analyzed by confocal microscopy using an anti-myc antibody and an anti-insulin antibody. (A and B) Anti-myc; (C and D) anti-insulin; (E and F) overlay of the signal obtained with the anti-myc and anti-insulin antibodies.
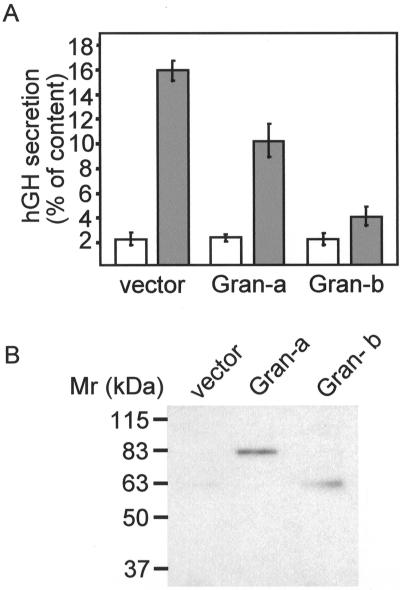
The subcellular localization of granuphilins prompted us to test their potential role in the regulation of insulin exocytosis. For this purpose, the hamster pancreatic β-cell line HIT-T15 was transiently cotransfected with granuphilin-a or -b and with hGH. Because in transfected cells, hGH is targeted to insulin-containing secretory granules, hGH release allows us to monitor selectively the exocytotic process of cells overexpressing the granuphilin isoforms (Coppola et al. 1999; Joberty et al. 1999; Iezzi et al. 2000). As shown in Figure 4A, overexpression of granuphilin-a and -b did not significantly alter basal secretion. In contrast, hGH release triggered by glucose and depolarizing K+ concentrations was greatly impaired. Although the two granuphilin isoforms were expressed at similar levels (Figure 4B), granuphilin-b caused a more pronounced inhibition of exocytosis. Similar experiments were also performed in INS-1 cells. In this cell line, the response to a mixture of secretagogues was smaller, but overexpression of granuphilins also resulted in a strong reduction in stimulated secretion. Thus, in INS-1 cells cotransfected with hGH and an empty vector, a mixture of forskolin (10 μM), IBMX (1 mM), and glucose (10 mM) enhanced hGH release by 2.7 ± 0.3-fold (n = 3). In contrast, in INS-1 cells overexpressing granuphilin-b, the same secretagogues increased hGH secretion by only 1.5 ± 0.1-fold (n = 3).
Figure 4.
Effect of granuphilin-a and -b on exocytosis. HIT-T15 cells were transiently cotransfected with a plasmid encoding hGH and either an empty vector (vector) or plasmids encoding granuphilin-a (Gran-a) or granuphilin-b (Gran-b). After 3 days in culture, the cells were incubated for 10 min under basal conditions (open bars) or in the presence of stimulatory concentrations of K+ and glucose (filled bars). The total amount of hGH expressed by the cells and the fraction released during the incubation period were determined by ELISA. (A) Percentage of hGH present in the cells that is secreted under basal or stimulatory conditions. (B) Expression level of granuphilin-a and -b assessed by Western blotting using an antimyc antibody.
