Abstract
Angio-based microvascular resistance (AMR) as a potential alternative to the index of microcirculatory resistance (IMR) and its relationship with microvascular obstruction (MVO) and other cardiac magnetic resonance (CMR) parameters still lacks comprehensive validation. This study aimed to validate the correlation between AMR and CMR-derived parameters and to construct an interpretable machine learning (ML) model, incorporating AMR and clinical data, to forecast MVO in ST-segment elevation myocardial infarction (STEMI) patients undergoing primary percutaneous coronary intervention (PPCI). We enrolled 452 STEMI patients from Nanjing Drum Tower Hospital between 2018 and 2022, who received both PPCI and CMR. After PPCI, AMR measurements and CMR-derived parameters were recorded, and clinical data were gathered. The ML workflow comprised feature selection using the Boruta algorithm, model construction with seven classifiers, hyperparameter optimization via ten-fold cross-validation, model comparison based on the area under the curve (AUC), and a Shapley additive explanations (SHAP) analysis to analyze the significance of different features. 32.29% of patients showed inconsistency between AMR and MVO, but we successfully constructed a predictive model for MVO. Among the classifiers, Extreme gradient boosting (XGBoost) post hyperparameter optimization displayed superior performance, achieving an AUC of 0.911 and 0.846 in the training and validation sets, respectively. SHAP analysis identified AMR as a pivotal predictor of MVO. Although we observed the inconsistency between AMR and MVO but the ML-based construction of MVO prediction model is feasible, which brings the possibility of timely prediction of patients with MVO and timely imposition of interventions during PPCI.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-87828-5.
Keywords: ST-segment elevation myocardial infarction, Angio-based microvascular resistance, Cardiac magnetic resonance, Microvascular obstruction, Machine learning
Subject terms: Cardiology, Interventional cardiology
Introduction
Timely primary percutaneous coronary intervention (PPCI) is crucial for patients with ST-segment elevation myocardial infarction (STEMI), as it rapidly restores epicardial perfusion and minimizes myocardial damage caused by ischemia/reperfusion (I/R).1 However, post-PPCI patients can still experience varying degrees of infarct size (IS), microvascular damage, and cardiac function deterioration2,3. Cardiac magnetic resonance (CMR), a non-invasive diagnostic tool, provides quantitative analysis of several parameters, including IS, left ventricular ejection fraction (LVEF), microvascular obstruction (MVO), and intramyocardial hemorrhage (IMH), aiding in the risk stratification of STEMI patients following PPCI4. MVO occurs in more than 50% of STEMI patients following PPCI and may be associated with intraluminal obstructive and extravascular compressive pathologies from reperfusion of the culprit vessel5. Additionally, there is a strong correlation between the zones of MVO and myocardial necrosis5. Previous research indicates that the presence and extent of MVO post-PPCI are linked to major adverse cardiovascular events (MACEs) and is independent predictor of adverse left ventricular remodeling6,7. Nevertheless, the challenge remains that CMR-derived parameters, an after-the-fact diagnosis, are not effective for assessing the risk in real-time during PPCI, hindering proactive intervention to improve patient outcomes.
The index of microcirculatory resistance (IMR), an invasive coronary artery physiological indicator, quantitatively measures microcirculation resistance during PPCI and is the gold standard for diagnosing coronary microvascular dysfunction (CMD)8. Previous studies have observed a significant linear correlation between IMR and the degree of MVO and IS, enabling the prediction of CMR phenotypes in STEMI patients based on IMR obtained during PPCI9,10. However, the efficacy of IMR in predicting MVO post-PPCI in STEMI patients has proven suboptimal, with discrepancies between IMR and MVO observed in over 30% of case9. The necessity of a pressure-temperature sensor wire and hyperemic agents in IMR measurement constrains its clinical utility, thus limiting opportunities for deeper investigation into the relationship between IMR and MVO presence.
Angio-based microvascular resistance (AMR) is a novel pressure-wire-free, adenosine-free and angiography-derived metric that has been shown to be a viable alternative to pressure wire-based IMR11. In this study, we will investigate the correlation between AMR and CMR-derived parameters and hope to construct a machine learning model (ML) based on available clinical data that can predict the occurrence of MVO during PPCI.
