Abstract
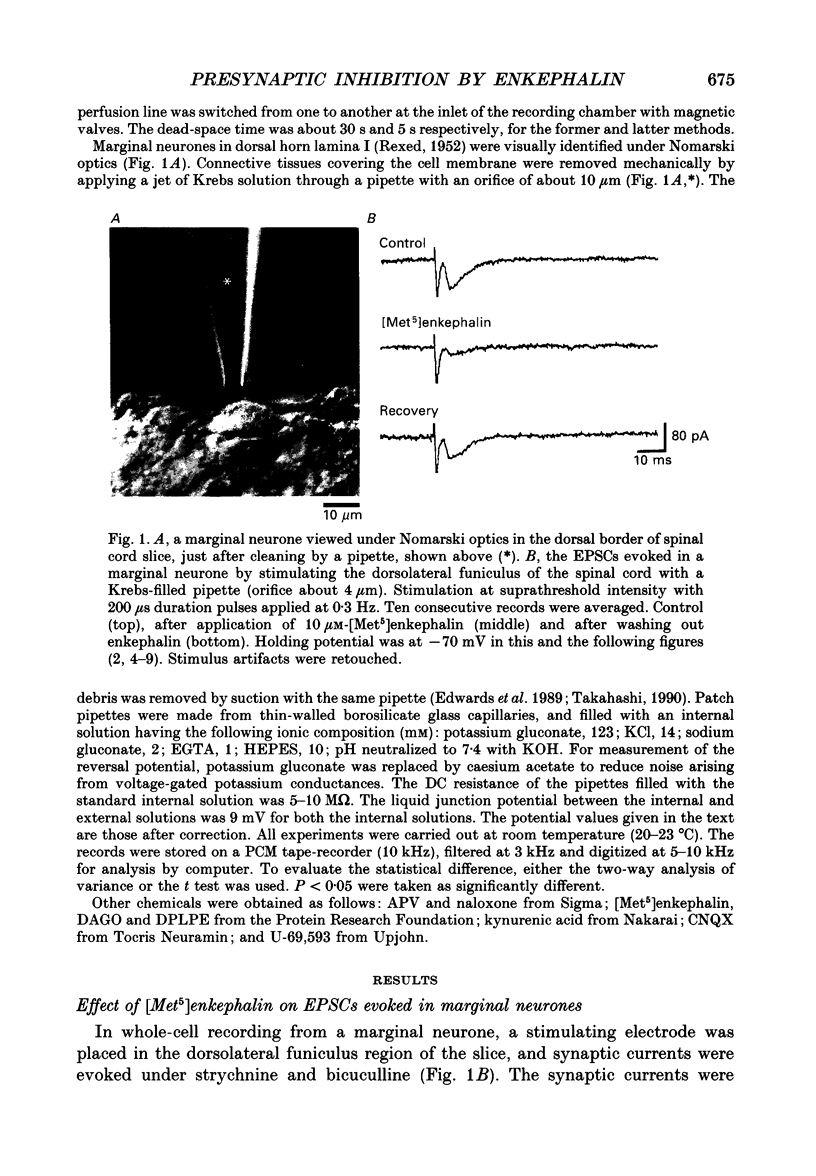
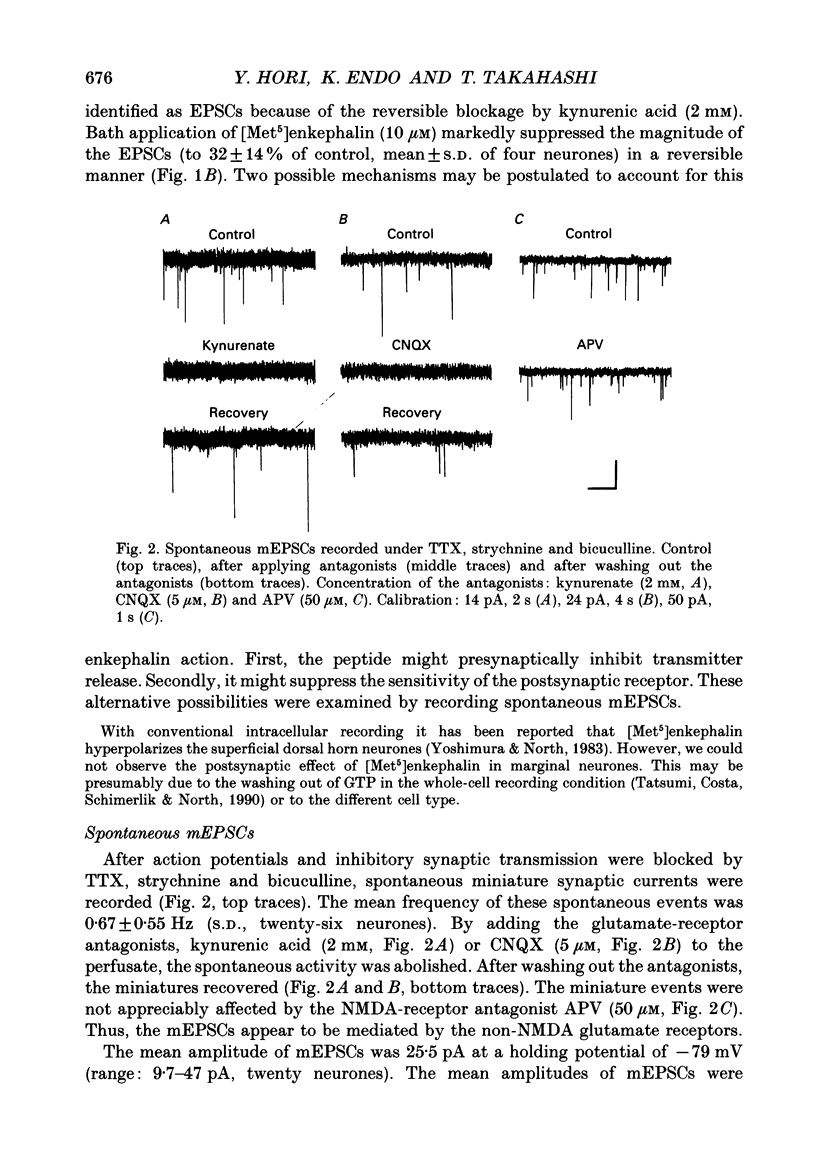
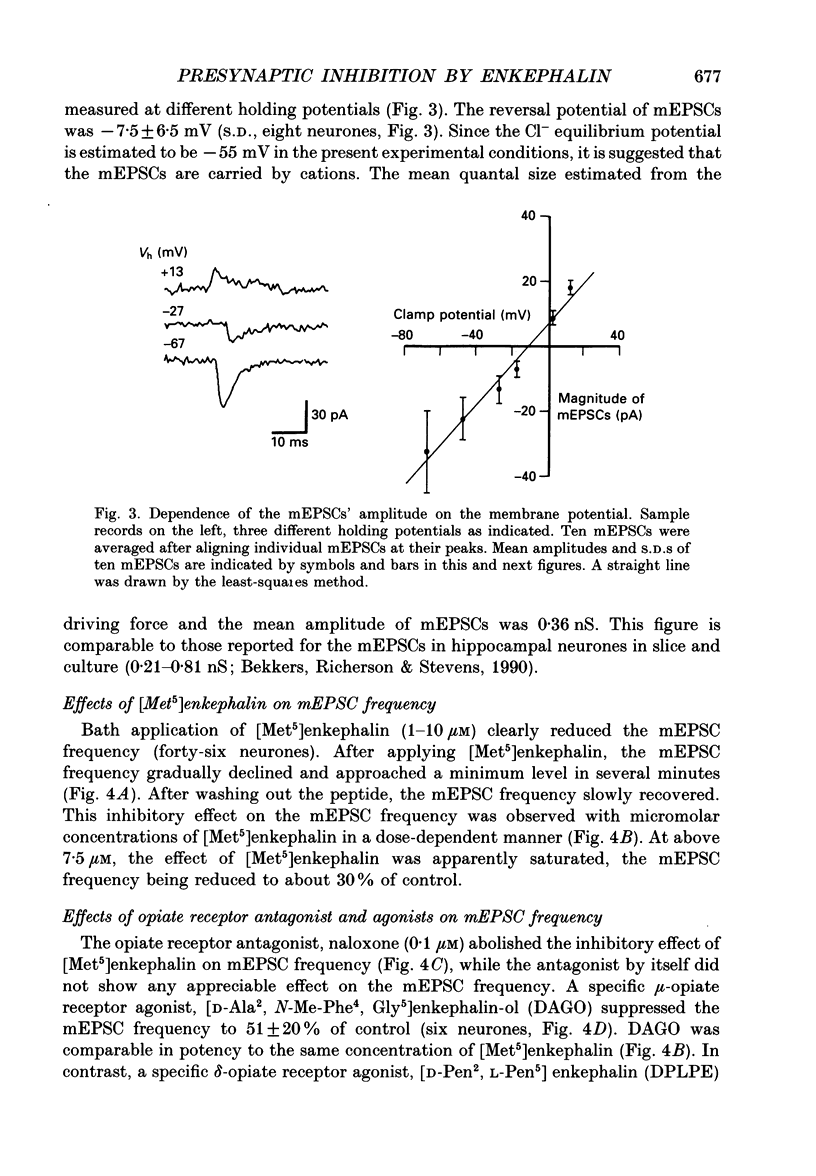
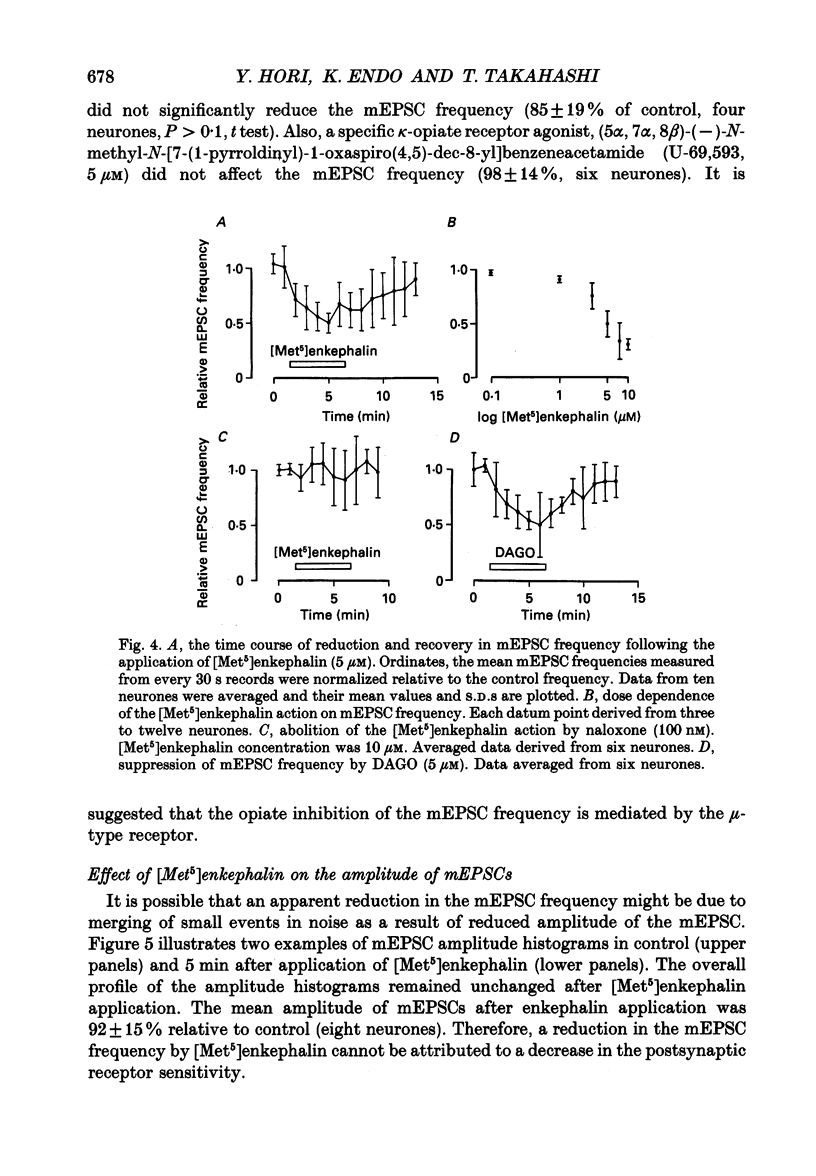
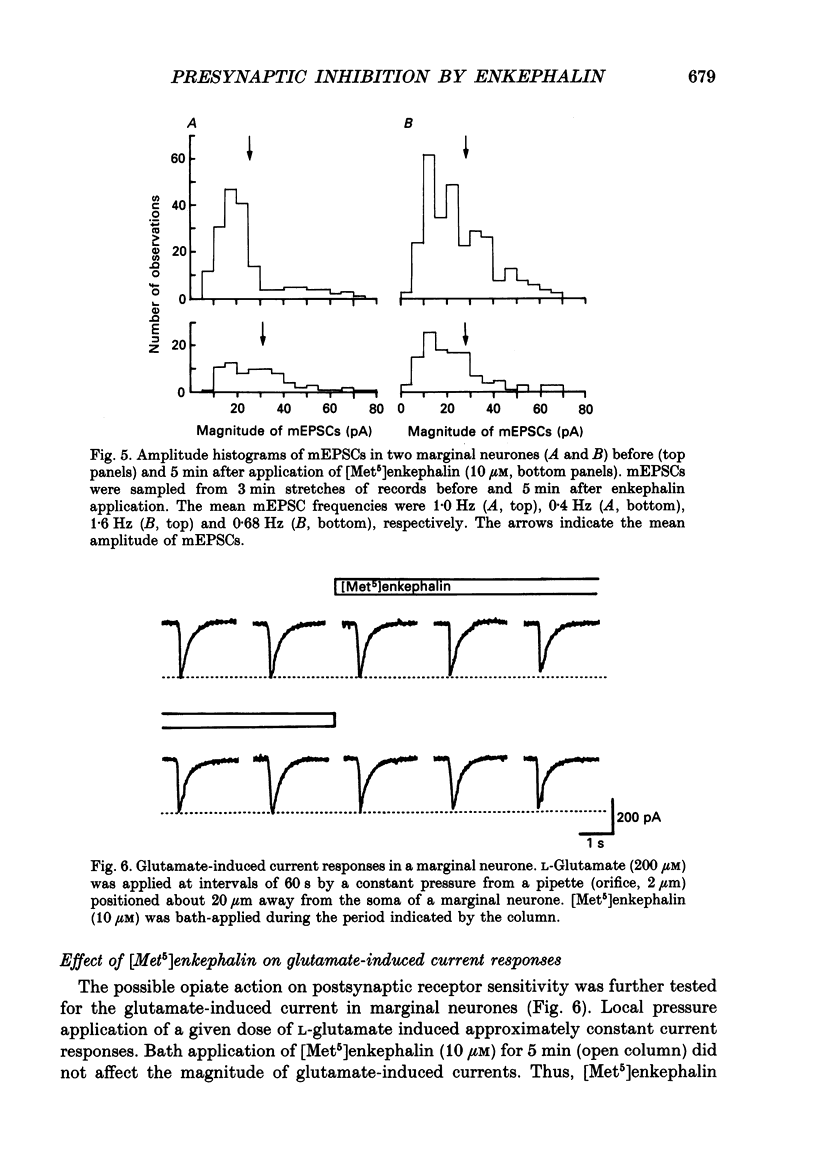
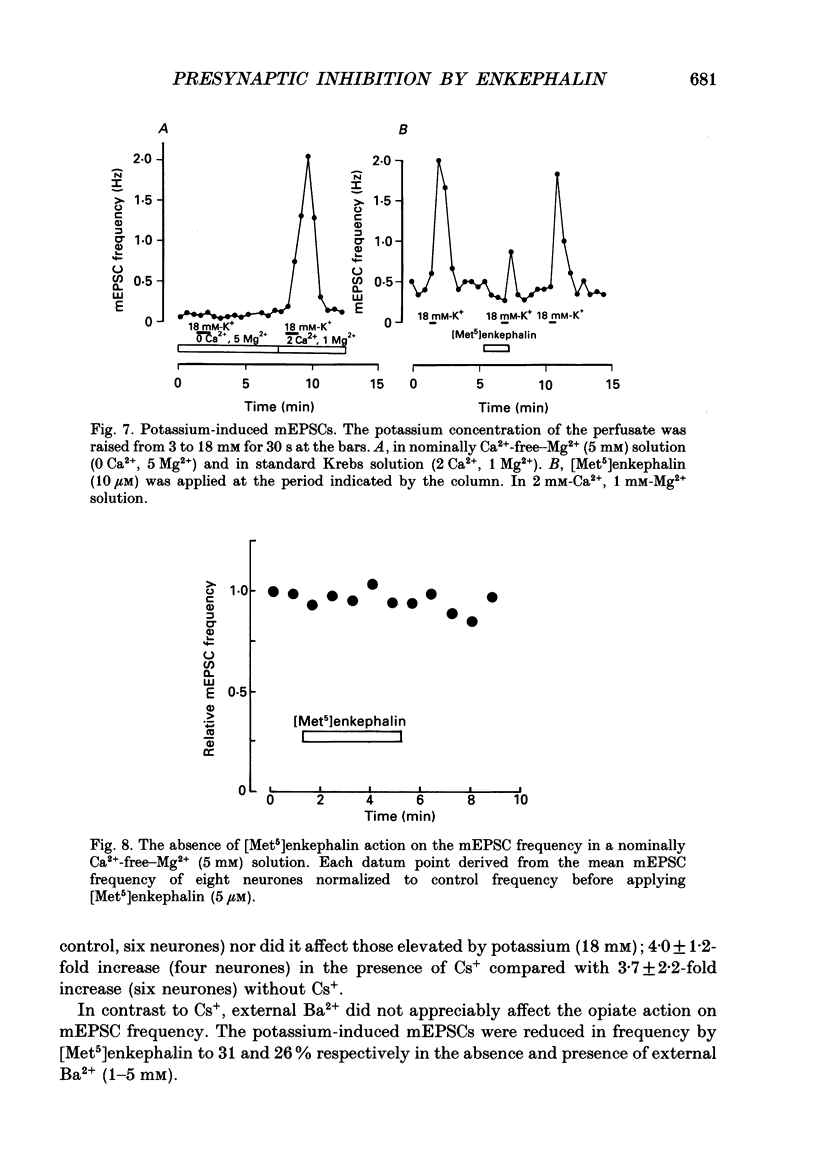
1. Tight-seal whole-cell recordings were made from marginal neurones visually identified in thin slices of 1- to 2-week-old rat lumbar spinal cord. Excitatory postsynaptic currents (EPSCs), either evoked by extracellular stimulation or those arising spontaneously in tetrodotoxin, i.e. miniature EPSCs (mEPSCs), were recorded after blocking inhibitory synaptic inputs with strychnine and bicuculline. 2. The EPSCs were abolished reversibly by kynurenic acid or 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) but not affected by (+)-2-amino-5-phosphonovalerate (APV), suggesting that they were mediated by non-NMDA (N-methyl-D-aspartate) glutamate receptors. Micromolar concentrations of methionine [Met5]enkephalin reversibly reduced the magnitude of evoked EPSCs and the frequency of mEPSCs. 3. The enkephalin action on the mEPSC frequency was blocked by naloxone. A specific agonist of mu-opiate receptor, [D-Ala2,N-Me-Phe4, Gly5]enkephalin-ol (DAGO) suppressed the mEPSC frequency. In contrast, neither a delta-opiate receptor agonist, [D-Pen2, L-Pen5]enkephalin (DPLPE) nor a kappa-opiate receptor agonist, (5 alpha, 7 alpha, 8 beta)-(-)-N-methyl-N-[7-(1-pyrrolidinyl)-1- oxaspiro(4,5)-dec-8-yl]benzeneacetamide (U-69,593) significantly affected the mEPSC frequency. 