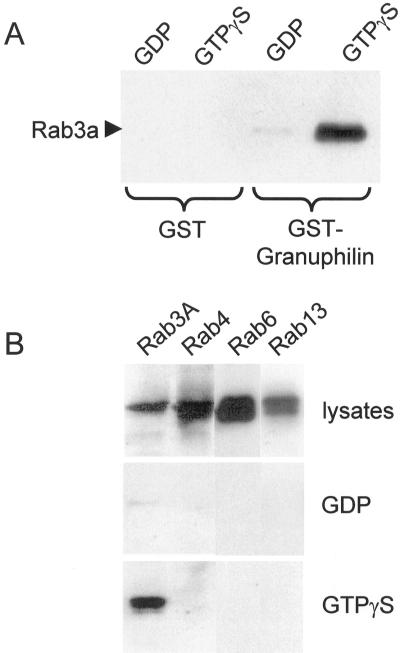
Granuphilins display the same structural organization as and some sequence homology with the Rab3-binding protein rabphilin-3A. The identity between the Rab3-binding region of rabphilin-3A and the first 120 amino acids of granuphilins is ∼30%. Interestingly, most of the amino acids that play a key role in the interaction between Rab3 and rabphilin-3A are conserved in granuphilins. In addition, the conformation of the first 120 amino acids of granuphilins predicted by three-dimensional alignment by use of the Swiss-Model algorithm is very similar to the Rab3-binding domain of rabphilin-3A. In agreement with these observations, we found that recombinant Rab3A binds in a GTP-dependent manner to a GST-fusion protein containing the N-terminus of granuphilins (Figure 5A). No binding was observed with GST alone. To test the ability of granuphilins to interact with native, prenylated Rab GTPases, HIT-T15 cells were transfected with the wild-type form of Rab3A, Rab4, Rab6, and Rab13. The cells were then homogenized and the lysate incubated in the presence of GDP or GTPγS. As shown in Figure 5B, the GTP-bound form of Rab3A was retained on the GST-granuphilin column. In contrast, the binding of Rab3A loaded with GDP was much less efficient. Neither the GDP- nor the GTP-bound forms of Rab4, Rab6, or Rab13, which belong to different Rab subfamilies (Pereira-Leal and Seabra, 2001), associated with GST-granuphilin (Figure 5B).
Figure 5.
The N-terminus of granuphilin binds to Rab3. (A) GST or GST-granuphilin 1–300 were immobilized on glutathione-agarose beads and incubated with purified wild-type Rab3A loaded with GDP or with GTPγS. The proteins remaining associated with the affinity columns were visualized by Western blotting using a monoclonal antibody against Rab3A. (B) HIT-T15 cells were transiently transfected with wild-type Rab3A, Rab4, Rab6, or Rab13. Two days later, the cells were homogenized and the cell lysates incubated either with GDP or GTPγS for 15 min at 30°C. They were then loaded onto GST-granuphilin 1–300 affinity columns. The proteins interacting with GST-granuphilin 1–300 were detected by Western blotting using an antibody against the epitope tag.
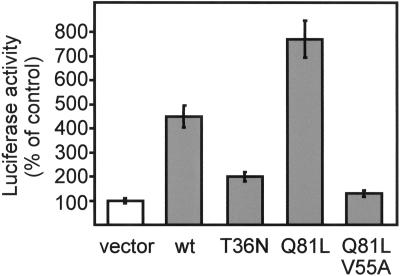
The interaction of granuphilins with Rab3A in living cells was assessed in a mammalian two-hybrid system (Figure 6). HIT-T15 cells were transiently cotransfected with a fusion protein between VP16 and full-length granuphilin-b, with a fusion protein between GAL4 and different Rab3A mutants, and with a luciferase reporter gene under the control of a GAL promoter. Using this approach, we found that full-length granuphilin-b forms a complex with wild-type Rab3A and, even more efficiently, with Rab3AQ81L, a GTPase-deficient mutant that is locked in the GTP-bound conformation (Brondyk et al., 1993). As was the case for the association with rabphilin-3A or RIM (Coppola et al., 1999), the interaction of Rab3AQ81L with granuphilin-b was prevented by a mutation in the effector loop of the GTPase (Rab3AQ81L, V55A). The association of granuphilin-b with Rab3AT36N, a mutant that is predominantly in the GDP-bound conformation (Brondyk et al., 1993), was very weak. Similar results were obtained with a VP16–granuphilin-a construct and with GAL4 fusion proteins of the other Rab3 isoforms (Rab3B, -C and -D) (data not shown). Together, these results demonstrate that granuphilins are Rab3 targets.
Figure 6.