Methods
Study design
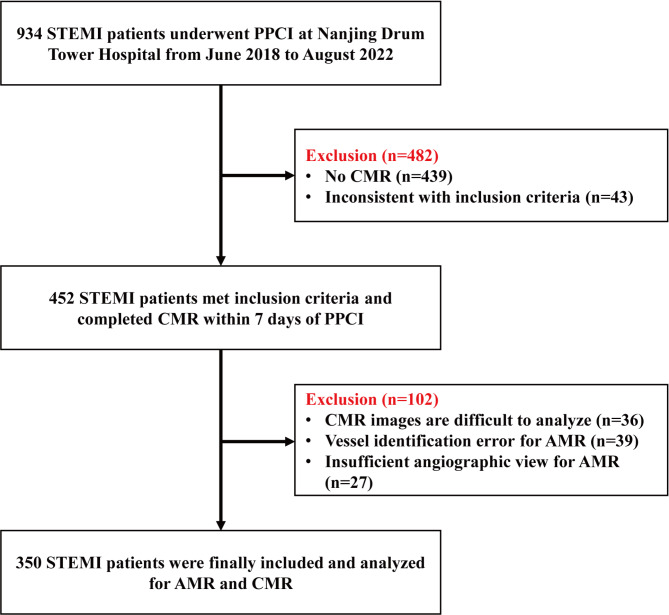
This study prospectively enrolled STEMI patients who underwent CMR during their hospitalization at Nanjing Drum Tower Hospital from June 2018 to August 2022, and a total of 350 patients diagnosed with STEMI and treated with PPCI were evaluated for inclusion in the study. (Fig. 1) The diagnostic criteria for STEMI are based on the ESC/ACCF/AHA/WHF consensus document. Exclusion criteria included cardiogenic shock, previous acute myocardial infarction or any form of revascularization, contraindication to CMR and inability to assess AMR. All procedures were performed in accordance with the Declaration of Helsinki. The Medical Ethics Committee of Nanjing Drum Tower Hospital approved the protocol of this study (No.2021-531-02). All participants provided written informed consent participate.
Fig. 1.
Study flow.
Measurement of AMR
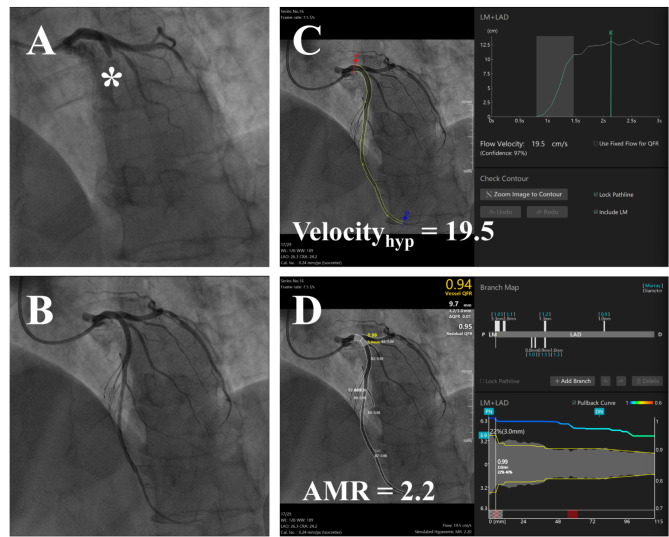
Commercial software (Angio Plus Core, version V3, Shanghai Pulse Medical Technology Inc., Shanghai, China) was utilized for analyzing Murray law-based Quantitative Flow Ratio (µQFR), hyperemic flow velocity (Velocityhyp), and AMR of the culprit vessel following successful recanalization. Analysis was conducted independently by an experienced analyst and a cardiovascular physician, each with experienced clinical experience, both blinded to the patients’ clinical data and CMR results. These methods are elaborated in previous studies11,12. The software automatically delineates the lumen of the culprit vessel by selecting angiographic images in optimal positions, minimizing vessel overlap. Velocityhyp is calculated by dividing the centerline length of the culprit vessel by the contrast fill time. Key frames, chosen manually for their clear contrast fill and complete lumen visualization, enable the software to sketch the lumen outlines of the culprit vessel and major branches, with manual adjustments if necessary. Ultimately, the software computes the AMR and µQFR, of the culprit vessel based on specific equations. (Fig. 2)
Fig. 2.
Measurement Process of Angio-based Microvascular Resistance (A) Coronary angiography images before reperfusion. (B) Coronary angiography images of completed reperfusion. (C) The culprit vessel contour was automatically circled and manually corrected after the keyframe was selected, and Velocityhyp was calculated during this process. (D) After circling and correcting for branch vessels of the culprit vessel, the software calculated µQFR and AMR.
