Abstracts
Objectives
The emergence of respiratory infectious diseases (ERID) poses a significant threat to global public health. However, effectively managing ERID outbreaks in large cities remains a challenge.
Methods
An age-structured Susceptible-Exposed-Infectious-Removed (SEIR) model was developed to predict the effectiveness of non-pharmaceutical interventions (NPIs) in controlling ERID outbreaks. Four ERID outbreak scenarios were created based on varying levels of infectivity and pathogenicity. Based on the World Health Organization's (WHO) categorization for responding to the influenza pandemic, the combinations of NPIs were classified into five levels: base, any, moderate, high, and extraordinary levels (from mild to severe). The simulated progression of ERID outbreaks in a megacity were compared across different levels of NPI.
Results
Our findings indicate that the response strategies should be formulated based on the epidemiological characteristics of ERID. In the low transmission scenarios, the mandatory NPIs were unnecessary to control ERID outbreaks regardless of their pathogenicity. However, even with low pathogenicity, severe NPIs are required to control the spread of ERID and minimize harm to the public in high transmission scenarios.
Conclusion
The NPIs for the EIRD outbreak in a city should be tailored to the epidemiological characteristics to control its impact and protect public health.
Keywords: Non-pharmaceutical interventions, ERID pandemic, Epidemiology characteristic, Public health, Healthcare system
Highlights
-
•
NPIs in megacities should match the outbreak's epidemiological features.
-
•
Intensive mandatory interventions may not be needed in low-transmission outbreaks.
-
•
High-transmission outbreaks require strict NPIs to minimize harm.
1. Introduction
Over the past two decades, the pandemic of several emerging respiratory infectious diseases (ERIDs), such as SARS-CoV I, MERS, and SARS-CoV II (COVID-19) have posed significant challenges to global public health and economic development. The severe acute respiratory syndrome coronavirus (SARS-CoV, 2003), a newly emerging respiratory disease, resulted in over 8000 cases and 900 deaths across 32 countries or regions in 2003 [1]. Similarly, the newly discovered H1N1 influenza virus led to 18,449 laboratory-confirmed deaths from April 2009 to August 2010 [2]. In particular, the COVID-19 pandemic triggered a global crisis, threatening the health of billions and disrupting economic growth in all countries from 2019 to 2022 [3]. More than 772 million infections and over 6.9 million deaths have been caused by COVID-19 by the time the WHO declared the end of COVID-19 in 2023 [4]. To combat these ERIDs, various control measures have been proposed to control the pandemic of these diseases, including vaccines, drugs, and NPIs. However, the appropriate response to the initial outbreak of ERIDs remains highly debated.
Countries have implemented various interventions to control ERID outbreaks based on the epidemiological characteristics of diseases. For instance, most countries included medication and treatments in response to the H1N1 pandemic, considering its low fatality rate (about 0.2 %) and limited transmissibility (R0 is 1.4) [5]. In contrast, early COVID-19 was characterized by a high fatality rate of 5.73%–6.09 % and a quick spread speed (R0 is over 2.2) in Wuhan 2020, China [6]. As a result, the control strategy for the COVID-19 outbreak shifted to strict non-pharmaceutical interventions (NPIs), including internal travel restrictions, reactive school closures, proactive school closures, reactive workplace closures, home working, and reducing meetings in many countries. So, when we face the outbreak of future ERIDs, it is urgent to identify its epidemiology parameters and implement proper responses quickly. Our study hypothesizes that tailoring NPIs to the specific epidemiological characteristics of an ERID, such as transmission rates and age-specific vulnerabilities, will enhance the ability to control disease spread and reduce the burden on healthcare systems [7].
Mathematical modelling can be used to simulate the effectiveness of potential interventions in the battle against the outbreak of ERID. In particular, the epidemic model could estimate and predict the demand for hospital beds and intensive care units (ICUs) [8,9]. Several studies utilize models to assess the effectiveness of NPIs in controlling outbreaks, such as social distancing, mask-wearing policies, etc. [10]. Mathematical modelling has also been crucial in guiding policy decisions during ERID outbreaks, particularly in the COVID-19 pandemic, where it has informed strategies to balance health protection and minimize socio-economic disruptions [11]. By simulating detailed projections of infection spread, healthcare resource needs, and the potential effectiveness of various public health interventions, the models enable informed and timely decision-making during the outbreak of ERID [12].
In this study, we developed an age-structured mathematical model to assessed the effectiveness of employing various interventions in different scenarios during the outbreak of an ERID in a simulated megacity based on Chinese population data. We estimated the demand for hospital beds and ICUs and evaluated the impact of NPIs to reduce the potential ERID burden and preventing the collapse of the local healthcare system. Our findings offer guidelines for future interventions to control new ERID outbreaks.
2. Methods
2.1. A dynamic model for emerging respiratory infectious disease outbreak
We developed an expanded age-structured Susceptible-Exposed-Infectious-Removed (SEIR) model to simulate ERID transmission in a hypothetical megacity based on Chinese population data. This model includes the susceptible, exposed, infectious, and removed populations, further groups infectious individuals into asymptomatic, symptomatic, and unquarantined symptomatic categories (Fig. 1). Our model simulates the transition of susceptible individuals (S) to the exposed (E) compartment upon infection using a stochastic chain binomial process. This process calculates the new exposures using binomial sampling at each time step, reflecting the inherent stochasticity in disease transmission. We conducted 200 simulations with 95 % confidence intervals to capture the stochastic variability. We applied a Gamma distribution to represent the variability in incubation periods to model the transition from latent (E) to infectious states (I). The average duration of the latent period (1/γE) was 4.1 (range: 3.4–5.0) days. The likelihood of progressing to either asymptomatic or symptomatic states is determined by the symptomatic probability (Ps = 81.2 %) [13]. Asymptomatic and symptomatic individuals are assumed to remain infectious for an average of 1/γI = 7 (range: 7–10) days before moving to the removed compartment. Both asymptomatic and symptomatic individuals were assumed to be equally infectious [14]. The model assumes that 2.5 % of the population has pre-existing immunity due on cross-immunity from other respiratory diseases [15]. We also conducted sensitivity analyses with 5 % and 10 % immunity levels.
Fig. 1.
Flowchart of the proposed extended SEIR transmission model. All states and parameters are defined in Table 1.
Five epidemiological indicators were considered as outcomes of this model: infections, symptomatic cases, hospitalizations, ICUs, and deaths in different scenarios. Symptomatic cases require hospital beds and ICUs. In contrast, the remaining symptomatic cases and all asymptomatic infections recover naturally. The average time from symptom onset to hospital admission was 1/γsh = 4 or 12.5 days. It is assumed that hospitalized patients do not transmit the virus. Based on the corresponding mortality risk, patients in the ICUs (or hospital beds) could stay there until they recover or die. It's assumed that all deaths occur among hospitalized patients.