Granuphilin interacts with the GTP-bound form of Rab3 in living cells. HIT-T15 cells were cotransfected with a fusion protein of VP16 with granuphilin-b, a fusion protein of GAL4 with the indicated mutants of Rab3A, and a plasmid containing five binding sites for GAL4 upstream of the firefly luciferase gene. The interaction between granuphilin-b and the Rab3A mutants was quantified by measurement of firefly luciferase activity. The luciferase activity produced in cells transfected with VP16/granuphilin-b and a GAL4 empty vector was set to 100%. The results are the mean ± SD of three independent experiments performed in duplicate. wt, wild-type.
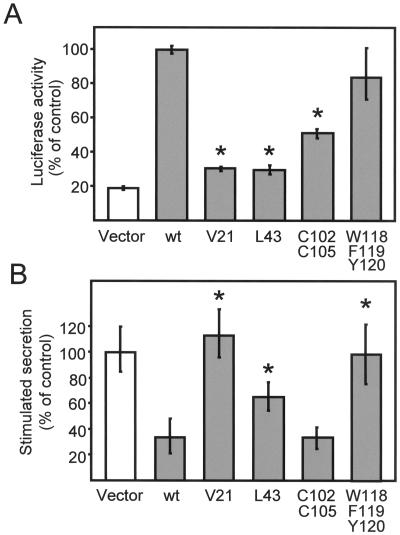
Next, we tested whether the interaction with Rab3 is required for the inhibition of exocytosis observed in cells overexpressing granuphilins. For this purpose, using the mammalian two-hybrid system, we engineered a series of amino acid substitutions within the N-terminus of granuphilin-b and tested the ability of these mutants to interact with Rab3A. The amino acids to be mutated were selected by taking advantage of the knowledge about the properties of the Rab3A/rabphilin-3A complex. Alanine substitutions of V57 and L79 in rat rabphilin-3A (corresponding to V61 and L83 in bovine rabphilin-3A) abolish the interaction with Rab3A (Joberty et al., 1999; Coppola et al., 2001). Replacement of the corresponding amino acids in granuphilin-b, V21 and L43, dramatically impaired the binding of Rab3A (Figure 7A). As was the case with rabphilin-3A (McKiernan et al., 1996), substitution of two cysteines (C102 and C105) in the zinc-finger motif of granuphilin-b reduced the interaction with Rab3A by ∼50%. In contrast, mutation of three amino acids (W118/F119/Y120) in the sequence homologous to the SGAWFF motif of rabphilin-3A did not significantly alter the capacity of granuphilin-b to associate with Rab3A.
Figure 7.
Effect of different point mutations of granuphilin on Rab3 binding and on exocytosis. (A) HIT-T15 cells were cotransfected with a fusion protein of GAL4 with the GTPase-deficient mutant Rab3AQ81L, a fusion protein of VP16 with the indicated mutants of granuphilin-b and a plasmid containing five binding sites for GAL4 upstream of the firefly luciferase gene. The interaction of the GTP-bound form of Rab3A with the mutants of granuphilin-b was assessed by measuring firefly luciferase activity. The luciferase activity produced in cells expressing the fusion proteins of GAL4 with Rab3AQ81L and VP16 with wild-type (wt) granuphilin-b was set to 100%. The results are the mean ± SD of three independent experiments performed in duplicate. Asterisks indicate the mutants whose interaction with Rab3A is significantly (p < 0.01) different from wild-type granuphilin-b. (B) HIT-T15 cells were transiently transfected with the indicated mutants of granuphilin-b and with a plasmid encoding hGH. After 3 days in culture, the cells were incubated under basal conditions or in the presence of depolarizing concentrations of K+ and glucose. The amount of hGH released into the medium under basal and stimulatory conditions was determined by ELISA. The response of the cells transfected with the hGH plasmid together with an empty vector (vector) was set to 100%. The figure shows the mean ± SD of at least five independent experiments measured in triplicate. Asterisks indicate the mutants whose effect on exocytosis is significantly (p < 0.01) different from that of wild-type granuphilin-b.