CMR protocol and image analysis
All patients underwent CMR at 3.0 T (Ingenia CX 3.0T; Philips Healthcare, Best, The Netherlands) within 7 days after PPCI. All CMR images were analyzed using cvi42 software (Circle Cardiovascular Imaging Inc., Calgary, Canada)13. Detailed CMR protocol and image analysis are provided in the supplementary material.
MVO prediction model constructed based on machine learning
Our objective was to develop a predictive model for determining the likelihood of MVO occurrence post-PPCI in patients with STEMI, utilizing angiography-derived index and clinical data available during the PPCI procedure. We retrospectively gathered demographic data, emergency room conditions, and angiographic characteristics from all patient records. The patient dataset was randomly segmented into a training set, comprising 70% of the data, and a validation set, constituting the remaining 30%. The training set was employed for model development and cross-validation processes, while the validation set was dedicated to internal validation. In our dataset, discrete variables were subjected to one-hot encoding, and continuous variables were normalized to ensure data uniformity and accuracy in model development.
To reduce the risk of overfitting and enhance the interpretability of our model, we employed the Boruta algorithm for feature selection and visualization. The Boruta algorithm is a robust feature selection method that identifies relevant variables by generating randomly shaded counterparts and iteratively evaluating their importance in comparison to the actual variables, utilizing the variable importance measure from a random forest (RF) model14. Briefly, Boruta duplicates all features, shuffles them, and constructs an RF model to calculate the Z-score for each feature during each iteration. The algorithm identifies the highest Z-score among the shaded attributes and designates features exceeding this threshold as significant, continuing this process until all features are evaluated or the predetermined model run limit is reached. For our study, we input 32 collected features, including gender, age, AMR, among others (detailed in Supplementary Table 1), into the Boruta function for selection. Consequently, variables such as AMR, µQFR, systolic blood pressure in the emergency room, thrombus aspiration, pre-TIMI flow grade (pre-TFG), and TIMI thrombus grade were selected for the construction of our machine learning model.
All machine learning-based classifications in our study were executed using R Studio (version 4.3.1, package: mlr3verse). We employed a range of algorithms as learners, including Logistic Regression (LR), Random Forest (RF), Decision Tree, Extreme Gradient Boosting (XGBoost), Support Vector Machine (SVM), K-Nearest Neighbor (KNN), and Naive Bayes (NB). Hyperparameter Optimization (HPO) across all models was conducted via random search, coupled with ten-fold cross-validation, to identify the hyperparameter combination with the highest Area Under the Curve (AUC). The performance of these algorithms was assessed based on various metrics: accuracy, AUC, precision, recall, F1 score, and confusion matrix. XGBoost was finally chosen as the learner for this study because it has the best diagnostic performance among all learners. XGBoost is renowned for being a scalable and efficient end-to-end tree boosting system, extensively utilized in data science15. To further evaluate the model, we generated calibration plots and Decision Curve Analysis (DCA) for both the training and validation sets. Additionally, we applied the Shapley Additive Explanations (SHAP) method, incorporating visualizations to enhance the interpretability of the model. This method elucidates the significance of each feature and its contribution to the model’s performance by calculating SHAP values16.
Statistical analysis
Continuous variables with symmetric distributions were written as mean ± SD, and variables with skewed distributions were presented as median (interquartile ranges). The frequency and percentage of categorical variables are used in their expression. And Categorical variables between two groups were tested by chi-square test or Fisher’s exact test. Comparison of two sets of continuous variables using Student’s t test or Mann-Whitney U test. Correlation analysis between the two sets of continuous variables was performed using Pearson’s correlation coefficient analysis or Spearman’s correlation coefficient analysis. Plotting receiver operating characteristic curve (ROC) and calculating AUC to evaluate the predictive efficacy of AMR in predicting MVO and of different learners in predicting MVO. Univariate logistic-regression analysis was used to determine predictors of MVO. Covariates with P < 0.10 in the univariate analysis were used as covariates in the multivariate logistic regression to determine whether AMR was an independent predictor of MVO. Further subgroup analyses explored whether AMR was an independent predictor of developing MVO after PCI in different subgroups of STEMI patients and were visualized using forest plots. All analyses were two-tailed, with P < 0.05 indicating a significant difference. All statistical analyses were performed using R studio (version 4.3.1).
