Abstract
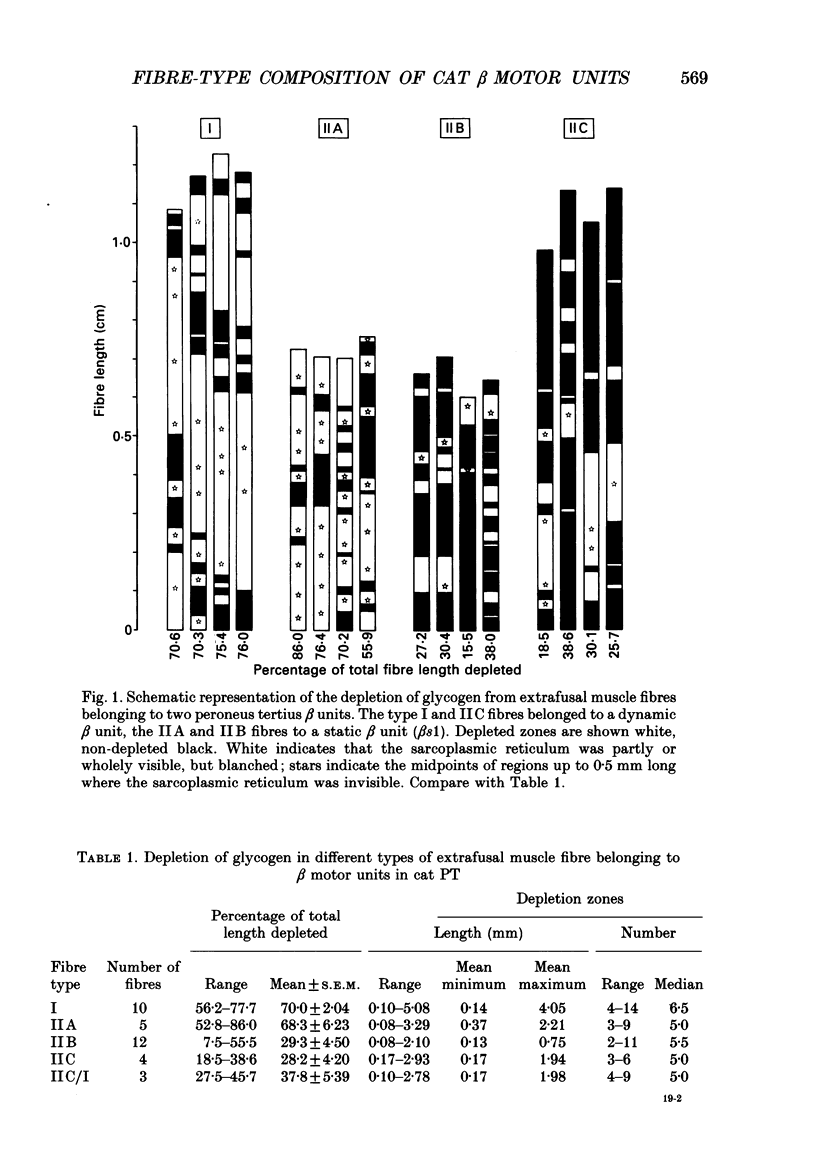
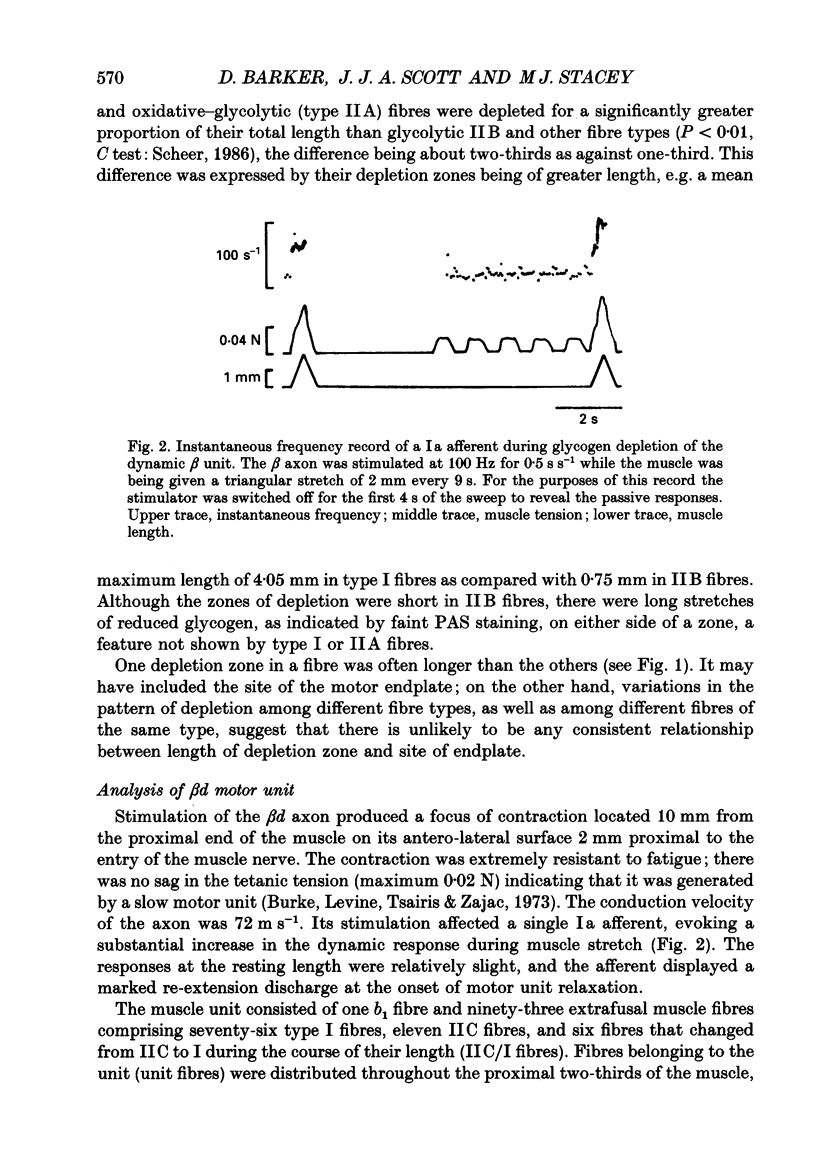
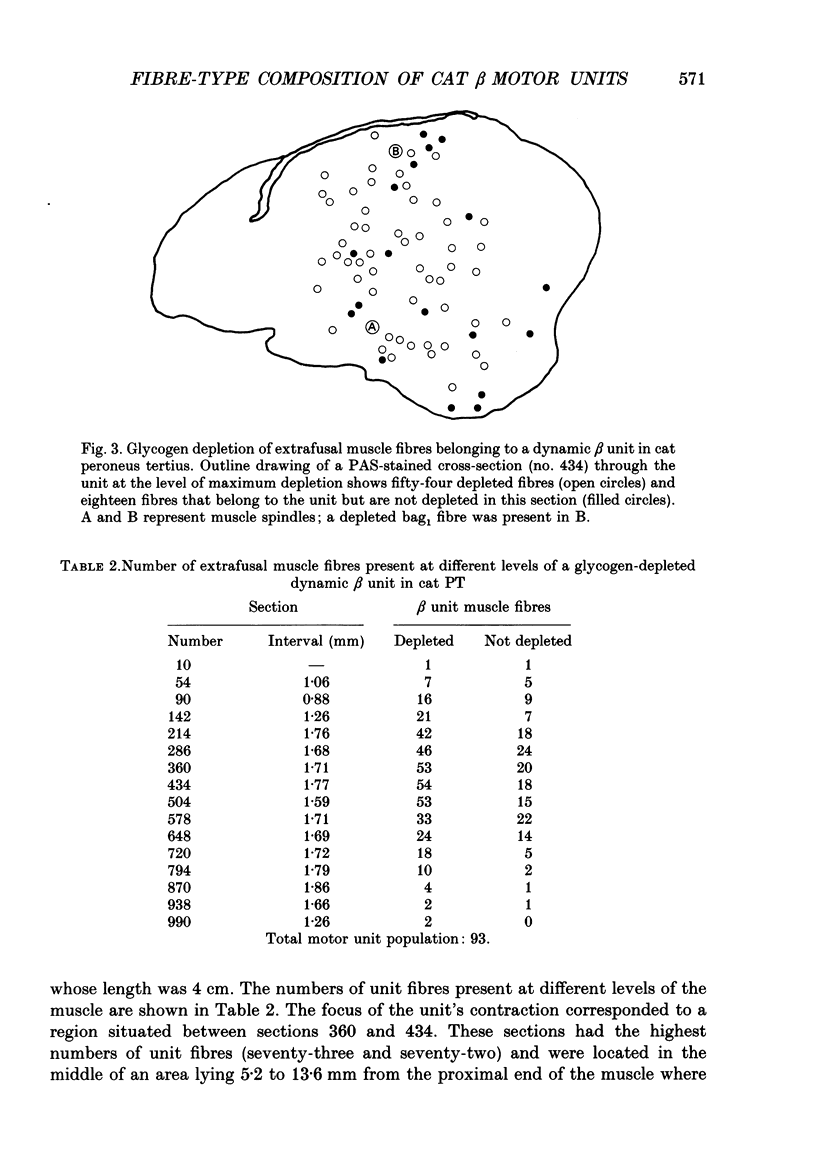
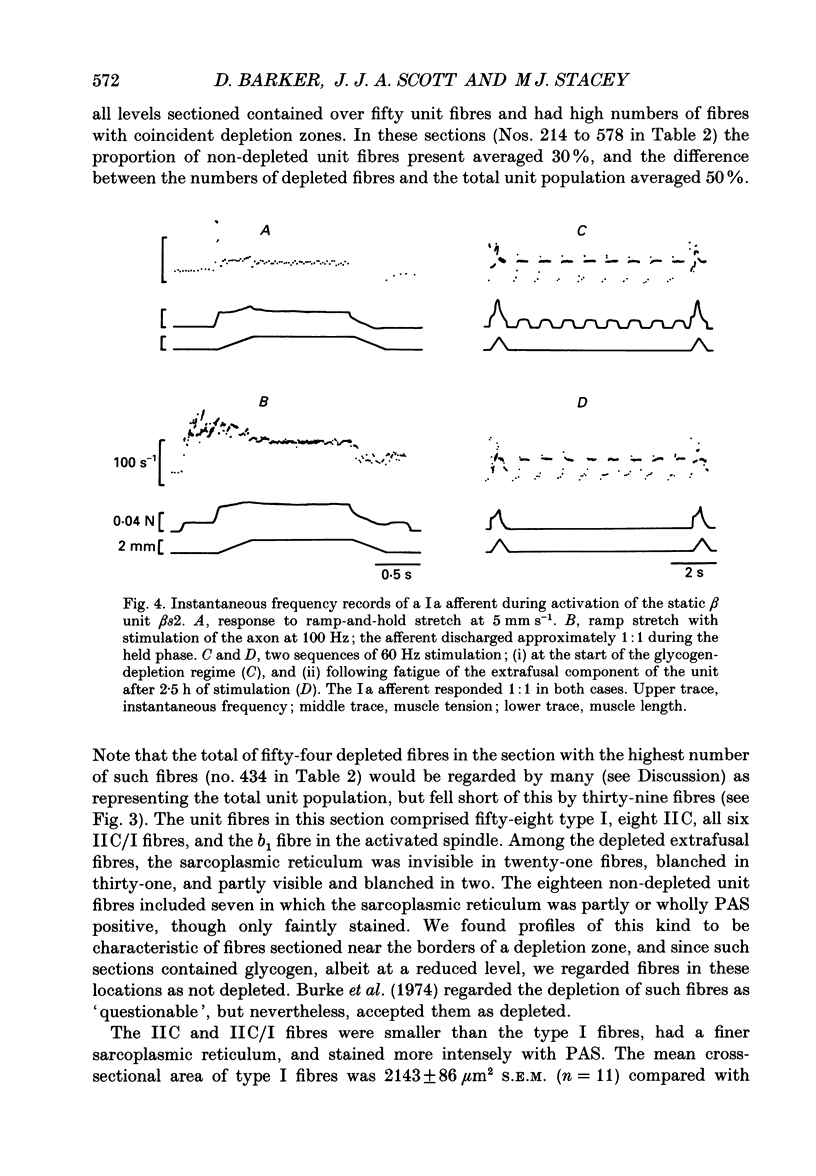
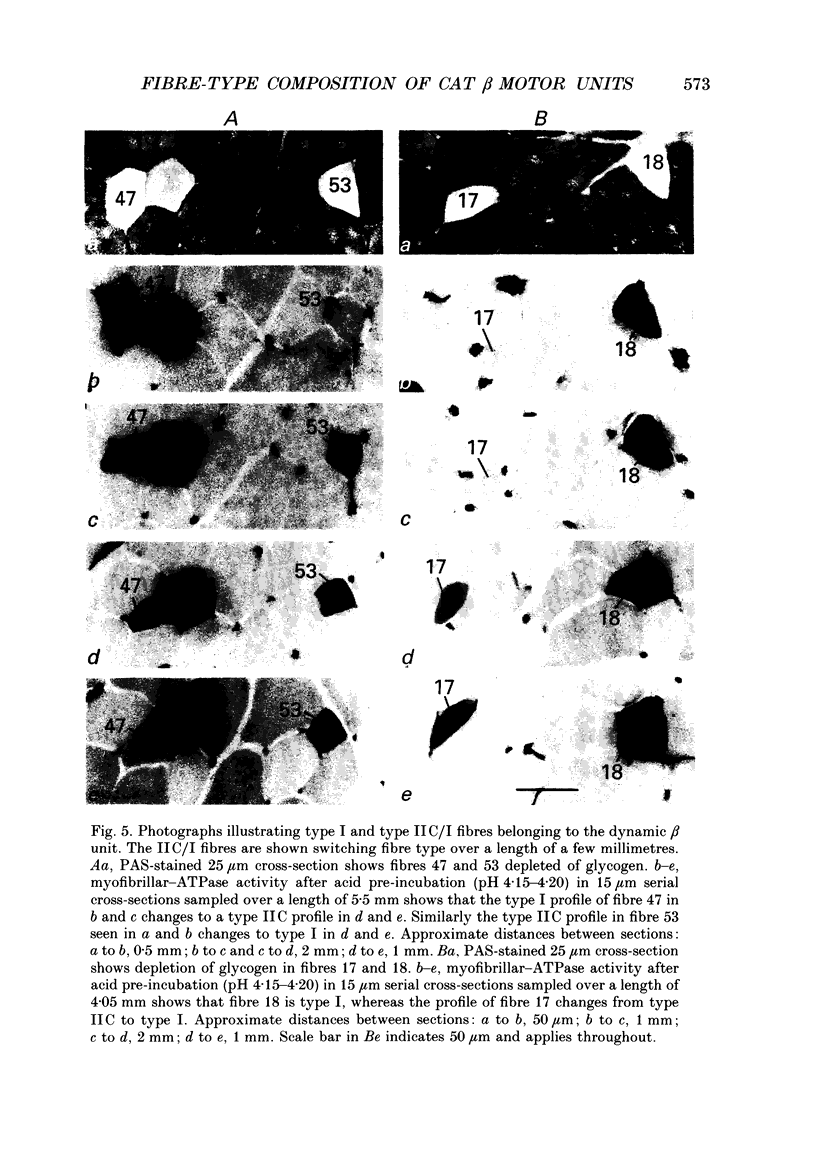
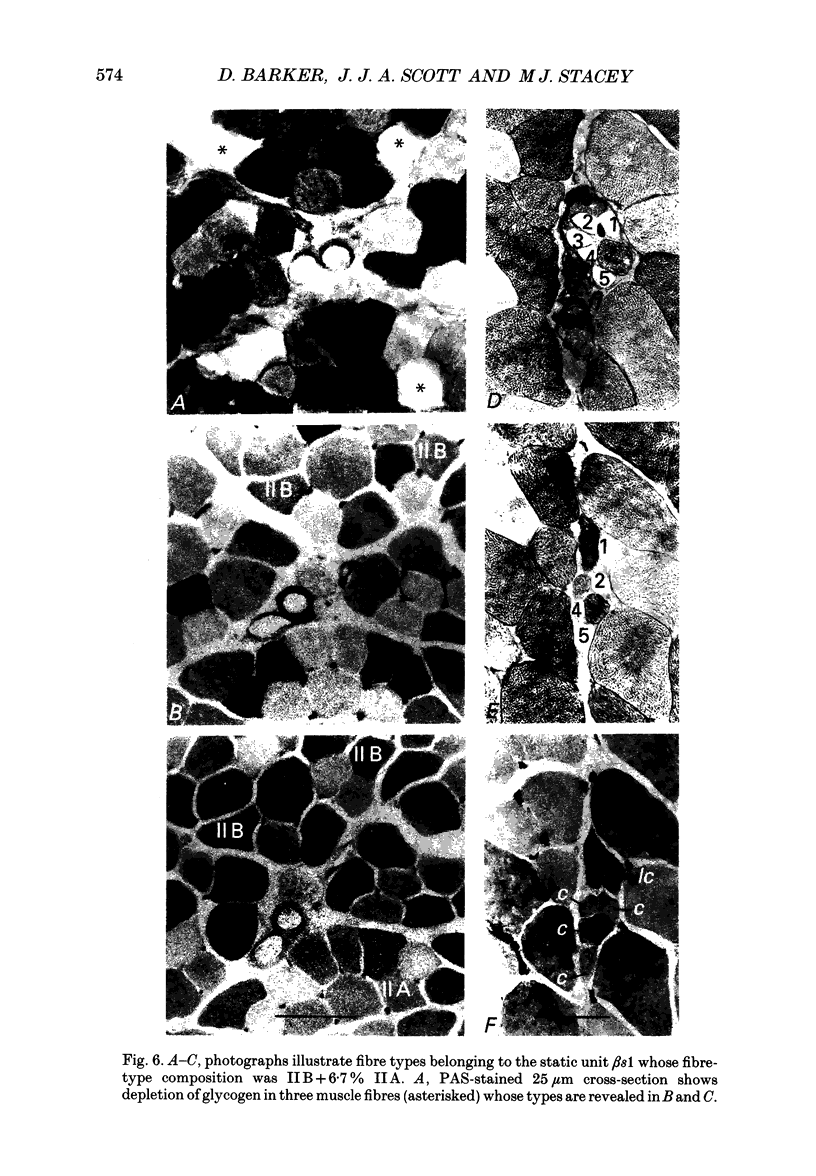
1. We have used the glycogen-depletion technique, combined with myofibrillar ATPase (mATPase) staining for muscle fibre type, to study the fibre-type composition of four skeleto-fusimotor (beta) units in cat peroneus tertius, namely, one beta dynamic (beta d) unit and three beta static (beta s) units. 2. Depletion of glycogen was observed in serial cross-sections of thirty-four beta-unit extrafusal muscle fibres of various types traced from origin to insertion. No fibre was depleted of glycogen throughout its length; depletion was restricted to a number of zones, usually about five. Oxidative (type I) and oxidative-glycolytic (type IIA) fibres were depleted for a significantly greater proportion of their total length than glycolytic IIB fibres. 