The different mutants of granuphilin-b were then transfected in HIT-T15 cells and tested for their ability to inhibit exocytosis. Before measuring the effect on exocytosis, we verified the expression level and the subcellular distribution of each mutant. All the constructs were expressed at equal levels, and none of the amino acid substitutions affected the association of granuphilin-b with secretory granules (not shown). The inhibitory effect of granuphilin on exocytosis was either completely (V21) or partially (L43) lost in the two mutants unable to bind Rab3A (Figure 7B). However, the C102/C105 mutant, which only partially retains the capacity to associate with Rab3A, nevertheless inhibited hormone release as efficiently as wild-type granuphilin-b (Figure 7B). Thus, although a complete loss of granuphilin/Rab3 binding reduces the inhibition of exocytosis, a partial loss has no effect. The W118/F119/Y120 mutant, which fully retains the capacity to bind Rab3A, did not affect exocytosis (Figure 7B). Therefore, the ability to form a complex with Rab3 is not in itself sufficient to explain the decrease in secretion in granuphilin-expressing cells. This indicates the existence of additional binding partners of granuphilin that participate in the control of exocytosis.
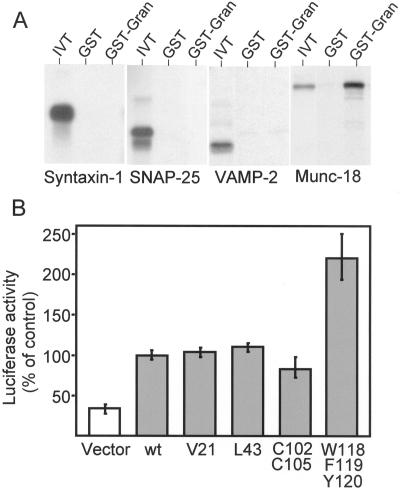
In an attempt to identify other potential partners of granuphilins, we produced by in vitro translation different components of the machinery of exocytosis of pancreatic β-cells. The radioactively labeled proteins were then tested for their ability to bind to GST-granuphilin (1–300) and to GST alone. Using this approach, we found that the components of the SNARE complex, syntaxin-1, SNAP-25, and VAMP-2, do not interact with the N-terminus of granuphilin (Figure 8A). In contrast, the SNARE-binding protein N-Sec-1/Munc-18 was efficiently retained on the GST-granuphilin affinity column but not on the GST control column. A weak but highly reproducible interaction of full-length granuphilin-b with Munc-18 was also detectable in living HIT-T15 cells by use of the mammalian two-hybrid system (Figure 8B). In view of these results, we analyzed the effect of granuphilin-b mutations on the association with Munc-18. The three granuphilin mutants displaying a reduced Rab3-binding activity (see Figure 7) did not show any statistical difference in the interaction with Munc-18 (Figure 8B). In contrast, the W118/F119/Y120 mutant was found to bind to Munc-18 more efficiently than the wild-type protein (Figure 8B).
Figure 8.
Granuphilin interacts with Munc-18 in vitro and in living cells. (A) GST or GST-granuphilin 1–300 (GST-Gran) were immobilized on glutathione agarose beads and incubated with in vitro translated 35S-labeled syntaxin-1, SNAP-25, VAMP-2, and Munc-18 (IVT). The proteins remaining associated with the affinity columns were separated by SDS-PAGE and visualized by autoradiography. (B) HIT-T15 cells were cotransfected with a fusion protein of GAL4 with Munc-18, a fusion protein of VP16 with the indicated mutants of granuphilin-b, and a firefly luciferase reporter gene. The luciferase activity produced in cells expressing the fusion proteins of GAL4 with Munc-18 and VP16 with wild-type (wt) granuphilin-b was set to 100%. The results are the mean ± SD of three independent experiments performed in duplicate.
