Abstract
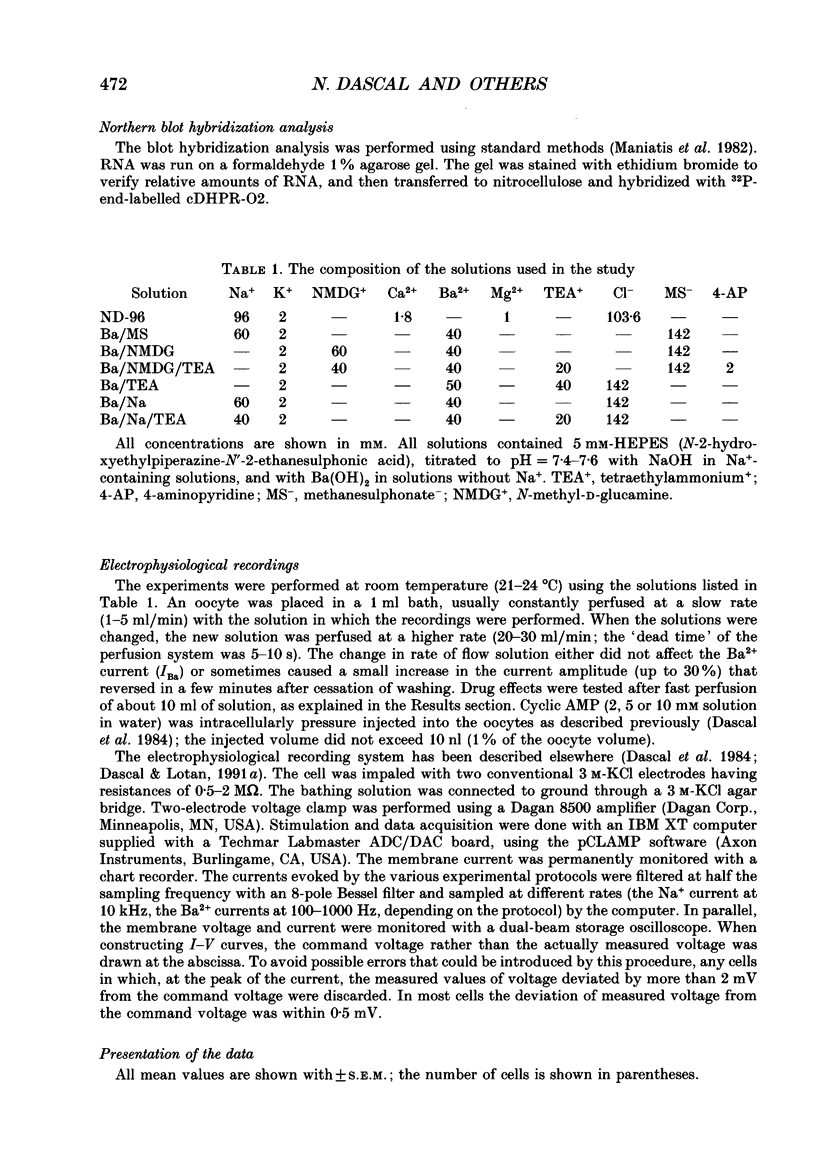
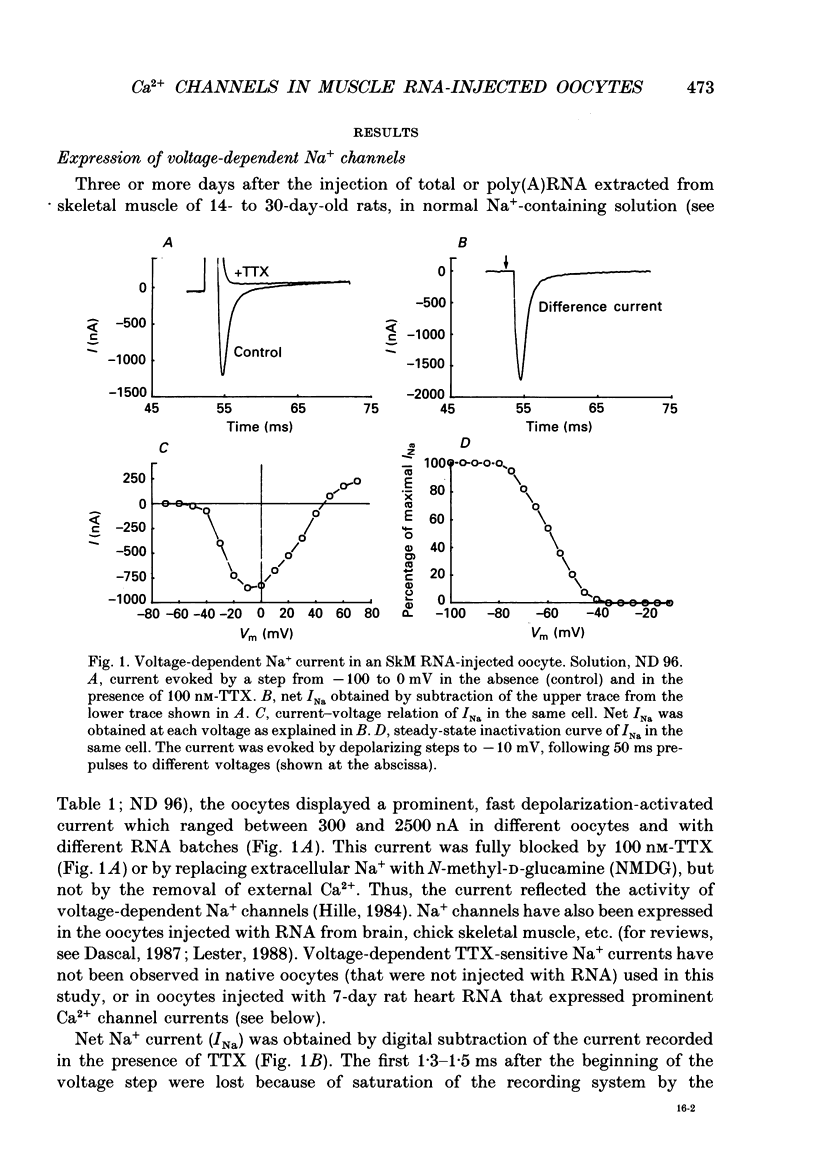
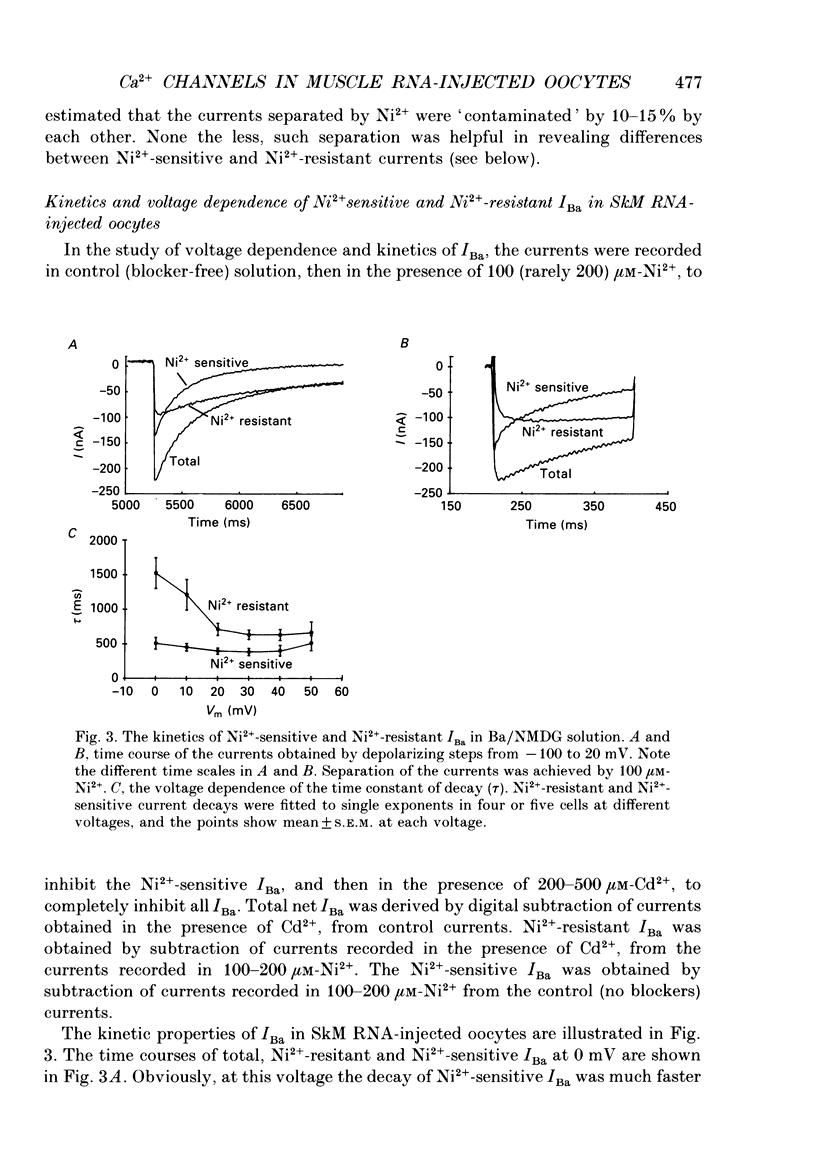
1. Ba2+ currents (IBa) through voltage-dependent Ca2+ channels were studied in Xenopus laevis oocytes injected with heterologous RNA extracted from skeletal muscle (SkM) of young rats, using the two-electrode voltage clamp technique. 2. With 40 or 50 mM-extracellular Ba2+, native oocytes of most frogs displayed IBa between -5 and -20 nA at 0 mV. However, in 'variant' native oocytes of four frogs, IBa exceeded -30 nA and reached up to -100 nA. In oocytes injected with SkM RNA, IBa of up to -250 nA was observed. 3. In SkM RNA-injected oocytes and 'variant' native oocytes, the decay of IBa displayed two kinetic components. The faster component was selectively blocked by 40-100 microM-Ni2+ and thus was termed the Ni(2+)-sensitive