3. The fibre-type composition of the beta d unit was determined by tracing its fibres from end to end. The muscle unit consisted of one intrafusal bag1 fibre and ninety-three extrafusal muscle fibres comprising seventy-six type I fibres, eleven IIC fibres, and six fibres that changed from IIC to I during the course of their length (IIC/I fibres). The extrafusal fibre-type composition was thus 81.7% I plus 18.3% IIC and IIC/I. 4. The three beta s units (beta s1, beta s2, beta s3) were all fast-contracting and fatigued rapidly. Identification of their extrafusal fibre types, made in 1 mm2 areas sampled from different parts of each unit, gave mixed compositions as follows: beta s1, IIB + 6.7% IIA; beta s2, IIB + 5.8% IIA; beta s3, IIB + 29.9% IIA. The intrafusal component of each unit included either one or two long chain fibres. 5. In a discussion of the results, the fact that the continuous stimulation of extrafusal muscle fibres does not deplete them of glycogen throughout their length is examined in relation to the work of others who have assumed that it did. With regard to the finding of mixed extrafusal fibre types in the beta units, a distinction is drawn between minimal (around 5%) and moderate mixing. It is suggested that minimal mixing may occur in any motor unit as the outcome of endplate degeneration with foreign replacement, but that moderate mixing indicates an on-going process of conversion from one fibre type to another which in the adult may prove to occur only among beta units.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker D., Emonet-Dénand F., Harker D. W., Jami L., Laporte Y. Distribution of fusimotor axons to intrafusal muscle fibres in cat tenuissimus spindles as determined by the glycogen-depletion method. J Physiol. 1976 Sep;261(1):49–69. doi: 10.1113/jphysiol.1976.sp011548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D., Emonet-Dénand F., Harker D. W., Jami L., Laporte Y. Types of intra- and extrafusal muscle fibre innervated by dynamic skeleto-fusimotor axons in cat peroneus brevis and tenuissimus muscles, as determined by the glycogen-depletion method. J Physiol. 1977 Apr;266(3):713–726. doi: 10.1113/jphysiol.1977.sp011789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D., Milburn A. Development and regeneration of mammalian muscle spindles. Sci Prog. 1984 Spring;69(273):45–64. [PubMed] [Google Scholar]