DISCUSSION
Granuphilin-a and -b are two splicing variants of a gene identified in a screening for mRNAs differentially expressed between pancreatic α- and β-cells (Wang et al., 1999). Western blotting and immunohistochemical analysis using an antibody that recognizes granuphilin-a revealed that the protein is expressed selectively in pancreatic β-cells and in the pituitary gland (Wang et al., 1999). In this study, we demonstrate that pancreatic β-cell lines also express high levels of granuphilin-b and that this protein is not detectable in other organs, including the brain. Interestingly, we found that granuphilin-a and -b are not present in the endocrine cell lines PC12, STC-1, and GLUTag, which have been shown to share with pancreatic β-cells most of the components of the machinery for exocytosis (Némoz-Gaillard et al., 1998; Lang, 1999; Gevrey et al., 2001). These findings suggest that granuphilins play a role in specialized functions of pancreatic β-cells. Subcellular fractionation studies indicated that granuphilin-a is associated with insulin-containing secretory granules (Wang et al., 1999). We have confirmed by confocal microscopy that both granuphilin-a and -b colocalize with dense core granules of pancreatic β-cells. Our data suggest that granuphilins associate preferentially with the subpopulation of secretory granules located at the periphery of the cells. In view of this observation, it is tempting to speculate that granuphilins are recruited at the surface of insulin-containing granules only at later stages of the secretory process. Verification of this hypothesis will require a detailed characterization of the different subpopulations of secretory granules present in pancreatic β-cells.
The association of granuphilins with mature secretory granules is compatible with a role of the protein in insulin release. Indeed, we found that overexpression of granuphilins has profound effects on exocytosis. Basal secretion was not affected, but the release of the granule content in stimulated cells was strongly reduced. Granuphilin (Slp-4) belongs to the synaptotagmin-like protein family (Fukuda and Mikoshiba, 2001; Fukuda et al., 2001). The members of this family are characterized by the presence of C2 domains located at the C-terminus of the protein and of two conserved sequences designated Slp homology domains 1 (SHD1) and 2 (SHD2) (Fukuda et al., 2001). These domains have been proposed to constitute protein–protein interaction sites (Fukuda et al., 2001). Indeed, we found that mutations of conserved amino acids within granuphilin SHD1 (V21 and L43) and SHD2 (W118/F119/Y120) cause the loss of the inhibitory function. In this study, we identified two proteins that interact with the SHD domains of granuphilin, Rab3 and Munc-18. The interaction with Munc-18 is not affected by mutations in SHD1 but is enhanced by substitutions in SHD2. The role of Munc-18 in the secretory process is complex and still not fully understood. With the data currently available, it is difficult to ascertain whether the lack of effect of the W118/F119/Y120 mutant in exocytosis is related to an increase in the binding to Munc-18 or to a failure to interact with another, as yet unidentified, partner of granuphilin. Munc-18 is believed to control the docking of secretory vesicles at the plasma membrane by binding to syntaxin-1 (Dulubova et al., 1999; Voets et al., 2001). Future investigations will have to determine whether the interaction with granuphilins influences the binding of Munc-18 to syntaxin-1 and, in turn, the docking of secretory granules.
In contrast to Munc-18, the association of granuphilins to Rab3 involves SHD1. Granuphilins possess all the criteria to be considered bona fide Rab3 effectors. First, they are able to interact with Rab3 isoforms both in vitro and in vivo. Second, granuphilins associate efficiently only with the active form of Rab3. Third, the interaction with Rab3 occurs through the effector loop of the GTPase. Fourth, the domain of granuphilins involved in Rab3 binding is predicted to assume a three-dimensional conformation very similar to that of the corresponding regions of rabphilin-3A. Accordingly, mutations of key amino acids in the predicted binding interface of granuphilins disrupt the association with Rab3. These mutants gave us the opportunity to investigate the functional role of the granuphilin/Rab3 interaction. The two point mutations in SHD1 that prevent Rab3 binding (V21 and L43) cause a decrease in the capacity to inhibit secretion, suggesting that the interaction with the GTPase is required for the control of exocytosis. However, the C102/C105 granuphilin mutant, which possesses a reduced affinity for Rab3, inhibits exocytosis as well as wild-type granuphilin. This apparent discrepancy could be explained by the fact that even with a partial reduction in the affinity for GTPase, the granuphilin/Rab3 complexes formed in cells overexpressing the C102/C105 mutant are still sufficient to cause full inhibition of exocytosis. The W118/F119/Y120 mutant retains the capacity to bind Rab3 but does not affect exocytosis. This indicates that in itself, the granuphilin/Rab3 complex is not sufficient for the inhibition of secretion. In view of this observation, it can be predicted that SHD2 constitutes a protein–protein interface to accommodate additional granuphilin partners involved in the exocytotic process. The identification of such proteins will be an important task for future investigations.