4. The amplitude of mEPSCs or of currents induced by exogenous L-glutamate, was not affected by [Met5]enkephalin. It is suggested that [Met5]enkephalin presynaptically inhibits glutamatergic EPSCs by activating the mu-opiate receptor. 5. The frequency of mEPSC was reduced by about 50% by replacement of external Ca2+ with Mg2+ or by addition of Cd2+. In Ca(2+)-free-Mg2+ solution, [Met5]enkephalin did not reduce the remaining mEPSCs' frequency any further. 6. It is concluded that the opiates may suppress presynaptic Ca2+ entry, thereby inhibiting synaptic transmission.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYD I. A., MARTIN A. R. Spontaneous subthreshold activity at mammalian neural muscular junctions. J Physiol. 1956 Apr 27;132(1):61–73. doi: 10.1113/jphysiol.1956.sp005502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers J. M., Richerson G. B., Stevens C. F. Origin of variability in quantal size in cultured hippocampal neurons and hippocampal slices. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5359–5362. doi: 10.1073/pnas.87.14.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. H., Wong R. K., Prince D. A. Spontaneous miniature synaptic potentials in hippocampal neurons. Brain Res. 1979 Nov 9;177(1):194–199. doi: 10.1016/0006-8993(79)90931-4. [DOI] [PubMed] [Google Scholar]