We ranked the interventions in descending order according to their outcome: lower infections, symptomatic cases, hospitalizations, ICUs, and deaths. We tested different levels of NPIs by adjusting their intensity, and performed sensitivity analyses on varying initial infection levels (5, 15, 20, 50, and 100). Simultaneously, we explore mitigation interventions to reduce the ERID burden to prevent the local healthcare system from being overwhelmed.
All compartments and parameters are listed in Table 1. A stochastic chain binomial process simulates the transitions between compartments [16]. For example, susceptible individuals move to the exposed compartment at a rate of Δt ∼ Binomial (S(t), 1-e-λ(t)), where λ(t) is the force of infection at time t. The next-generation matrix method was used to calculate β from the R0, representing the infectivity of carriers [17]. We used a contact matrix from a study conducted in Shanghai, China, aggregated into three age groups: 0–17 years, 18–59 years, and 60+ years. The total population was set at 10 million, based on the age distribution from the 2022 China Statistical Yearbook [18]. The expanded SEIR set of equations for ERID is as follows:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
Table 1.
| Summary of parameter values in the mathematical model.
| Parameter | Description | Value | rang | Source |
|---|---|---|---|---|
| R0 | Basic reproduction number | 1.5 (low) | 1.3–1.7 | 2009 pandemic H1N1 influenza [1] |
| 9.5 (high) | 5.5–24 | Coronavirus disease 2019 [2] | ||
| K | Fatality rate | 0.2 % (low) | 0.2%–1.3 % | 2009 pandemic H1N1 influenza [3] |
| 34.5 % (high) | Worldwide: 34.5 % and South Korea: 20.4 % | Middle Eastern respiratory syndrome coronavirus [4] | ||
| n | Initial number of infections | 20 | / | Assumed value (5, 15, and 50 as sensitivity analysis) |
| λ(t) | Force of infection at time t | / | / | The next-generation matrix method [5] |
| β | Transmission rate in the absence of NPIs, inferred from the value of R0 | / | / | Inferred from the value of the reproduction number R0 |
| φ | Reduction of the transmission rate due to NPIs | 20 %, 50 %, 80 % | / | Assumed value |
| Ps | Proportion of infections who developed symptoms | 81.2 % | 74.9%–87.5 % | Middle Eastern respiratory syndrome [6] |
| ϴ | Infectivity of an asymptomatic individual relative to a symptomatic individual | 100 % | / | Assumed value (35 % as sensitivity analysis) |
| ρ | Proportion of infections quarantined per day | 10 %, 20 % | / | Reference [7] |
| Ph | Proportion of symptomatic infections requiring hospitalizations | 15.4 % (high) | 2.672%–15.465 % | Reference [8] |
| 2.672 % (low) | 2.672%–15.465 % | Reference [8] | ||
| Phd | Fatality rate among hospitalized (non-ICU) patients | 39.1 % (high) | 37.2%–41.1 % | Reference [9] |
| 3 % (low) | 3%–15 % | Reference [10] | ||
| Phc | The proportion of hospitalized patients requiring ICU | 44.71 % (high) | 5%–44.71 % | Reference [8] |
| 5 % (low) | 5%–44.71 % | Reference [8] | ||
| Pcd | Fatality rate among ICU patients | 30 % (high) | / | Calibrated against the COVID-2019 outbreak in China |
| 15 % (low) | / | Calibrated against the COVID-2019 outbreak in China | ||
| 1/γE | Average duration of latent period (days) | 4.1 (3.4–5.0) | 3.4–5.0 | Reference [11] |
| 1/γI | Average infectious periods (days) | 7 (7–10) | 7–10 | Calibrated against the COVID-2019 outbreak in China |
| 1/γsh | Average time from symptom onset to hospitalization (days) | 12.5 (high) | 10.3–14.8 | Reference [12] |
| 4 (low) | / | Reference [13] | ||
| 1/γhd | Average time from hospital (non-ICU) admission to death (days) | 7.5 | / | Reference [12] |
| 1/γhr | Length of hospital stay before recovery (days) | 14 | / | Calibrated against the COVID-2019 outbreak in China |
| 1/γcd | Average time from ICU admission to death (days) | 7 | / | Calibrated against the COVID-2019 outbreak in China |
| 1/γcr | Length of hospital stay before recovery (days) | 8 | / | Calibrated against the COVID-2019 outbreak in China |
WHO – World Health Organization.
The meanings of the parameters in the equations above are listed in in Table 1. Based on previous research, we hypothesize that the first 100 days are crucial for controlling the epidemic [19]. The modelling simulations were initialized with 20 imported infections and ran forward for 100 days. Sensitivity analyses were performed with 5, 15, 50, and 100 initial infections to evaluate the robustness of the results under various conditions.
2.2. The emerging respiratory infectious disease scenarios
We have summarized the transmissibility and pathogenicity of several emerging respiratory infectious diseases, including SARS, H1N1, MERS, and COVID-19 (in Table 1). The transmissibility of ERID was defined by the basic reproduction number (R0). An R0 value of 1.5 (1.3–1.7) from H1N1 was selected as low transmissibility, and an R0 value of 9.5 (5.5–24) from the Omicron (B.1.351) has been selected as high transmissibility [6]. The pathogenicity of ERID was defined by the case fatality rate while also considering factors such as hospitalization and ICUs rates post-infection. A case fatality rate of 0.2 % (0.2%–1.3 %) from H1N1 was selected as indicative of low pathogenicity, and a case fatality rate of 34.5 % from MERS was selected as indicative of high pathogenicity [20]. Four scenarios were constructed to evaluate the coping strategy for ERID based on the potential transmissibility and fatality of ERID, including Scenario 1 (high transmission, high fatality), Scenario 2 (high transmission, low fatality), Scenario 3 (low transmission, high fatality) and Scenario 4 (low transmission, low fatality) [21](Supplementary Fig. 1).
2.3. Interventions for the outbreak of ERID
We referred to the WHO's NPIs for influenza outbreaks, categorized into four types: personal, community, environmental measures, and travel-related measures. We also do not consider adopting NPIs that the WHO does not recommend at any time, such as ultraviolet light, modifying humidity, contact tracing, quarantine of exposed individuals, entry and exit screening, and border closure [22].
2.4. Personal
In this classification, we considered five interventions: hand hygiene, respiratory etiquette, face masks for symptomatic individuals, isolation of sick individuals, and face masks for the public. During the pandemic, face masks were proven effective means to reduce transmission. Studies have shown that face masks for symptomatic individuals can reduce transmission by 22 %, and face masks for the public can reduce it by 25 % [23,24]. Isolation of sick individuals is also an effective measure, with studies indicating an 11 % reduction in transmission [23]. Experimental research targeting influenza has shown that hand hygiene effectively reduces influenza virus on hands and theoretically reduces transmission, though sufficient quantitative evidence is still lacking. Similarly, studies on the influenza virus show no evidence that adhering to respiratory etiquette reduces transmission, though its potential effectiveness is mechanistically plausible [22].