To the best of our knowledge, this is the first report on structure–function studies concerning members of the Slp family. The presence of SHD1 and SHD2 is a characteristic feature of all members of the Slp family (Fukuda et al., 2001). Moreover, the amino acids that play a key role in granuphilin-mediated inhibition of secretion are very well conserved among other Slp family members. In view of this, it can be predicted that most, if not all, Slp family members participate in the control of exocytosis in different cell systems. It should be noted that the cysteine-rich sequence corresponding to amino acids 47–107 in granuphilin is missing in Slp1 and Slp2a. Because C102/C105 are involved in Rab3 binding, the ability to interact with Rab3 is probably a peculiarity of granuphilin and not a general feature of all Slp family members.
In conclusion, we have demonstrated that granuphilins associate with a subpopulation of secretory granules and potently modulate exocytosis of pancreatic β-cells. We were able to identify two important components of the secretory machinery of pancreatic β-cells that bind to granuphilins, Rab3 and Munc-18. Our data suggest that the interaction with Rab3 is important for granuphilin function. However, in itself, the binding to Rab3 and Munc-18 is not sufficient to explain the effect of granuphilins on exocytosis, suggesting the existence of additional binding partners. Screening for the granuphilin partners susceptible to regulating insulin exocytosis will certainly be facilitated by the knowledge of the properties of granuphilin mutants gathered in this study.
ACKNOWLEDGMENTS
We thank Dr. C. Bonny for providing the INS-1 cDNA library and Drs. M. Cormont, B. Goud, A. Zahraoui, R. Scheller, and F. Senic-Matuglia for the supply of plasmids. We are also grateful to Dr. J. Abello for GLUTag and STC-1 cell extracts and to Dr. P. Clarke for critical reading of the manuscript. This work was supported by the Swiss National Science Foundation, grant 32–61400.00 (R.R.).
Abbreviations used:
- hGH
human growth hormone
- SHD
Slp homology domain
- SNAP
soluble N-ethylmaleimide-sensitive factor attachment protein
- SNARE
SNAP receptor
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02–02–0025. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.02–02–0025.
REFERENCES
- Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130:167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- Brondyk WH, McKiernan CJ, Burstein ES, Macara IG. Mutants of Rab3A analogous to oncogenic Ras mutants. J Biol Chem. 1993;268:9410–9415. [PubMed] [Google Scholar]
- Chung SH, Takai Y, Holz RW. Evidence that the Rab3a-binding protein, rabphilin3a, enhances regulated secretion. J Biol Chem. 1995;270:16714–16717. doi: 10.1074/jbc.270.28.16714. [DOI] [PubMed] [Google Scholar]
- Coppola T, Hirling H, Perret-Menoud V, Gattesco S, Catsicas S, Joberty G, Macara IG, Regazzi R. Rabphilin dissociated from Rab3 promotes endocytosis through interaction with rabaptin-5. J Cell Sci. 2001;114:1757–1764. doi: 10.1242/jcs.114.9.1757. [DOI] [PubMed] [Google Scholar]