IBa. The slower component was Ni2+ resistant, being inhibited only 10-20% by 100-200 microM-Ni2+. The half-activation and the half-inactivation voltages of the Ni(2+)-sensitive IBa were more negative (by 14.5 and 28.7 mV, respectively) than those of the Ni(2+)-resistant IBa. 4. Neither Ni(2+)-sensitive nor Ni(2+)-resistant IBa in native or SkM RNA-injected oocytes were affected by dihydropyridine antagonists nifedipine and (+) PN 200-110 (1-10 microM), by the dihydropyridine agonist (-)Bay K 8644 (0.01-2 microM), or by verapamil below 50 microM. IBa was blocked by diltiazem (half-block at about 500 microM). Thus, the pharmacology of IBa in SkM RNA-injected and in native oocytes was not characteristic of the L-type Ca2+ channel abundant in the skeletal muscle. 5. Destruction of the RNA coding for the channel-forming alpha 1-subunit of the SkM L-type Ca2+ channel using a hybrid arrest method failed to selectively suppress the appearance of either Ni(2+)-sensitive or Ni(2+)-resistant IBa in SkM RNA-injected oocytes. 6. Our results suggest that the appearance of large voltage-dependent Ba2+ currents in SkM RNA-injected oocytes is not due to the expression of the alpha 1-subunit of the SkM L-type Ca2+ channel. The possibility that the expression of a channel-forming subunit of another Ca2+ channel type underlies one of these currents cannot be rejected. However, since the Ba2+ currents in SkM RNA-injected oocytes resemble those observed in native oocytes, we suggest that their appearance may be the result of an enhanced activity of the native Ca2+ channels, possibly due to the expression of the 'auxiliary' subunits of the SkM Ca2+ channel that form complexes with a native alpha 1-subunit.