2.5. Community
In this category, we considered four types of interventions: school measures and closures, workplace measures and closures, avoiding crowding, and internal travel restrictions. Research has shown that school closures and measures can reduce transmission by 14 %, while workplace closures and measures can decrease it by 11 %. Avoiding crowding can lower transmission by 2 %, and Internal travel restrictions can reduce it by 3 % [23].
2.6. Environmental measures
In this category, we considered two types of interventions: cleaning of surface and object cleaning and increased ventilation. While there is no evidence of these interventions reducing transmission in influenza, their potential effectiveness is mechanistically plausible.
2.7. Travel related measures
In this category, we considered the measure of travel advice. Research on influenza has found no scientific evidence supporting the effectiveness of travel advice during a pandemic. However, providing information to travellers is simple, feasible, and acceptable.
Based on the WHO's recommendations for responding to an influenza pandemic, we categorized response strategies into five levels: base, any, moderate, high, and extraordinary levels of NPI [22]. Each level includes the aforementioned different interventions, and the intensity of the same intervention varies across levels. We have hypothesized different effects for each level of intervention. We assume that implementing interventions at the levels of daily, any, moderate, high, and extraordinary, can reduce transmission by 0 %, 10 %, 25 %, 50 %, and 70 %, respectively. By isolating symptomatic patients at home, their transmission can be reduced by 0 %, 15 %, 25 %, 50 %, and 75 % respectively (Table 2). In addition to formal NPIs, we also account for natural human adaptive behaviours, such as voluntary social distancing, hygiene improvements, and avoidance of crowded places. Based on the literature, we assumed that such behaviours could reduce transmission rates by 10 % [25,26]. Sensitivity analyses were conducted with reductions in the transmission of 0 %, 10 %, 20 %, and 40 % to reflect different levels of adaptive behaviour.
Table 2.
| Summary of different levels of NPI used in the simulations under four scenarios over the simulated 100-day period.
| Category | Specific intervention | Reduction | Severitya |
||||
|---|---|---|---|---|---|---|---|
| Base | Any | Moderate | High | Extraordinary | |||
| Personal | |||||||
| personal protective measures for everyday use | (1)Hand hygiene | / | + | + | + | + | |
| (2)Respiratory etiquette | / | + | + | + | + | ||
| (3)Face masks for symptomatic individuals | 22 % [14] | + | + | + | + | ||
| personal protective measures Reserved for Pandemics | (4)Isolation of sick individuals | 11 % [15] | + | + | + | + | |
| (5)Face masks for public | 25 % [16] | + | + | ||||
| Community | |||||||
| School closures and dismissals | (6)School measures and closures | 14 % [15] | + | + | |||
| social distancing measures | (7)Workplace measures and closures | 11 % [15] | |||||
| (8)Avoiding crowding | 2 % [15] | + | + | + | |||
| (9)Internal travel restrictions | 3 % [15] | + | |||||
| Environmental measures | |||||||
| Environmental Surface Cleaning Measures | (10) Surface and object cleaning | / | + | + | + | + | |
| Other environmental measures | (11)Increased ventilation | / | + | + | + | + | |
| Travel related measures | |||||||
| (12)Travel advise | / | + | + | + | + | ||
| Reduction on translation (all) | 0 % | 10 % | 25 % | 50 % | 70 % | ||
| Isolation rate of symptomatic | 0 % | 15 % | 25 % | 50 % | 75 % | ||
Recommendations on the use of NPIs by severity level based on the World Health Organization in 2019 [14].
We evaluated the effects of different intervention strategies by varying the reduction values of transmission in the extended SEIR equations, Eqs. (1), (2), (3), (4), (5), (6), (7), (8), (9), making the parameters time-dependent.
2.8. Model calibration using the MCMC algorithm
Based on the comprehensive epidemiological data from Wuhan in JAN 2020 [27], we estimated the parameters , , using a Markov Chain monte Carlo (MCMC) method. Data of COVID-19 cases in Wuhan in January 2020 were used to calibrate the model parameters using MCMC [28,29]. Calculate the correlation between the estimated cases and the reported number from January 1 to January 22, 2020 (because few interventions were implemented before January 23). We assumed that the transmission and ascertainment rates did not change.
2.9. Sensitivity analyses
To evaluate the use of interventions at the different times of effect, five, 15, 50, and 100 seeds of infections are considered sensitivity analyses [30] (Supplementary Figs. 6–8). The main analysis assumes that the infectiousness of asymptomatic and symptomatic cases are identical. Here, it's assumed that the asymptomatic individuals were considered to be 65 % less infectious than symptomatic ones as a sensitivity analysis (Supplementary Figs. 9–11). Natural immunity levels (0 %, 5 %, and 10 %) and human adaptive behaviours (with transmission reductions of 0 %, 10 %, 20 %, and 40 %) were both evaluated through sensitivity analyses (Supplementary Figs. 12–17).
2.10. Data analysis
For each scenario, 200 stochastic model realizations were performed. The number of model simulations used in the analysis was empirically determined to guarantee the stability of the results. We defined 95 % credible intervals as quantiles 0.025 and 0.975 of the estimated distributions. Microsoft Visual Studio Enterprise 2019 (.NET Framework 4.5) was used to build the model code. The model output was analyzed in R (version 4.3.2).
3. Results
This model fits the data well (Supplementary Fig. 18). The overall correlation between the estimated cases and the reported cases from January 1 to January 22, 2020, was also significant (P < 0.001, R2 = 0.966). In each scenario, we quantify the effects of different NPI levels on infections, symptomatic cases, hospitalizations, ICU admissions, and deaths. Below, we present the results for each scenario, categorized into three age groups: 0–17 years, 18–59 years, and 60+ years.
3.1. Scenario 1 (high transmission, high fatality)
For the 0–17 age group, under the base NPI level, infections are 21,895.95 (95 % CI, 21,895.51–21,896.41), symptomatic cases are 17,779.51 (95 % CI, 17,779.15–17,779.88), hospitalizations are 676.64 (95 % CI, 676.62–676.65), ICU cases are 547.16 (95 % CI, 547.15–547.17), and deaths are 548.23 (95 % CI, 548.20–548.25). Any NPI level reduces infections by 1.08 %, and at the extraordinary level, infections, hospitalizations, ICU cases, and deaths are reduced by 99.99 %. For the 18–59 age group, the base NPI level results in 43,725.98 (95 % CI, 43,725.97–43,725.98) infections, 35,505.50 (95 % CI, 35,505.49–35,505.50) symptomatic cases, 1351.24 (95 % CI, 1351.24) hospitalizations, 1092.67 (95 % CI, 1092.67) ICU cases, and 1095.02 (95 % CI, 1094.97–1095.05) deaths. NPI measures at any level reduce infections and deaths by 0.10 % and, at the extraordinary level, by 99.99 %. For the 60+ age group, the base NPI level results in 23,601.45 (95 % CI, 23,601.40–23,601.48) infections, 19,164.38 (95 % CI, 19,164.33–19,164.40) symptomatic cases, 729.34 (95 % CI, 729.34) hospitalizations, 589.78 (95 % CI, 589.78) ICU cases, and 591.04 (95 % CI, 591.02–591.06) deaths. The extraordinary NPI level reduces all outcomes by over 99.99 %.