- Christensen B. N., Perl E. R. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol. 1970 Mar;33(2):293–307. doi: 10.1152/jn.1970.33.2.293. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Fields H. L., Emson P. C., Leigh B. K., Gilbert R. F., Iversen L. L. Multiple opiate receptor sites on primary afferent fibres. Nature. 1980 Mar 27;284(5754):351–353. doi: 10.1038/284351a0. [DOI] [PubMed] [Google Scholar]
- Glazer E. J., Basbaum A. I. Immunohistochemical localization of leucine-enkephalin in the spinal cord of the cat: enkephalin-containing marginal neurons and pain modulation. J Comp Neurol. 1981 Mar 1;196(3):377–389. doi: 10.1002/cne.901960303. [DOI] [PubMed] [Google Scholar]
- HUBBARD J. I. The effect of calcium and magnesium on the spontaneous release of transmitter from mammalian motor nerve endings. J Physiol. 1961 Dec;159:507–517. doi: 10.1113/jphysiol.1961.sp006824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Hunt S. P., Kelly J. S., Emson P. C. The electron microscopic localization of methionine-enkephalin within the superficial layers (I and II) of the spinal cord. Neuroscience. 1980;5(11):1871–1890. doi: 10.1016/0306-4522(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Iversen L. L. Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature. 1977 Aug 11;268(5620):549–551. doi: 10.1038/268549a0. [DOI] [PubMed] [Google Scholar]