Full text
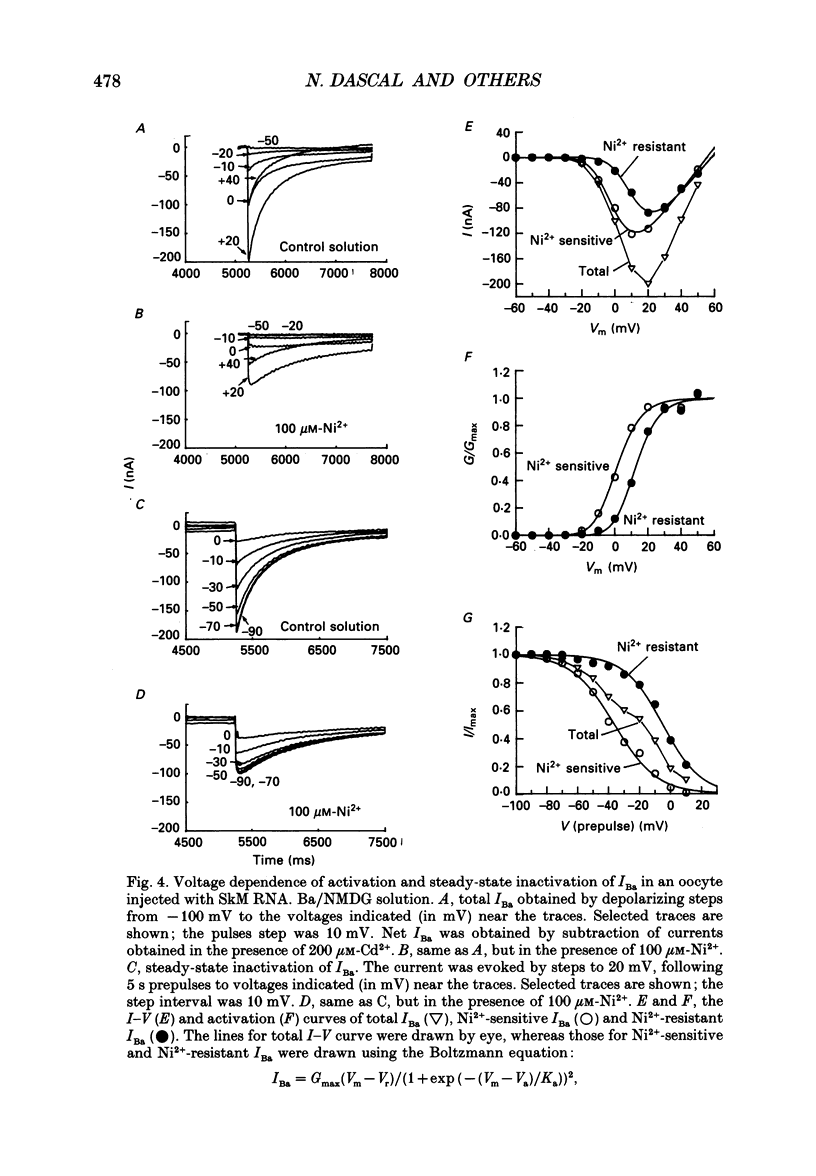
PDF






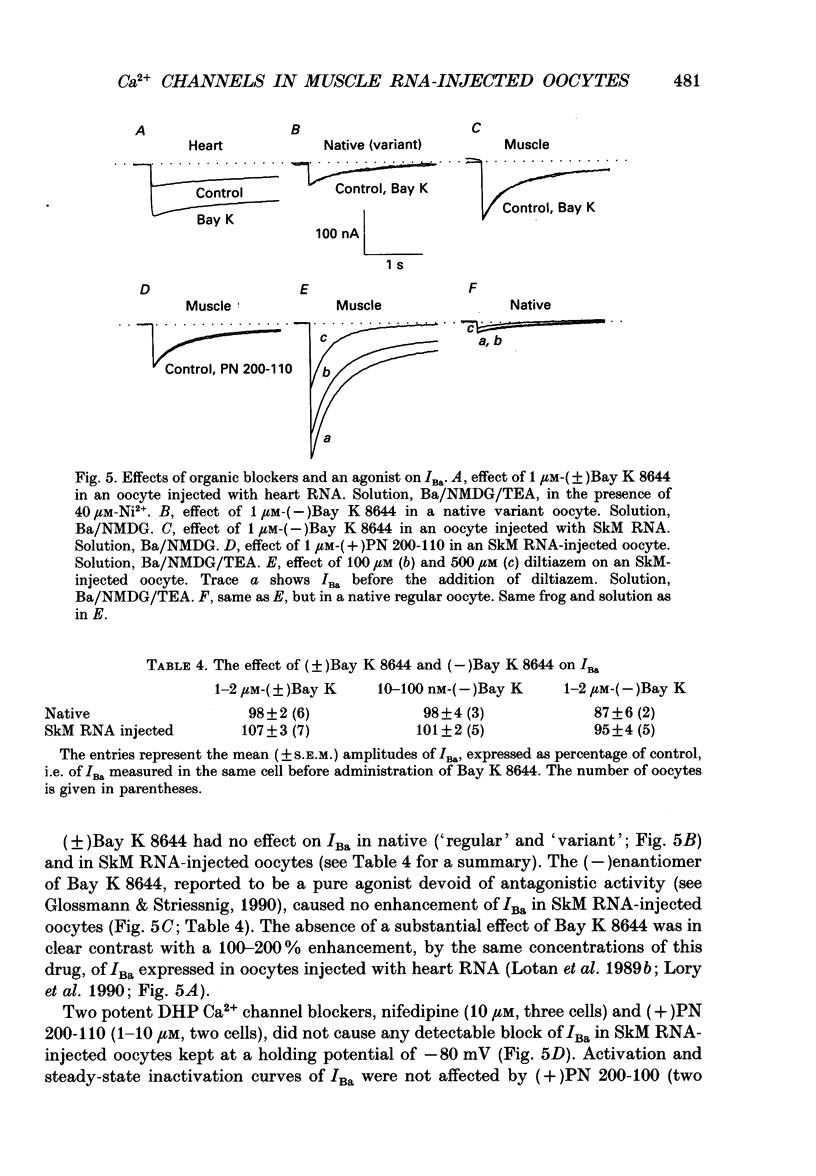

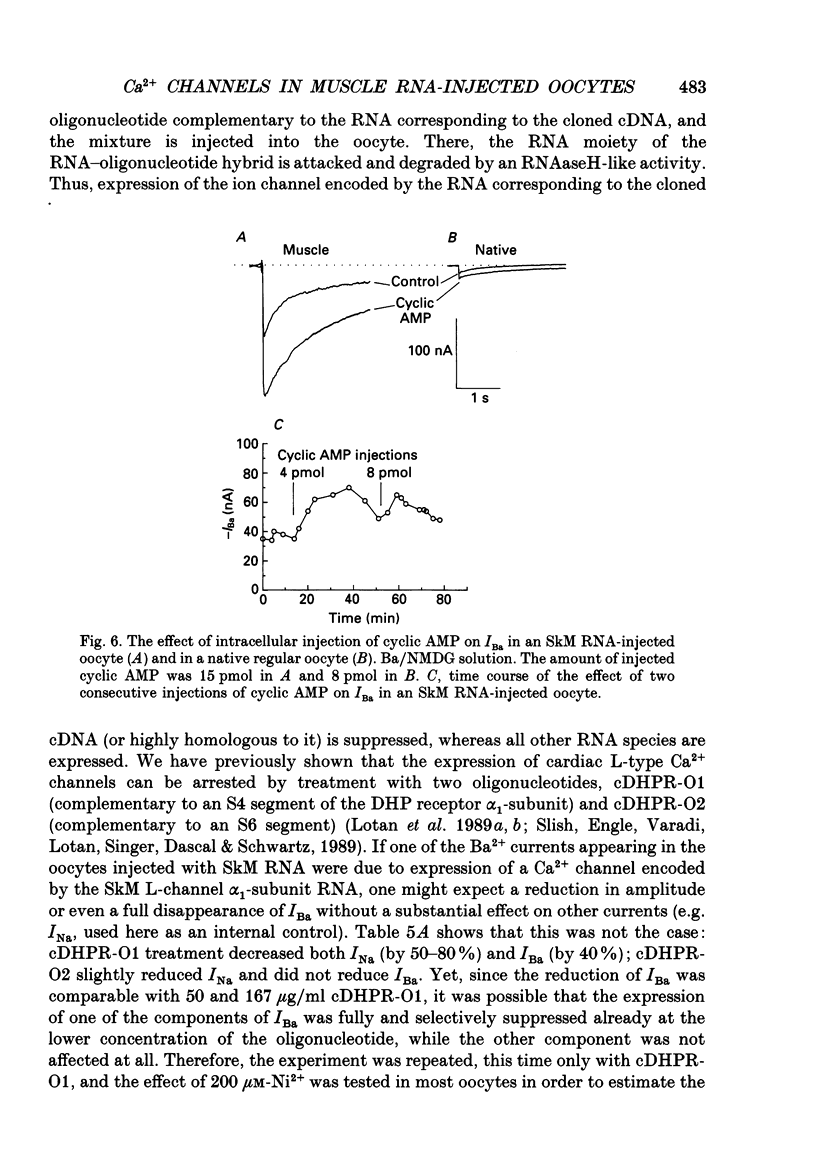
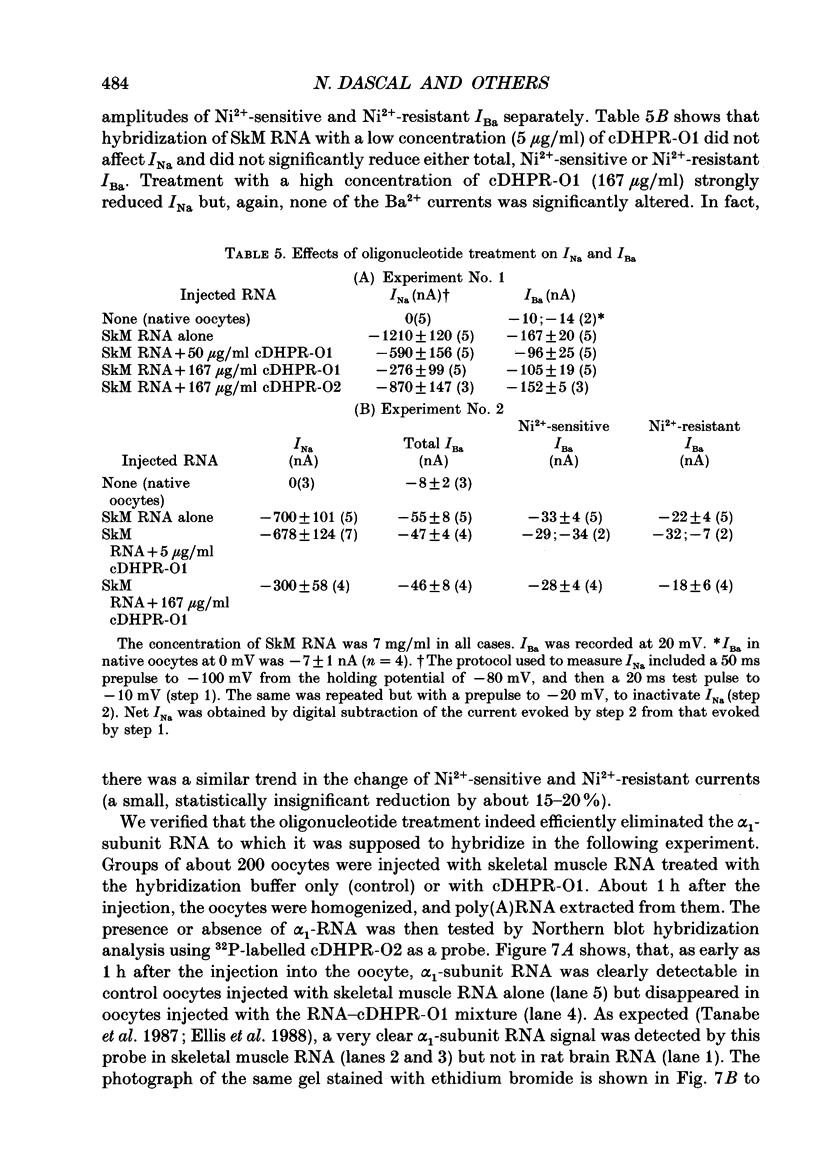





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. A., Beam K. G. A novel calcium current in dysgenic skeletal muscle. J Gen Physiol. 1989 Sep;94(3):429–444. doi: 10.1085/jgp.94.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Palade P. T. Slow calcium and potassium currents across frog muscle membrane: measurements with a vaseline-gap technique. J Physiol. 1981 Mar;312:159–176. doi: 10.1113/jphysiol.1981.sp013622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arreola J., Calvo J., García M. C., Sánchez J. A. Modulation of calcium channels of twitch skeletal muscle fibres of the frog by adrenaline and cyclic adenosine monophosphate. J Physiol. 1987 Dec;393:307–330. doi: 10.1113/jphysiol.1987.sp016825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G., Knudson C. M. Calcium currents in embryonic and neonatal mammalian skeletal muscle. J Gen Physiol. 1988 Jun;91(6):781–798. doi: 10.1085/jgp.91.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G., Knudson C. M. Effect of postnatal development on calcium currents and slow charge movement in mammalian skeletal muscle. J Gen Physiol. 1988 Jun;91(6):799–815. doi: 10.1085/jgp.91.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Biel M., Ruth P., Bosse E., Hullin R., Stühmer W., Flockerzi V., Hofmann F. Primary structure and functional expression of a high voltage activated calcium channel from rabbit lung. FEBS Lett. 1990 Sep 3;269(2):409–412. doi: 10.1016/0014-5793(90)81205-3. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-sensitive ion channels. Science. 1988 Oct 7;242(4875):50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Cognard C., Lazdunski M., Romey G. Different types of Ca2+ channels in mammalian skeletal muscle cells in culture. Proc Natl Acad Sci U S A. 1986 Jan;83(2):517–521. doi: 10.1073/pnas.83.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N. Analysis and functional characteristics of dihydropyridine-sensitive and -insensitive calcium channel proteins. Biochem Pharmacol. 1990 Sep 15;40(6):1171–1178. doi: 10.1016/0006-2952(90)90380-4. [DOI] [PubMed] [Google Scholar]
- Dascal N., Landau E. M., Lass Y. Xenopus oocyte resting potential, muscarinic responses and the role of calcium and guanosine 3',5'-cyclic monophosphate. J Physiol. 1984 Jul;352:551–574. doi: 10.1113/jphysiol.1984.sp015310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N., Lotan I. Activation of protein kinase C alters voltage dependence of a Na+ channel. Neuron. 1991 Jan;6(1):165–175. doi: 10.1016/0896-6273(91)90131-i. [DOI] [PubMed] [Google Scholar]
- Dascal N., Snutch T. P., Lübbert H., Davidson N., Lester H. A. Expression and modulation of voltage-gated calcium channels after RNA injection in Xenopus oocytes. Science. 1986 Mar 7;231(4742):1147–1150. doi: 10.1126/science.2418503. [DOI] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22(4):317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Donaldson P. L., Beam K. G. Calcium currents in a fast-twitch skeletal muscle of the rat. J Gen Physiol. 