Results
Baseline characteristics
This study ultimately enrolled 350 STEMI patients underwent PPCI at Nanjing Drum Tower Hospital, who completed measurements of CMR and AMR. 85.71% of patients were male, and the average age of all patients was 59 (51–67) years old. The median AMR, Velocityhyp and µQFR were 2.48 (2.18–2.84), 16 (12.8–18.5), and 0.94 (0.90–0.97), respectively. The median IS mass, IS/LV mass, MVO/LV mass and LVEF were 19.75 g (12.30–32.52 g), 15.53 (10.26–24.56), 0.78 (0-2.71) and 51 (42–58) respectively. (Supplementary Table 2) Supplementary Table 3 compares the differences in incorporated features between the training and validation sets. Patients with Killip class 1 were higher in the training set than in the validation set, and there were more patients with multivessel disease in the training set. While the rest of the characteristics were not statistically different between the two groups.
Correlation of AMR and CMR-derived parameters
All patients in the cohort completed CMR after PPCI, and the median time from PPCI to CMR was 5 (3–6) days. In all patients, there was a significant linear correlation between AMR and IS/LV mass (Rho = 0.266, P < 0.001) (Fig. 3A). In patients with the presence of MVO diagnosed by CMR after PPCI, AMR showed a significant linear correlation with MVO/LV mass (Rho = 0.219, P = 0.001) (Fig. 3B). There was a weak but statistically significant linear correlation between AMR and LVEF in all patients (Rho = -0.128, P = 0.019) (Fig. 3C). Patients with MVO have higher AMR value (2.580(2.335–2.950) vs. 2.275(2.060–2.645), P < 0.001) (Fig. 3D). Univariate logistic regression analysis showed that 15 variables, including µQFR (OR: 2.129, 95%CI: 1.452–3.121), AMR (OR: 3.698, 95%CI: 2.217–6.167), and pre-infarction angina (OR: 0.562, 95%CI: 0.364–0.868), were associated with MVO (Supplementary Table 4). Multivariate logistic regression suggests that AMR remains an independent predictor of MVO after adjusting for covariates (OR: 3.276, 95%CI: 1.397–7.680). The forest map shows that the risk of MVO increases with the increase of AMR in all subgroups except diabetes patients (Fig. 3E).
Fig. 3.
Correlation of AMR and CMR-derived parameters (A) Scatterplot demonstrating the correlation between IS/LV mass with AMR. (B) Scatterplot demonstrating the correlation between MVO/LV mass with AMR in patients with MVO. (C) Scatterplot demonstrating the correlation between LVEF with AMR. (D) Violin diagram of AMR in patients with and without MVO. (E) Forest plot showing the association between AMR and MVO risk in each subgroup.
In the ROC curve, the cut-off value of AMR for predicting MVO is 2.36 (sensitivity: 74.30%, specificity: 57.35%, AUC: 0.671, 95%CI: 0.619–0.720) (Supplementary Fig. 1A). Patients were categorized into High-AMR and Low-AMR based on the cut-off value, 159 patients (73.27%) in the High-AMR group had MVO and 78 patients (58.65%) in the Low-AMR group did not have MVO. However, the total number of patients in the High-AMR group who did not have MVO and the total number of patients in the Low-AMR group who had MVO was 113 (32.29%) (Supplementary Fig. 1B). Discordance between AMR and MVO was observed in 32.29% of patients.