- Bessou P., Emonet-Dénand F., Laporte Y. Motor fibres innervating extrafusal and intrafusal muscle fibres in the cat. J Physiol. 1965 Oct;180(3):649–672. doi: 10.1113/jphysiol.1965.sp007722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Ribchester R. R., Ridge R. M. Competitive mechanisms underlying synapse elimination in the lumbrical muscle of the rat. J Neurobiol. 1990 Jan;21(1):1–17. doi: 10.1002/neu.480210102. [DOI] [PubMed] [Google Scholar]
- Bodine-Fowler S., Garfinkel A., Roy R. R., Edgerton V. R. Spatial distribution of muscle fibers within the territory of a motor unit. Muscle Nerve. 1990 Dec;13(12):1133–1145. doi: 10.1002/mus.880131208. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Salcman M., Tsairis P. Motor units in cat soleus muscle: physiological, histochemical and morphological characteristics. J Physiol. 1974 May;238(3):503–514. doi: 10.1113/jphysiol.1974.sp010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Tsairis P. Anatomy and innervation ratios in motor units of cat gastrocnemius. J Physiol. 1973 Nov;234(3):749–765. doi: 10.1113/jphysiol.1973.sp010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Tsairis P. Histochemical and physiological profile of a skeletofusimotor (beta) unit in cat soleus muscle. Brain Res. 1977 Jul 1;129(2):341–345. doi: 10.1016/0006-8993(77)90013-0. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Nieto-Sampedro M., Harris E. W. Synapse replacement in the nervous system of adult vertebrates. Physiol Rev. 1981 Jul;61(3):684–784. doi: 10.1152/physrev.1981.61.3.684. [DOI] [PubMed] [Google Scholar]
- Edström L., Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essén B., Henriksson J. Glycogen content of individual muscle fibres in man. Acta Physiol Scand. 1974 Mar;90(3):645–647. doi: 10.1111/j.1748-1716.1974.tb05629.x. [DOI] [PubMed] [Google Scholar]
- Gates H. J., Ridge R. M., Rowlerson A. Motor units of the fourth deep lumbrical muscle of the adult rat: isometric contractions and fibre type compositions. J Physiol. 1991 Nov;443:193–215. doi: 10.1113/jphysiol.1991.sp018830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker D. W., Jami L., Laporte Y., Petit J. Fast-conducting skeletofusimotor axons supplying intrafusal chain fibers in the cat peroneus tertius muscle. J Neurophysiol. 1977 Jul;40(4):791–799. doi: 10.1152/jn.1977.40.4.791. [DOI] [PubMed] [Google Scholar]