1983 Oct;82(4):449–468. doi: 10.1085/jgp.82.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S. B., Williams M. E., Ways N. R., Brenner R., Sharp A. H., Leung A. T., Campbell K. P., McKenna E., Koch W. J., Hui A. Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science. 1988 Sep 23;241(4873):1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glossmann H., Striessnig J. Molecular properties of calcium channels. Rev Physiol Biochem Pharmacol. 1990;114:1–105. doi: 10.1007/BFb0031018. [DOI] [PubMed] [Google Scholar]
- Guy H. R., Conti F. Pursuing the structure and function of voltage-gated channels. Trends Neurosci. 1990 Jun;13(6):201–206. doi: 10.1016/0166-2236(90)90160-c. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda A. E., Kim H. S., Ruth P., Perez-Reyes E., Flockerzi V., Hofmann F., Birnbaumer L., Brown A. M. Normalization of current kinetics by interaction between the alpha 1 and beta subunits of the skeletal muscle dihydropyridine-sensitive Ca2+ channel. Nature. 1991 Aug 8;352(6335):527–530. doi: 10.1038/352527a0. [DOI] [PubMed] [Google Scholar]
- Leonard J. P., Nargeot J., Snutch T. P., Davidson N., Lester H. A. Ca channels induced in Xenopus oocytes by rat brain mRNA. J Neurosci. 1987 Mar;7(3):875–881. doi: 10.1523/JNEUROSCI.07-03-00875.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A. Heterologous expression of excitability proteins: route to more specific drugs? Science. 1988 Aug 26;241(4869):1057–1063. doi: 10.1126/science.2457947. [DOI] [PubMed] [Google Scholar]