Model construction and interpretation
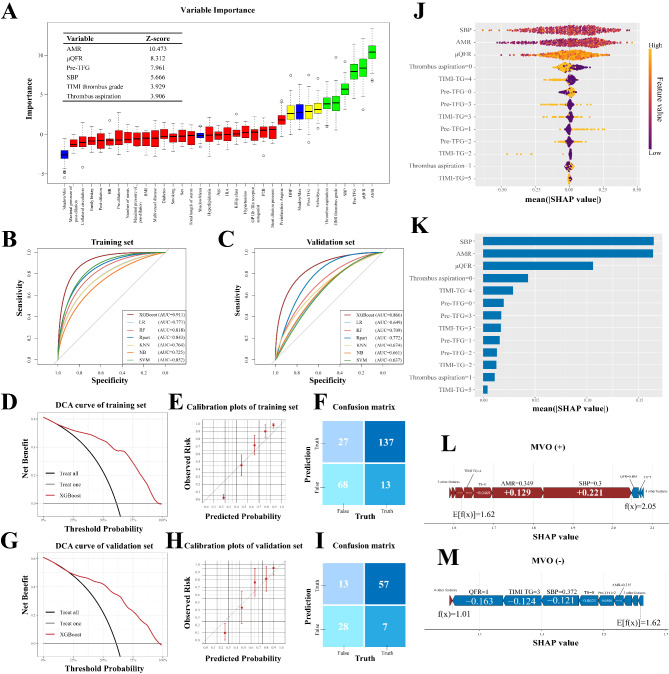
Feature selection was performed using the Boruta algorithm. AMR, µQFR, Pre-TFG, SBP, TIMI thrombus grade and thrombus aspiration were selected as significant variables with Z-scores of 10.473, 8.312, 7.961, 5.666, 3.929 and 3.906 respectively (Fig. 4A). Based on the variables selected by feature selection, we used several common supervised machine algorithms to construct a predictive model of MVO after PPCI in STEMI patients. We construct a dichotomous model in which the target is set to the presence or absence of MVO in CMR. After optimal parameter tuning, the AUC of XGBoost, LR, RF, decision tree, KNN, naive bayes, and SVM in the validation set are 0.866, 0.649, 0.709, 0.772, 0.674, 0.661 and 0.637, respectively. Accuracy, precision, recall, AUC and F1 value for all models are listed in Table 1. Figure 4B and C demonstrate that all models show effectiveness in predicting MVO in both the training and validation set, with XGBoost having the best prediction performance with AUC of 0.911 and 0.866, respectively. The prediction was confirmed by the confusion matrix (Fig. 4F). As shown in the confusion matrix of Fig. 4I, MVO was predicted to be present in 57 (89.06%) of the 105 patients, with 7 (10.94%) missed diagnoses. The DCA curves of the training and validation sets are shown in Fig. 4D and G. The DCA curves show that predicting MVO using the tuned XGBoost has greater net benefit when the threshold probability of an individual in the training set is greater than 13%, and when the threshold of an individual in the validation set is greater than 20%. The obtained calibration curve suggested good calibration of the XGBoost model in the training and validation sets (Fig. 4E and H).
Fig. 4.
Interpretable Machine Learning Model Construction based on Shapley Additive Explanations (A) Plot of feature selection process based on Boruta algorithm. (B) ROC curves for seven machine learning models in the training set. (C) ROC curves for seven machine learning models in the validation set. (D) DCA curve for the training set of XGBoost. (G) DCA curve for the validation set of XGBoost. (E) Calibration plot for the training set of XGBoost. (H) Calibration plot for the validation set of XGBoost. (F) Confusion matrix for the training set of XGBoost. (I) Confusion matrix for the validation set of XGBoost. (J) The bee-swarm plot shows the SHAP values of all the variables in the model, with higher SHAP values representing greater influence and contribution to the mode. (K) The importance ranking of all variables in model by the mean value of |SHAP value|. (L & M) The force plots for patient with MVO and without MVO, respectively. (The values of each variable are normalized values.)
Table 1.
Performance of machine learning algorithms.