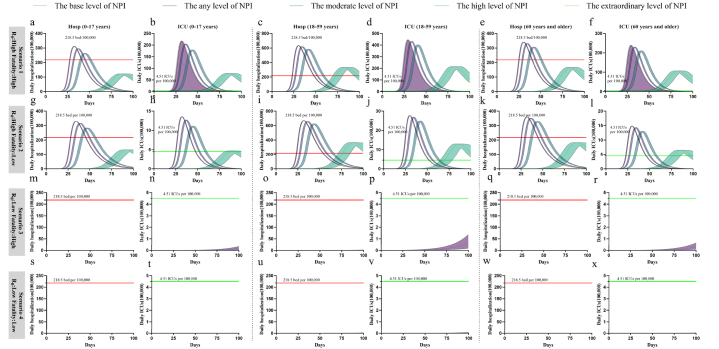
With high-level NPI measures, hospital bed demand is below the national capacity for the age group (0–17 years) and the age group (60+ years). Only at the extraordinary NPI level dose the demand for hospital and ICU beds below the average capacity in China (218.5 beds/100,000 and 4.51 ICU beds/100,000) [31].
3.2. Scenario 2 (high transmission, low fatality)
For the 0–17 age group, under the base NPI level, infections are 21,895.85 (95 % CI, 21,895.28–21,896.31), symptomatic cases are 17,779.43 (95 % CI, 17,778.97–17,779.80), hospitalizations are 650.88 (95 % CI, 650.86–650.89), ICU cases are 34.26 (95 % CI, 34.26), and deaths are 41.05 (95 % CI, 41.04–41.05). Any NPI level reduces infections by 1.08 %, and at the extraordinary level, infections, hospitalizations, ICU cases, and deaths are reduced by 99.99 %. For the 18–59 age group, the base NPI level results in 43,725.98 (95 % CI, 43,725.97–43,725.98) infections, 35,505.50 (95 % CI, 35,505.49–35,505.50) symptomatic cases, 1299.81 (95 % CI, 1299.81) hospitalizations, 68.41 (95 % CI, 68.41) ICU cases, and 82.04 (95 % CI, 82.02–82.05) deaths. NPI measures at any level reduce infections and deaths by 0.10 % and, at the extraordinary level, by 99.99 %. For the 60+ age group, the base NPI level results in 23,601.45 (95 % CI, 23,601.41–23,601.49) infections, 19,164.38 (95 % CI, 19,164.35–19,164.41) symptomatic cases, 701.58 (95 % CI, 701.58) hospitalizations, 36.93 (95 % CI, 36.93) ICU cases, and 44.28 (95 % CI, 44.27–44.29) deaths. The extraordinary NPI level reduces all outcomes by over 99.99 %.
With high-level NPI measures, hospital bed demand is below the national capacity for the age group (0–17 years) and the age group (60+ years). Only at the extraordinary NPI level dose the demand for hospital and ICU beds below the average capacity in China.
3.3. Scenario 3 (low transmission, high fatality)
For the 0–17 age group, under the base NPI level, infections are 29.95 (95 % CI, 8.70–65.58), symptomatic cases are 24.32 (95 % CI, 7.06–53.25), hospitalizations are 0.63 (95 % CI, 0.18–1.39), ICU cases are 0.51 (95 % CI, 0.15–1.12), and deaths are 0.34 (95 % CI, 0.10–0.73). The extraordinary NPI level reduces all outcomes by 99.99 %. For the 18–59 age group, the base NPI level results in 119.50 (95 % CI, 35.36–266.46) infections, 97.03 (95 % CI, 28.71–216.37) symptomatic cases, 2.53 (95 % CI, 0.76–5.67) hospitalizations, 2.04 (95 % CI, 0.62–4.59) ICU cases, and 1.35 (95 % CI, 0.42–3.00) deaths. NPI measures at the extraordinary level reduce all outcomes by over 99.99 %. For the 60+ age group, the base NPI level results in 57.16 (95 % CI, 16.80–125.79) infections, 46.41 (95 % CI, 13.64–102.14) symptomatic cases, 1.21 (95 % CI, 0.37–2.68) hospitalizations, 0.98 (95 % CI, 0.30–2.17) ICU cases, and 0.64 (95 % CI, 0.20–1.41) deaths. The extraordinary NPI level reduces all outcomes by over 99.99 %.
In all levels of NPIs, the demand for beds and ICUs is less than the existing average bed and ICU resources in China for all age groups.
3.4. Scenario 4 (low transmission, low fatality)
For the 0–17 age group, under the base NPI level, infections are 27.06 (95 % CI, 7.47–58.92), symptomatic cases are 21.97 (95 % CI, 6.07–47.84), hospitalizations are 0.56 (95 % CI, 0.15–1.20), ICU cases are 0.03 (95 % CI, 0.01–0.06), and deaths are 0.02 (95 % CI, 0.01–0.04). The extraordinary NPI level reduces all outcomes by 99.99 %. For the 18–59 age group, the base NPI level results in 111.16 (95 % CI, 29.96–236.79) infections, 90.26 (95 % CI, 24.32–192.27) symptomatic cases, 2.25 (95 % CI, 0.62–4.81) hospitalizations, 0.12 (95 % CI, 0.03–0.25) ICU cases, and 0.08 (95 % CI, 0.02–0.18) deaths. NPI measures at the extraordinary level reduce all outcomes by 99.99 %. For the 60+ age group, the base NPI level results in 52.32 (95 % CI, 14.36–112.66) infections, 42.48 (95 % CI, 11.66–91.48) symptomatic cases, 1.07 (95 % CI, 0.30–2.29) hospitalizations, 0.06 (95 % CI, 0.02–0.12) ICU cases, and 0.04 (95 % CI, 0.01–0.08) deaths. The extraordinary NPI level reduces all outcomes by over 99.99 %.
In all levels of NPIs, the demand for beds and ICUs is less than the existing average bed and ICU resources in China for all age groups.
All results are calculated as relative values based on every 100,000 people in these four scenarios. Detailed results are in Fig. 2, Supplementary Figs. 3–5, and Table 3.
Fig. 2.
Projected demand and shortage of hospital beds and ICUs when adopting the base level, any level, moderate level, high level, and extraordinary level of NPI under four scenarios over the simulated 100-day period. a, Daily demand of hospital (non-ICU) beds. b, Daily demand of ICUs. In a, c, e, g, i, k, m, o, q, s, u, w, The red dashed line indicates the number of ICUs available in China per 100,000 (China Health Statistics Yearbook 2021). In b, d, f, h, j, l, n, p, r, t, v, x, The green dashed line indicates the number of hospital (non-ICU) beds available in China per 100,000 (China Health Statistics Yearbook 2021). Four scenarios: scenario 1 (R0 = 9.5, Fatality = 34.5 %), scenario 2 (R0 = 9.5, Fatality = 0.2 %), scenario 3 (R0 = 1.5, Fatality = 34.5 %), scenario 4 (R0 = 1.5, Fatality = 0.2 %). Data are presented as median with 2.5 % and 97.5 % quantiles of n = 200 simulations.