- Coppola T, Perret-Menoud V, Lüthi S, Farnsworth CC, Glomset JA, Regazzi R. Disruption of Rab3-calmodulin interaction, but not other effector interactions, prevents Rab3 inhibition of exocytosis. EMBO J. 1999;18:5885–5891. doi: 10.1093/emboj/18.21.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Südhof TC, Rizo J. A conformational switch in syntaxin during exocytosis: role of munc-18. EMBO J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easom RA. β-Granule transport and exocytosis. Cell Dev Biol. 2000;11:253–266. doi: 10.1006/scdb.2000.0174. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Mikoshiba K. Synaptotagmin-like protein 1–3: a novel family of C-terminal-type tandem C2 proteins. Biochem Biophys Res Commun. 2001;281:1226–1233. doi: 10.1006/bbrc.2001.4512. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Saegusa C, Mikoshiba K. Novel splicing isoforms of synaptotagmin-like proteins 2 and 3: identification of the Slp homology domain. Biochem Biophys Res Commun. 2001;283:513–519. doi: 10.1006/bbrc.2001.4803. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Stevens CF, Südhof TC. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature. 1997;387:810–814. doi: 10.1038/42954. [DOI] [PubMed] [Google Scholar]
- Gevrey J-C, Laurent S, Saurin J-C, Némoz-Gaillard E, Regazzi R, Chevrier A-M, Chayvalle J-A, Abello J. Rab3a controls exocytosis in cholecystokinin-secreting cells. FEBS Lett. 2001;503:19–24. doi: 10.1016/s0014-5793(01)02683-7. [DOI] [PubMed] [Google Scholar]
- Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Guex N, Diemand A, Peitsch MC. Protein modeling for all. Trends Biochem Sci. 1999;24:364–367. doi: 10.1016/s0968-0004(99)01427-9. [DOI] [PubMed] [Google Scholar]
- Haynes LP, Evans GJO, Morgan A, Burgoyne RD. A direct inhibitory role for the Rab3-specific effector, Noc2, in Ca2+-regulated exocytosis in neuroendocrine cells. J Biol Chem. 2001;276:9726–9732. doi: 10.1074/jbc.M006959200. [DOI] [PubMed] [Google Scholar]
- Holz R, Brondyk WH, Senter RA, Kuzion L, Macara IG. Evidence for the involvement of Rab3A in Ca2+-dependent exocytosis from adrenal chromaffin cells. J Biol Chem. 1994;269:10229–10234. [PubMed] [Google Scholar]
- Iezzi M, Escher G, Meda M, Charollais A, Baldini G, Darchen F, Wollheim CB, Regazzi R. Subcellular distribution and function of Rab3A, B, C, and D isoforms in insulin-secreting cells. Mol Endocrinol. 1999;13:202–212. doi: 10.1210/mend.13.2.0228. [DOI] [PubMed] [Google Scholar]
- Iezzi M, Regazzi R, Wollheim CB. The Rab3-interacting molecule RIM is expressed in pancreatic β-cells and is implicated in insulin exocytosis. FEBS Lett. 2000;474:66–70. doi: 10.1016/s0014-5793(00)01572-6. [DOI] [PubMed] [Google Scholar]
- Jahn R, Südhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- Joberty G, Stabila PF, Coppola T, Macara IG, Regazzi R. High affinity Rab3 binding is dispensable for rabphilin-dependent potentiation of stimulated secretion. J Cell Sci. 1999;112:3579–3587. doi: 10.1242/jcs.112.20.3579. [DOI] [PubMed] [Google Scholar]
- Johannes L, Lledo PM, Roa M, Vincent JD, Henry J-P, Darchen F. The GTPase Rab3a negatively controls calcium-dependent exocytosis in neuroendocrine cells. EMBO J. 1994;13:2029–2037. doi: 10.1002/j.1460-2075.1994.tb06476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J. Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur J Biochem. 1999;259:3–17. doi: 10.1046/j.1432-1327.1999.00043.x. [DOI] [PubMed] [Google Scholar]
- Lupashin VV, Waters MG. t-SNARE activation through transient interaction with a Rab-like guanosine triphosphatase. Science. 1997;276:1255–1258. doi: 10.1126/science.276.5316.1255. [DOI] [PubMed] [Google Scholar]
- Martelli AM, Baldini G, Tabellini G, Koticha D, Bareggi R, Baldini G. Rab3A and Rab3D control the total granule number and the fraction of granules docked at the plasma membrane in PC12 cells. Traffic. 2000;1:976–986. [PubMed] [Google Scholar]