- Jessell T., Tsunoo A., Kanazawa I., Otsuka M. Substance P: depletion in the dorsal horn of rat spinal cord after section of the peripheral processes of primary sensory neurons. Brain Res. 1979 May 25;168(2):247–259. doi: 10.1016/0006-8993(79)90167-7. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H., Takahashi T. Characterization of miniature inhibitory post-synaptic potentials in rat spinal motoneurones. J Physiol. 1985 Nov;368:627–640. doi: 10.1113/jphysiol.1985.sp015880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamotte C., Pert C. B., Snyder S. H. Opiate receptor binding in primate spinal cord: distribution and changes after dorsal root section. Brain Res. 1976 Aug 13;112(2):407–412. doi: 10.1016/0006-8993(76)90296-1. [DOI] [PubMed] [Google Scholar]
- Landau E. M. The interaction of presynaptic polarization with calcium and magnesium in modifying spontaneous transmitter release from mammalian motor nerve terminals. J Physiol. 1969 Aug;203(2):281–299. doi: 10.1113/jphysiol.1969.sp008864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R. L., Werz M. A. Dynorphin A decreases voltage-dependent calcium conductance of mouse dorsal root ganglion neurones. J Physiol. 1986 Aug;377:237–249. doi: 10.1113/jphysiol.1986.sp016184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake M., Christie M. J., North R. A. Single potassium channels opened by opioids in rat locus ceruleus neurons. Proc Natl Acad Sci U S A. 1989 May;86(9):3419–3422. doi: 10.1073/pnas.86.9.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge A. W., Leeman S. E., Fischbach G. D. Enkephalin inhibits release of substance P from sensory neurons in culture and decreases action potential duration. Proc Natl Acad Sci U S A. 1979 Jan;76(1):526–530. doi: 10.1073/pnas.76.1.