Table 3.
| Median and 95 % Confidence Intervals (2.5 % and 97.5 % Percentiles) of the estimated cumulative number of infections, symptomatic cases, hospitalization (non-ICU), ICU, and deaths per 100,000 individuals under four scenarios over the simulated 100-day period. Data are presented as median with 2.5 % and 97.5 % quantiles of n = 200 simulations.
| Scenarios | Severity | Infections | Symptomatic cases | Hospitalization | ICU | Deaths |
|---|---|---|---|---|---|---|
| R0:high Fatality: high (0–17 years) | Base | 21895.95(21895.51–21896.41) | 17779.51(17779.15–17779.88) | 676.64(676.62–676.65) | 547.16(547.15–547.17) | 548.23(548.20–548.25) |
| Any | 21659.48(21656.26–21662.78) | 17587.49(17584.88–17590.18) | 669.32(669.22–669.43) | 541.25(541.16–541.33) | 541.99(541.87–542.10) | |
| Moderate | 20777.16(20770.05–20785.67) | 16871.05(16865.28–16877.96) | 642.02(641.81–642.29) | 519.17(518.99–519.38) | 518.80(518.44–519.17) | |
| High | 13884.15(13174.34–14115.99) | 11273.93(10697.56–11462.18) | 395.30(347.45–412.34) | 319.66(280.97–333.44) | 253.97(195.51–279.68) | |
| Extraordinary | 0.21(0.06–1.06) | 0.17(0.05–0.86) | 0.01(0.00–0.03) | 0.01(0.00–0.02) | 0.01(0.00–0.02) | |
| R0:high Fatality: low (0–17 years) | Base | 21895.85(21895.28–21896.31) | 17779.43(17778.97–17779.80) | 650.88(650.86–650.89) | 34.26(34.26–34.26) | 41.05(41.04–41.05) |
| Any | 21641.15(21636.98–21644.04) | 17572.61(17569.23–17574.96) | 643.30(643.18–643.39) | 33.86(33.85–33.86) | 40.47(40.44–40.49) | |
| Moderate | 20703.97(20696.08–20711.48) | 16811.62(16805.21–16817.72) | 615.39(615.15–615.61) | 32.39(32.38–32.40) | 38.44(38.37–38.50) | |
| High | 13209.95(11813.56–13602.31) | 10726.48(9592.61–11045.07) | 348.32(270.65–374.68) | 18.33(14.24–19.72) | 14.73(9.16–17.42) | |
| Extraordinary | 0.20(0.06–0.79) | 0.16(0.05–0.64) | 0.01(0.00–0.02) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| R0:low Fatality: high (0–17 years) | Base | 29.95(8.70–65.58) | 24.32(7.06–53.25) | 0.63(0.18–1.39) | 0.51(0.15–1.12) | 0.34(0.10–0.73) |
| Any | 0.18(0.07–0.55) | 0.14(0.06–0.44) | 0.01(0.00–0.02) | 0.00(0.00–0.01) | 0.00(0.00–0.01) | |
| Moderate | 0.10(0.06–0.18) | 0.08(0.05–0.14) | 0.00(0.00–0.01) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| High | 0.07(0.06–0.10) | 0.06(0.05–0.08) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| Extraordinary | 0.06(0.06–0.09) | 0.05(0.05–0.07) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| R0:low Fatality: low (0–17 years) | Base | 27.06(7.47–58.92) | 21.97(6.07–47.84) | 0.56(0.15–1.20) | 0.03(0.01–0.06) | 0.02(0.01–0.04) |
| Any | 0.18(0.08–0.46) | 0.15(0.06–0.37) | 0.01(0.00–0.01) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| Moderate | 0.10(0.06–0.19) | 0.08(0.05–0.15) | 0.00(0.00–0.01) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| High | 0.07(0.06–0.10) | 0.06(0.05–0.08) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| Extraordinary | 0.06(0.06–0.09) | 0.05(0.05–0.07) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| R0:high Fatality: high (18–59 years) | Base | 43725.98(43725.97–43725.98) | 35505.50(35505.49–35505.50) | 1351.24(1351.24–1351.24) | 1092.67(1092.67–1092.67) | 1095.02(1094.97–1095.05) |
| Any | 43717.08(43716.50–43717.59) | 35498.27(35497.79–35498.68) | 1350.96(1350.94–1350.97) | 1092.45(1092.43–1092.46) | 1094.38(1094.26–1094.48) | |
| Moderate | 43515.89(43511.59–43518.51) | 35334.90(35331.41–35337.03) | 1344.72(1344.59–1344.80) | 1087.40(1087.29–1087.47) | 1087.95(1087.57–1088.28) | |
| High | 36772.23(35798.89–37062.70) | 29859.05(29068.70–30094.91) | 1082.20(997.92–1109.08) | 875.11(806.96–896.85) | 737.06(605.15–789.69) | |
| Extraordinary | 0.67(0.14–3.27) | 0.54(0.11–2.66) | 0.02(0.00–0.09) | 0.02(0.00–0.08) | 0.02(0.00–0.07) | |
| R0:high Fatality: low (18–59 years) | Base | 43725.98(43725.97–43725.98) | 35505.50(35505.49–35505.50) | 1299.81(1299.81–1299.81) | 68.41(68.41–68.41) | 82.04(82.02–82.05) |
| Any | 43715.36(43714.57–43716.13) | 35496.87(35496.23–35497.49) | 1299.49(1299.46–1299.51) | 68.39(68.39–68.40) | 81.88(81.83–81.91) | |
| Moderate | 43488.26(43485.09–43491.69) | 35312.47(35309.89–35315.25) | 1292.70(1292.61–1292.80) | 68.04(68.03–68.04) | 81.06(80.94–81.14) | |
| High | 35777.36(33636.48–36313.82) | 29051.22(27312.82–29486.82) | 985.42(830.89–1030.43) | 51.86(43.73–54.23) | 45.00(30.73–51.15) | |
| Extraordinary | 0.57(0.13–2.42) | 0.46(0.10–1.96) | 0.02(0.00–0.07) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| R0:low Fatality: high(18–59 years) | Base | 119.50(35.36–266.46) | 97.03(28.71–216.37) | 2.53(0.76–5.67) | 2.04(0.62–4.59) | 1.35(0.42–3.00) |
| Any | 0.55(0.22–1.60) | 0.44(0.17–1.30) | 0.02(0.01–0.05) | 0.01(0.01–0.04) | 0.01(0.01–0.04) | |
| Moderate | 0.22(0.12–0.37) | 0.18(0.10–0.30) | 0.01(0.00–0.01) | 0.01(0.00–0.01) | 0.01(0.00–0.01) | |
| High | 0.11(0.08–0.16) | 0.09(0.06–0.13) | 0.00(0.00–0.01) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| Extraordinary | 0.09(0.08–0.13) | 0.07(0.06–0.11) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| R0:low Fatality: low (18–59 years) | Base | 111.16(29.96–236.79) | 90.26(24.32–192.27) | 2.25(0.62–4.81) | 0.12(0.03–0.25) | 0.08(0.02–0.18) |