- Lory P., Rassendren F. A., Richard S., Tiaho F., Nargeot J. Characterization of voltage-dependent calcium channels expressed in Xenopus oocytes injected with mRNA from rat heart. J Physiol. 1990 Oct;429:95–112. doi: 10.1113/jphysiol.1990.sp018246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan I., Gigi A., Dascal N. Expression of voltage-dependent Ca channels from skeletal muscle in Xenopus oocytes. Ann N Y Acad Sci. 1989;560:183–184. doi: 10.1111/j.1749-6632.1989.tb24095.x. [DOI] [PubMed] [Google Scholar]
- Lotan I., Goelet P., Gigi A., Dascal N. Specific block of calcium channel expression by a fragment of dihydropyridine receptor cDNA. Science. 1989 Feb 3;243(4891):666–669. doi: 10.1126/science.2464853. [DOI] [PubMed] [Google Scholar]
- Lupu-Meiri M., Shapira H., Matus-Leibovitch N., Oron Y. Two types of intrinsic muscarinic responses in Xenopus oocytes. I. Differences in latencies and 45Ca efflux kinetics. Pflugers Arch. 1990 Dec;417(4):391–397. doi: 10.1007/BF00370658. [DOI] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Moorman J. R., Zhou Z., Kirsch G. E., Lacerda A. E., Caffrey J. M., Lam D. M., Joho R. H., Brown A. M. Expression of single calcium channels in Xenopus oocytes after injection of mRNA from rat heart. Am J Physiol. 1987 Oct;253(4 Pt 2):H985–H991. doi: 10.1152/ajpheart.1987.253.4.H985. [DOI] [PubMed] [Google Scholar]
- Mori Y., Friedrich T., Kim M. S., Mikami A., Nakai J., Ruth P., Bosse E., Hofmann F., Flockerzi V., Furuichi T. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991 Apr 4;350(6317):398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E., Kim H. S., Lacerda A. E., Horne W., Wei X. Y., Rampe D., Campbell K. P., Brown A. M., Birnbaumer L. Induction of calcium currents by the expression of the alpha 1-subunit of the dihydropyridine receptor from skeletal muscle. Nature. 1989 Jul 20;340(6230):233–236. doi: 10.1038/340233a0. [DOI] [PubMed] [Google Scholar]
- Singer D., Biel M., Lotan I., Flockerzi V., Hofmann F., Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991 Sep 27;253(5027):1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- Slish D. F., Engle D. B., Varadi G., Lotan I., Singer D., Dascal N., Schwartz A. Evidence for the existence of a cardiac specific isoform of the alpha 1 subunit of the voltage dependent calcium channel. FEBS Lett. 1989 Jul 3;250(2):509–514. doi: 10.1016/0014-5793(89)80786-0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Adams B. A., Niidome T., Numa S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature. 1990 Aug 9;346(6284):567–569. doi: 10.1038/346567a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Powell J. A., Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988 Nov 10;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Umbach J. A., Gundersen C. B. Expression of an omega-conotoxin-sensitive calcium channel in Xenopus oocytes injected with mRNA from Torpedo electric lobe. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5464–5468. doi: 10.1073/pnas.84.15.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi G., Lory P., Schultz D., Varadi M., Schwartz A. Acceleration of activation and inactivation by the beta subunit of the skeletal muscle calcium channel. Nature. 1991 Jul 11;352(6331):159–162. doi: 10.1038/352159a0. [DOI] [PubMed] [Google Scholar]