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A. Twelfth Gaddum memorial lecture. Drug receptors and the inhibition of nerve cells. Br J Pharmacol. 1989 Sep;98(1):13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J., Brandeis L., Cuello A. C. Depletion of substance P-containing axons in substantia gelatinosa of patients with diminished pain sensitivity. Nature. 1982 Jan 7;295(5844):61–63. doi: 10.1038/295061a0. [DOI] [PubMed] [Google Scholar]
- Pert C. B., Kuhar M. J., Snyder S. H. Autoradiograhic localization of the opiate receptor in rat brain. Life Sci. 1975 Jun 15;16(12):1849–1853. doi: 10.1016/0024-3205(75)90289-1. [DOI] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- Schneider S. P., Perl E. R. Comparison of primary afferent and glutamate excitation of neurons in the mammalian spinal dorsal horn. J Neurosci. 1988 Jun;8(6):2062–2073. doi: 10.1523/JNEUROSCI.08-06-02062.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. E., Fischbach P. S., Zheng D., McCleskey E. W. Activation of mu opioid receptors inhibits transient high- and low-threshold Ca2+ currents, but spares a sustained current. Neuron. 1991 Jan;6(1):13–20. doi: 10.1016/0896-6273(91)90117-i. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I., Tamarova Z. A. Synaptic activity in motoneurons of the immature cat spinal cord in vitro. Effects of manganese and tetrodotoxin. Brain Res. 1979 Jan 19;160(3):524–528. doi: 10.1016/0006-8993(79)91080-1. [DOI] [PubMed] [Google Scholar]