| Any | 0.53(0.20–1.28) | 0.43(0.16–1.04) | 0.02(0.01–0.04) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| Moderate | 0.23(0.12–0.37) | 0.18(0.10–0.30) | 0.01(0.00–0.01) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| High | 0.11(0.08–0.16) | 0.09(0.06–0.13) | 0.00(0.00–0.01) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| Extraordinary | 0.09(0.08–0.13) | 0.07(0.06–0.11) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| R0:high Fatality: high (60 years and older) | Base | 23601.45(23601.40–23601.48) | 19164.38(19164.33–19164.40) | 729.34(729.34–729.34) | 589.78(589.78–589.78) | 591.04(591.02–591.06) |
| Any | 23574.57(23573.42–23575.79) | 19142.55(19141.62–19143.54) | 728.51(728.47–728.55) | 589.10(589.08–589.14) | 590.14(590.07–590.20) | |
| Moderate | 23304.66(23300.55–23307.54) | 18923.38(18920.04–18925.72) | 720.15(720.03–720.24) | 582.35(582.25–582.42) | 582.57(582.36–582.80) | |
| High | 18608.01(18052.35–18780.94) | 15109.70(14658.51–15250.12) | 545.38(500.21–560.21) | 441.02(404.49–453.01) | 369.18(301.60–396.85) | |
| Extraordinary | 0.32(0.07–1.54) | 0.26(0.06–1.25) | 0.01(0.00–0.04) | 0.01(0.00–0.04) | 0.01(0.00–0.03) | |
| R0:high Fatality: low (60 years and older) | Base | 23601.45(23601.41–23601.49) | 19164.38(19164.35–19164.41) | 701.58(701.58–701.58) | 36.93(36.93–36.93) | 44.28(44.27–44.29) |
| Any | 23571.01(23570.07–23572.42) | 19139.66(19138.89–19140.81) | 700.67(700.64–700.71) | 36.88(36.88–36.88) | 44.15(44.12–44.16) | |
| Moderate | 23275.02(23271.40–23278.73) | 18899.32(18896.38–18902.33) | 691.85(691.74–691.96) | 36.41(36.41–36.42) | 43.37(43.31–43.41) | |
| High | 18023.83(16832.02–18330.42) | 14635.35(13667.60–14884.30) | 493.87(413.04–518.19) | 25.99(21.74–27.27) | 22.40(15.21–25.56) | |
| Extraordinary | 0.28(0.07–1.17) | 0.23(0.06–0.95) | 0.01(0.00–0.03) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| R0:low Fatality: high (60 years and older) | Base | 57.16(16.80–125.79) | 46.41(13.64–102.14) | 1.21(0.37–2.68) | 0.98(0.30–2.17) | 0.64(0.20–1.41) |
| Any | 0.27(0.10–0.77) | 0.22(0.08–0.62) | 0.01(0.00–0.02) | 0.01(0.00–0.02) | 0.01(0.00–0.02) | |
| Moderate | 0.11(0.07–0.22) | 0.09(0.05–0.17) | 0.00(0.00–0.01) | 0.00(0.00–0.01) | 0.00(0.00–0.01) | |
| High | 0.07(0.06–0.10) | 0.06(0.05–0.08) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| Extraordinary | 0.06(0.06–0.08) | 0.05(0.05–0.06) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| R0:low Fatality: low (60 years and older) | Base | 52.32(14.36–112.66) | 42.48(11.66–91.48) | 1.07(0.30–2.29) | 0.06(0.02–0.12) | 0.04(0.01–0.08) |
| Any | 0.26(0.10–0.66) | 0.21(0.08–0.53) | 0.01(0.00–0.02) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| Moderate | 0.12(0.07–0.21) | 0.10(0.06–0.17) | 0.00(0.00–0.01) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| High | 0.07(0.06–0.10) | 0.06(0.05–0.08) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | |
| Extraordinary | 0.06(0.06–0.08) | 0.05(0.05–0.06) | 0.00(0.00–0.00) | 0.00(0.00–0.00) | 0.00(0.00–0.00) |
4. Discussion
Our study highlights the importance of tailoring mitigation strategies to the epidemiological characteristics of a new ERID during an outbreak. For ERIDs with low transmissibility, regardless of their pathogenicity, NPIs targeting specific vulnerable populations, such as the elderly and those with chronic conditions, are still recommended. This ensures the reduction of disease burden, hospitalizations, deaths, and other social impacts, even when the overall transmission and fatality rates are low. NPIs are sufficient, and there is no need to resort to intensive NPIs to manage the outbreak. However, in the case of highly contagious ERIDs, promptly adopting NPIs with an extraordinary level of NPI is crucial to prevent the collapse of the healthcare system. Our study provides strategic options for Chinese cities during an ERID outbreak, which will help effectively respond to future ERID outbreaks in urban areas.
Our study reaffirms the critical role of NPIs in controlling new ERID outbreaks in a city. NPIs reduce the transmission of the virus by limiting contact between individuals and separating known infected individuals from those who are susceptible. In scenarios involving ERIDs with high transmissibility and high fatality rates, the significance of NPIs is further amplified, as highlighted in our findings and supported by Shengjie Lai et al. [32]. Lai's research underscored the pivotal role of NPIs in curtailing disease transmission during the COVID-19 pandemic. Our study aligns with this perspective, emphasizing the criticality of NPIs in managing high transmission risks. Similarly, for ERIDs with high transmission but lower fatality rates, our study corroborates the findings of Gao et al. [33]. Their studies demonstrated the effectiveness of NPIs in reducing influenza transmission in China and the United States. In contrast, vaccines and drugs are the main interventions for diseases with lower transmission rates but higher fatality rates, as noted by Hemachudha et al. [34]. Overall, our study aligns with these previous findings and serves as a valuable supplement, enriching further evidence on the intervention strategic application of NPIs in various ERID scenarios.