- Hintz C. S., Chi M. M., Fell R. D., Ivy J. L., Kaiser K. K., Lowry C. V., Lowry O. H. Metabolite changes in individual rat muscle fibers during stimulation. Am J Physiol. 1982 Mar;242(3):C218–C228. doi: 10.1152/ajpcell.1982.242.3.C218. [DOI] [PubMed] [Google Scholar]
- Jami L., Lan-Couton D., Malmgren K., Petit J. Histophysiological observations on fast skeleto-fusimotor axons. Brain Res. 1979 Mar 23;164:53–59. doi: 10.1016/0006-8993(79)90005-2. [DOI] [PubMed] [Google Scholar]
- Jami L., Murthy K. S., Petit J. A quantitative study of skeletofusimotor innervation in the cat peroneus tertius muscle. J Physiol. 1982 Apr;325:125–144. doi: 10.1113/jphysiol.1982.sp014140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami L., Murthy K. S., Petit J., Zytnicki D. Distribution of physiological types of motor units in the cat peroneus tertius muscle. Exp Brain Res. 1982;48(2):177–184. doi: 10.1007/BF00237213. [DOI] [PubMed] [Google Scholar]
- Kugelberg E. Adaptive transformation of rat soleus motor units during growth. J Neurol Sci. 1976 Mar;27(3):269–289. doi: 10.1016/0022-510x(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Matsuda H. Effects of external and internal K+ ions on magnesium block of inwardly rectifying K+ channels in guinea-pig heart cells. J Physiol. 1991 Apr;435:83–99. doi: 10.1113/jphysiol.1991.sp018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlerson A., Mascarello F., Barker D., Saed H. Muscle-spindle distribution in relation to the fibre-type composition of masseter in mammals. J Anat. 1988 Dec;161:37–60. [PMC free article] [PubMed] [Google Scholar]
- Scheer B. T. The significance of differences between means. An empirical study. Comp Biochem Physiol A Comp Physiol. 1986;83(3):405–408. doi: 10.1016/0300-9629(86)90123-4. [DOI] [PubMed] [Google Scholar]
- Scott J. J. Classification of muscle spindle afferents in the peroneus brevis muscle of the cat. Brain Res. 1990 Feb 12;509(1):62–70. doi: 10.1016/0006-8993(90)90309-y. [DOI] [PubMed] [Google Scholar]
- Snow D. H., Billeter R., Mascarello F., Carpenè E., Rowlerson A., Jenny E. No classical type IIB fibres in dog skeletal muscle. Histochemistry. 1982;75(1):53–65. doi: 10.1007/BF00492533. [DOI] [PubMed] [Google Scholar]