- Shimahara T., Icard-Liepkalns C. Activation of enkephalin receptors reduces calcium conductance in neuroblastoma cells. Brain Res. 1987 Jul 14;415(2):357–361. doi: 10.1016/0006-8993(87)90220-4. [DOI] [PubMed] [Google Scholar]
- Sugiura Y., Lee C. L., Perl E. R. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986 Oct 17;234(4774):358–361. doi: 10.1126/science.3764416. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Inhibitory miniature synaptic potentials in rat motoneurons. Proc R Soc Lond B Biol Sci. 1984 Mar 22;221(1222):103–109. doi: 10.1098/rspb.1984.0025. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Intracellular recording from visually identified motoneurons in rat spinal cord slices. Proc R Soc Lond B Biol Sci. 1978 Jul 26;202(1148):417–421. doi: 10.1098/rspb.1978.0076. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Membrane currents in visually identified motoneurones of neonatal rat spinal cord. J Physiol. 1990 Apr;423:27–46. doi: 10.1113/jphysiol.1990.sp018009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi H., Costa M., Schimerlik M., North R. A. Potassium conductance increased by noradrenaline, opioids, somatostatin, and G-proteins: whole-cell recording from guinea pig submucous neurons. J Neurosci. 1990 May;10(5):1675–1682. doi: 10.1523/JNEUROSCI.10-05-01675.