The key to controlling a new ERID in a city is to avoid the collapse of the healthcare system during the outbreak. The differences in medical resources between cities directly affect the intensity of the interventions required in the face of the same infectious disease, especially regarding the number of beds and ICUs. For example, there are only 612 beds/100,000 in Shanghai, China, compared to 735 beds/100,000 in Chongqing, China. Regarding ICUs, Shanghai has 3.36 ICU/100,000, slightly higher than Chongqing's 3.82 ICU/100,000 [31]. Cities with fewer medical resources may need stricter NPIs to avoid overwhelming their medical resources when dealing with highly transmissible diseases. Conversely, cities with more abundant resources may take mild interventions when facing the same scenario. However, even in resource-rich cities, NPIs should still be implemented for vulnerable populations to avoid unnecessary disease burdens. In responding to a new ERID, it is essential to consider the epidemiology characteristics of the disease and the available medical resources. Our study suggested the proper strategy against a new ERID in a city under various scenarios, which will help us respond to the outbreak of a new ERID in the future. Understanding the medical resource situation in different cities is crucial for developing effective and NPIs [35]. These measures are key to ensuring the effective control of the epidemic and maintaining the stable operation of the healthcare system.
There are several limitations to this study. Firstly, the model parameters were based on previously respiratory infectious diseases, and these parameters may change for future ERID diseases. However, the sensitivity analysis for the key parameters ensured the stability of the findings. Furthermore, our study provided a practical framework for the decision-making to control the outbreak of new ERID. Secondly, we have not discussed the specific NPIs but have categorized NPIs based on the effects of these measures, considering that the same NPI measure may have different effectiveness in different regions. Finally, the impact of NPI measures on social order and the public's acceptance of these measures were not considered. Therefore, the findings should be regarded only as a reference for decision-making. However, our analytical framework and parameters can be easily modified to be applied to the study of ERID in other places and countries.
In conclusion, it is necessary to develop and adjust intervention strategies based on the epidemiological characteristics and available interventions of ERID to address it. In the early stage of the ERID, there is not enough data and waiting time to support the model's accurate prediction ability. In uncertain prediction results, choosing the best interventions becomes a paramount concern. The present study demonstrates how to control the new ERID outbreaks in a city with proper NPIs. A comprehensive approach to NPIs will be essential for mitigating the impact of the outbreak of the new ERID and safeguarding public health.
CRediT authorship contribution statement
Zhiqun Lei: Writing – review & editing, Writing – original draft, Software, Data curation, Conceptualization. Ziwei Shi: Software, Data curation. Jiao Huang: Methodology. Xiaolong Yan: Data curation. Jiayao Luo: Software. Meng Xu: Data curation. Qiuyue Wang: Visualization. Rui Wang: Data curation. Qi Wang: Writing – original draft, Funding acquisition, Data curation. Qu Cheng: Writing – review & editing, Writing – original draft, Software, Data curation. Sheng Wei: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Data curation.
Ethical approval statement
Approval was not required.
Funding
This work was supported by the National Key Research and Development Program (grant no. 2022YFC2305103) and the National Natural Science Foundation of China (grant no. 72061137006).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors would like to express sincere gratitude to Huazhong University of Science and Technology Tongji Medical College for providing a comprehensive research platform, which greatly facilitated the progress of this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e41383.
Contributor Information
Qu Cheng, Email: chengqu@hust.edu.cn.
Sheng Wei, Email: ws2008cn@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Severe Acute Respiratory Syndrome (SARS).Available from:: https://www.who.int/health-topics/severe-acute-respiratory-syndrome [accessed April 21, 2024].
- 2.Simonsen L., Spreeuwenberg P., Lustig R., Taylor R.J., Fleming D.M., Kroneman M., Van Kerkhove M.D., Mounts A.W., Paget W.J. GLaMOR Collaborating Teams. Global mortality estimates for the 2009 Influenza Pandemic from the GLaMOR project: a modeling study. PLoS Med. 2013 Nov;10(11) doi: 10.1371/journal.pmed.1001558. PMID:24302890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ou S., He X., Ji W., Chen W., Sui L., Gan Y., Lu Z., Lin Z., Deng S., Przesmitzki S., Bouchard J. Erratum: author Correction: machine learning model to project the impact of COVID-19 on US motor gasoline demand. Nat. Energy. 2020;5(12):1051–1052. doi: 10.1038/s41560-020-00711-7. PMID:33052987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO COVID-19 dashboard. datadot.Available from: https://data.who.int/dashboards/covid19/cases [accessed April 21, 2024].
- 5.Li Z., Chen Q., Feng L., Rodewald L., Xia Y., Yu H., Zhang R., An Z., Yin W., Chen W., Qin Y., Peng Z., Zhang T., Ni D., Cui J., Wang Q., Yang X., Zhang M., Ren X., Wu D., Sun X., Li Y., Zhou L., Qi X., Song T., Gao G.F., Feng Z., China CDC COVID-19 Emergency Response Strategy Team Active case finding with case management: the key to tackling the COVID-19 pandemic. Lancet Lond Engl. 2020 July 4;396(10243):63–70. doi: 10.1016/S0140-6736(20)31278-2. PMID:32505220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020 Mar 26;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. PMID:31995857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paital B. Nurture to nature via COVID-19, a self-regenerating environmental strategy of environment in global context. Sci. Total Environ. 2020 August 10;729 doi: 10.1016/j.scitotenv.2020.139088. PMID:32388136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McBryde E.S., Meehan M.T., Adegboye O.A., Adekunle A.I., Caldwell J.M., Pak A., Rojas D.P., Williams B.M., Trauer J.M. Role of modelling in COVID-19 policy development. Paediatr. Respir. Rev. 2020 Sep;35:57–60. doi: 10.1016/j.prrv.2020.06.013. PMID:32690354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He M., Tang S., Xiao Y. Combining the dynamic model and deep neural networks to identify the intensity of interventions during COVID-19 pandemic. PLoS Comput. Biol. 2023 Oct;19(10) doi: 10.1371/journal.pcbi.1011535. PMID:37851640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai S., Ruktanonchai N.W., Zhou L., Prosper O., Luo W., Floyd J.R., Wesolowski A., Santillana M., Zhang C., Du X., Yu H., Tatem A.J. Effect of non-pharmaceutical interventions to contain COVID-19 in China. Nature. 2020 Sep;585(7825):410–413. doi: 10.1038/s41586-020-2293-x. PMID:32365354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott N., Abeysuriya R.G., Delport D., Sacks-Davis R., Nolan J., West D., Sutton B., Wallace E.M., Hellard M. COVID-19 epidemic modelling for policy decision support in Victoria, Australia 2020-2021. BMC Publ. Health. 