- McKiernan CJ, Stabila PF, Macara IG. Role of the Rab3A-binding domain in targeting of rabphilin-3a to vesicle membranes of PC12 cells. Mol Cell Biol. 1996;16:4985–4995. doi: 10.1128/mcb.16.9.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Némoz-Gaillard E, Bosshart A, Regazzi R, Bernard C, Cuber J-C, Takahashi M, Catsicas S, Chayvialle J-A, Abello J. Expression of SNARE proteins in enteroendocrine cell lines and functional role of tetanus toxin-sensitive proteins in cholecystokinin release. FEBS Lett. 1998;425:66–70. doi: 10.1016/s0014-5793(98)00209-9. [DOI] [PubMed] [Google Scholar]
- Ostermeier C, Brunger AT. Structural basis of Rab effector specificity: crystal structure of the small G protein Rab3A complexed with the effector domain of rabphilin-3A. Cell. 1999;96:363–374. doi: 10.1016/s0092-8674(00)80549-8. [DOI] [PubMed] [Google Scholar]
- Pereira-Leal JB, Seabra MC. Evolution of the Rab family of small GTP-binding proteins. J Mol Biol. 2001;313:889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- Regazzi R, Li G, Deshusses J, Wollheim CB. Stimulus-response coupling in insulin-secreting HIT cells. Effects of secretagogues on cytosolic Ca2+, diacylglycerol, and protein kinase C activity. J Biol Chem. 1990;265:15003–15009. [PubMed] [Google Scholar]
- Regazzi R, Ravazzola M, Iezzi M, Lang J, Zahraoui A, Andereggen E, Morel P, Takai Y, Wollheim CB. Expression, localization and functional role of small GTPases of the Rab3 family in insulin-secreting cells. J Cell Sci. 1996;109:2265–2273. doi: 10.1242/jcs.109.9.2265. [DOI] [PubMed] [Google Scholar]
- Roa M, Paumet F, Le Mao J, David B, Blank U. Involvement of the ras-like GTPase rab3d in RBL-2H3 mast cell exocytosis following stimulation via high affinity IgE receptors (FcεRI) J Immunol. 1997;159:2815–2823. [PubMed] [Google Scholar]
- Rothman JD. Mechanism of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Shirataki H, Kaibuchi K, Sakoda T, Kishida S, Yamaguchi T, Wada K, Miyazaki M, Takai Y. Rabphilin-3A, a putative target protein for smg p25A/rab3A p25 small GTP-binding protein related to synaptotagmin. Mol Cell Biol. 1993;13:2061–2068. doi: 10.1128/mcb.13.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirataki H, Kaibuchi K, Yamaguchi T, Wada K, Horiuchi H, Takai Y. A possible target protein for smg-25/rab3A small GTP-binding protein. J Biol Chem. 1992;267:10946–10949. [PubMed] [Google Scholar]
- Sun L, Bittner MA, Holz RW. Rab3a binding and secretion-enhancing domains in Rim1 are separate and unique. J Biol Chem. 2001;276:12911–12917. doi: 10.1074/jbc.M011110200. [DOI] [PubMed] [Google Scholar]
- Voets T, Toonen RF, Brian EC, de Wit H, Moser T, Rettig J, Südhof TC, Neher E, Verhage M. Munc18–1 promotes large dense-core vesicle docking. Neuron. 2001;31:581–591. doi: 10.1016/s0896-6273(01)00391-9. [DOI] [PubMed] [Google Scholar]
- Wang J, Takeuchi T, Yokota H, Izumi T. Novel rabphilin-3-like protein associates with insulin-containing granules in pancreatic beta cells. J Biol Chem. 1999;274:28542–28548. doi: 10.1074/jbc.274.40.28542. [DOI] [PubMed] [Google Scholar]
- Wang X, Hu B, Zimmermann B, Kilimann MW. Rim1 and rabphilin-3 bind Rab3-GTP by composite determinants partially related through N-terminal α-helix motifs. J Biol Chem. 2001;276:32480–32488. doi: 10.1074/jbc.M103337200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Okamoto M, Schmitz F, Hofmann K, Südhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]