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoo A., Yoshii M., Narahashi T. Block of calcium channels by enkephalin and somatostatin in neuroblastoma-glioma hybrid NG108-15 cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9832–9836. doi: 10.1073/pnas.83.24.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. T., Egan T. M., North R. A. Enkephalin opens potassium channels on mammalian central neurones. Nature. 1982 Sep 2;299(5878):74–77. doi: 10.1038/299074a0. [DOI] [PubMed] [Google Scholar]
- Williams J. T., North R. A., Tokimasa T. Inward rectification of resting and opiate-activated potassium currents in rat locus coeruleus neurons. J Neurosci. 1988 Nov;8(11):4299–4306. doi: 10.1523/JNEUROSCI.08-11-04299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimpey T. L., Chavkin C. Opioids activate both an inward rectifier and a novel voltage-gated potassium conductance in the hippocampal formation. Neuron. 1991 Feb;6(2):281–289. doi: 10.1016/0896-6273(91)90363-5. [DOI] [PubMed] [Google Scholar]
- Woolf C. J., Fitzgerald M. The properties of neurones recorded in the superficial dorsal horn of the rat spinal cord. J Comp Neurol. 1983 Dec 10;221(3):313–328. doi: 10.1002/cne.902210307. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Jessell T. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol. 1990 Nov;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M., North R. A. Substantia gelatinosa neurones hyperpolarized in vitro by enkephalin. Nature. 1983 Oct 6;305(5934):529–530. doi: 10.1038/305529a0. [DOI] [PubMed] [Google Scholar]