2023 May 27;23(1):988. doi: 10.1186/s12889-023-15936-w. PMID:37237343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grassly N.C., Fraser C. Mathematical models of infectious disease transmission. Nat. Rev. Microbiol. 2008 Jun;6(6):477–487. doi: 10.1038/nrmicro1845. PMID:18533288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breban R., Riou J., Fontanet A. Interhuman transmissibility of Middle East respiratory syndrome coronavirus: estimation of pandemic risk. Lancet Lond Engl. 2013 August 24;382(9893):694–699. doi: 10.1016/S0140-6736(13)61492-0. PMID:23831141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu S., Wang W., Wang Y., Litvinova M., Luo K., Ren L., Sun Q., Chen X., Zeng G., Li J., Liang L., Deng Z., Zheng W., Li M., Yang H., Guo J., Wang K., Chen X., Liu Z., Yan H., Shi H., Chen Z., Zhou Y., Sun K., Vespignani A., Viboud C., Gao L., Ajelli M., Yu H. Infectivity, susceptibility, and risk factors associated with SARS-CoV-2 transmission under intensive contact tracing in Hunan, China. Nat. Commun. 2021 March 9;12(1):1533. doi: 10.1038/s41467-021-21710-6. PMID:33750783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine P., Eames K., Heymann D.L. "herd immunity": a rough guide. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011 April 1;52(7):911–916. doi: 10.1093/cid/cir007. PMID:21427399. [DOI] [PubMed] [Google Scholar]
- 16.Viner R.M., Mytton O.T., Bonell C., Melendez-Torres G.J., Ward J., Hudson L., Waddington C., Thomas J., Russell S., van der Klis F., Koirala A., Ladhani S., Panovska-Griffiths J., Davies N.G., Booy R., Eggo R.M. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021 Feb 1;175(2):143–156. doi: 10.1001/jamapediatrics.2020.4573. PMID:32975552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diekmann O., Heesterbeek J.a.P., Roberts M.G. The construction of next-generation matrices for compartmental epidemic models. J R Soc Interface. 2010 June 6;7(47):873–885. doi: 10.1098/rsif.2009.0386. PMID:19892718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.China statistical Yearbook. 2022. https://www.stats.gov.cn/sj/ndsj/2022/indexeh.htm Available from:
- 19.Kaxiras E., Neofotistos G., Angelaki E. The first 100 days: modeling the evolution of the COVID-19 pandemic. Chaos, Solit. Fractals. 2020 Sep;138 doi: 10.1016/j.chaos.2020.110114. PMID:32834582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z., Harrich D., Li Z., Hu D., Li D. The unique features of SARS-CoV-2 transmission: comparison with SARS-CoV, MERS-CoV and 2009 H1N1 pandemic influenza virus. Rev. Med. Virol. 2021 Mar;31(2) doi: 10.1002/rmv.2171. PMID:33350025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piroth L., Cottenet J., Mariet A.-S., Bonniaud P., Blot M., Tubert-Bitter P., Quantin C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir. Med. 2021 Mar;9(3):251–259. doi: 10.1016/S2213-2600(20)30527-0. PMID:33341155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization . World Health Organization; Geneva: 2019. Non-pharmaceutical Public Health Measures for Mitigating the Risk and Impact of Epidemic and Pandemic Influenza.https://iris.who.int/handle/10665/329438 Available from: :978-92-4-151683-9. [Google Scholar]
- 23.Li Y., Campbell H., Kulkarni D., Harpur A., Nundy M., Wang X., Nair H. Usher Network for COVID-19 Evidence Reviews (UNCOVER) group. The temporal association of introducing and lifting non-pharmaceutical interventions with the time-varying reproduction number (R) of SARS-CoV-2: a modelling study across 131 countries. Lancet Infect. Dis. 2021 Feb;21(2):193–202. doi: 10.1016/S1473-3099(20)30785-4. PMID:33729915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng Q., Hao X., Wu D., Wang Q., Spear R.C., Wei S. Feasible intervention combinations for achieving a safe exit of the Zero-COVID policy in China and its determinants: an individual-based model study. BMC Infect. Dis. 2023 June 12;23(1):390. doi: 10.1186/s12879-023-08382-x. doi: 10/gtcdtm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.S F., E G., C W., Va J. The spread of awareness and its impact on epidemic outbreaks. Proc Natl Acad Sci U S A Proc Natl Acad Sci U S A. 2009 Apr 21;106(16) doi: 10.1073/pnas.0810762106. PMID:19332788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fenichel E.P., Castillo-Chavez C., Ceddia M.G., Chowell G., Parra P.A.G., Hickling G.J., Holloway G., Horan R., Morin B., Perrings C., Springborn M., Velazquez L., Villalobos C. Adaptive human behavior in epidemiological models. Proc Natl Acad Sci. 2011 Apr 12;108(15):6306–6311. doi: 10.1073/pnas.1011250108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan A., Liu L., Wang C., Guo H., Hao X., Wang Q., Huang J., He N., Yu H., Lin X., Wei S., Wu T. Association of public health interventions with the epidemiology of the COVID-19 outbreak in wuhan, China. JAMA. 2020 May 19;323(19):1915–1923. doi: 10.1001/jama.2020.6130. PMID:32275295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taghizadeh L., Karimi A., Heitzinger C. Uncertainty quantification in epidemiological models for the COVID-19 pandemic. Comput. Biol. Med. 2020 Oct;125 doi: 10.1016/j.compbiomed.2020.104011. PMID:33091766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Worobey M. Dissecting the early COVID-19 cases in Wuhan. Science. 2021 December 3;374(6572):1202–1204. doi: 10.1126/science.abm4454. PMID:34793199. [DOI] [PubMed] [Google Scholar]
- 30.Cai J., Deng X., Yang J., Sun K., Liu H., Chen Z., Peng C., Chen X., Wu Q., Zou J., Sun R., Zheng W., Zhao Z., Lu W., Liang Y., Zhou X., Ajelli M., Yu H. Modeling transmission of SARS-CoV-2 Omicron in China. Nat Med. 2022 Jul;28(7):1468–1475. doi: 10.1038/s41591-022-01855-7. PMID:35537471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Health Commission of China China Health Statistics Yearbook 2021. 2021 [Google Scholar]
- 32.Ge Y., Zhang W.-B., Wu X., Ruktanonchai C.W., Liu H., Wang J., Song Y., Liu M., Yan W., Yang J., Cleary E., Qader S.H., Atuhaire F., Ruktanonchai N.W., Tatem A.J., Lai S. Untangling the changing impact of non-pharmaceutical interventions and vaccination on European COVID-19 trajectories. Nat. Commun. 2022 Jun 3;13(1):3106. doi: 10.1038/s41467-022-30897-1. PMID:35661759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng L., Zhang T., Wang Q., Xie Y., Peng Z., Zheng J., Qin Y., Zhang M., Lai S., Wang D., Feng Z., Li Z., Gao G.F. Impact of COVID-19 outbreaks and interventions on influenza in China and the United States. Nat. Commun. 2021 May 31;12(1):3249. doi: 10.1038/s41467-021-23440-1. PMID:34059675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemachudha T., Ugolini G., Wacharapluesadee S., Sungkarat W., Shuangshoti S., Laothamatas J. Human rabies: neuropathogenesis, diagnosis, and management. Lancet Neurol. 2013 May;12(5):498–513. doi: 10.1016/S1474-4422(13)70038-3. PMID:23602163. [DOI] [PubMed] [Google Scholar]
- 35.Persad G., Phillips J., Emanuel E.J. Allocating medical resources in the time of Covid-19. Reply. N. Engl. J. Med. 2020 May 28;382(22) doi: 10.1056/NEJMc2009666. PMID:32343503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.