| Classifiers | AUC | Accuracy | Precision | Recall | F1 value | |
|---|---|---|---|---|---|---|
| XGBoost | Train | 0.911 | 0.837 | 0.840 | 0.716 | 0.773 |
| Test | 0.866 | 0.810 | 0.800 | 0.683 | 0.737 | |
| Logistic Regression | Train | 0.771 | 0.694 | 0.652 | 0.453 | 0.535 |
| Test | 0.649 | 0.648 | 0.571 | 0.390 | 0.463 | |
| Random Forest | Train | 0.818 | 0.747 | 0.720 | 0.568 | 0.635 |
| Test | 0.709 | 0.676 | 0.606 | 0.488 | 0.541 | |
| Decision Tree | Train | 0.843 | 0.808 | 0.773 | 0.716 | 0.743 |
| Test | 0.772 | 0.771 | 0.743 | 0.634 | 0.684 | |
| K-Nearest Neighbor | Train | 0.764 | 0.694 | 0.647 | 0.463 | 0.540 |
| Test | 0.674 | 0.638 | 0.548 | 0.415 | 0.472 | |
| Naive Bayes | Train | 0.725 | 0.685 | 0.615 | 0.505 | 0.555 |
| Test | 0.661 | 0.619 | 0.514 | 0.439 | 0.474 | |
| Support Vector Machines | Train | 0.852 | 0.759 | 0.790 | 0.516 | 0.624 |
| Test | 0.637 | 0.629 | 0.533 | 0.390 | 0.450 |
To further explore the effect of different variables on prediction of MVO, we use SHAP to interpret the model. The bee-swarm plot in Fig. 4J illustrates the ways in which the various variables in the predictive model affect prediction of MVO. High AMR, high µQFR, low SBP, low pre-TFG, thrombus aspiration and high thrombus burden have a high “positive” contribution to prediction of MVO. Figure 4K shows the average SHAP value for all features, sorted according to the SHAP value. SBP, AMR, and µQFR were the three most important variables with SHAP values greater than 0.1. Figure 4L and M show personalized feature mapping for two representative patients in model with and without MVO in the XGBoost model. The arrows indicate the effect of each variable on the prediction, with red indicating that the variable is a risk of increasing MVO and blue vice versa. Patients with MVO had higher predicted value (2.05), which were higher than the base value (1.62), while patients without MVO had lower predicted value (1.01), which were lower than the base value (Fig. 4L and M).
Discussion
In this study, we evaluated AMR and CMR-derived parameters in a cohort of STEMI patients undergoing PPCI to explore their interrelationships. Our main findings are as follows:
AMR showed a positive correlation with IS and MVO mass and the cut-off value of AMR for predicting MVO is 2.36.
Controlling for covariates, elevated AMR emerged as an independent predictor of MVO. Utilizing AMR to forecast MVO proved feasible, with a noted mismatch between AMR and MVO in over 30% of patients.
Of the seven models developed, the hyperparameter-optimized XGBoost model showed superior performance, effectively predicting MVO intraoperatively.
Analysis of the model using SHAP values revealed that high AMR, QFR, thrombus burden, and thrombus aspiration positively influenced MVO prediction, whereas high SBP and Pre-TFG exerted a negative impact.
Even with timely administration of PPCI, more than 60% of STEMI patients may still develop CMD8. Many studies have linked CMD to adverse prognosis, highlighting the urgency of early diagnosis and intervention in STEMI patients with CMD16–18. IMR, an invasive method of measuring microvascular resistance by means of a pressure wire, is the gold standard for the diagnosis of CMD. However, its widespread clinical application is hampered by cost-effectiveness and the necessity of vasoactive drugs19. In contrast, AMR, an angiography-derived index of microcirculation, has shown significant correlation with IMR11. The aim of our study was to use AMR to assess microcirculatory function after PPCI in STEMI patients.
In our study, AMR was positively correlated with IS and MVO mass. Although previous studies have attempted to establish a link between IMR and CMR-derived parameters9,10,20, our study aimed to delve deeper into this relationship using a larger sample size and an angiography-derived microvascular resistance index. Notably, our finding is similar to earlier studies, revealing statistically significant but weak correlations between AMR, IS and MVO mass20. Pathophysiologic changes in cardiomyocytes after myocardial infarction may lead to changes in intracoronary microcirculatory function21, as demonstrated in our study: the larger the IS or MVO mass, the higher the AMR. However, precise numerical agreement between AMR and IS or MVO mass is not achievable, probably due to intracoronary hemodynamic instability after reperfusion in STEMI patient22. Despite this limitation, AMR holds promise as a tool for risk stratification of STEMI patients, even if it does not accurately predict infarct or MVO size. It is worth noting that this study focused on STEMI patients undergoing PPCI. While the prognostic significance of IMR in STEMI patients is well-established9, recent research indicates that IMR/HMR lacks predictive value in primary CMD patients23,24 This discrepancy may be attributed to differing underlying mechanisms between primary CMD and post-PCI CMD following STEMI. Consequently, the clinical application of AMR should remain restricted until its role across diverse CMD subtypes is thoroughly validated.
Both IMR and MVO indicate the state of the microcirculation in STEMI patients after PPCI, but they exhibit different characteristics. IMR is an invasive measure that quantifies the microvascular resistance in culprit vessel at a specific time after PPCI. Previous studies have demonstrated dynamic changes in IMR in culprit vessel after PPCI, with the most pronounced decrease occurring within 24 h25. MVO is recognizable as visible anatomical microvascular dysfunction on CMR imaging, and is considered irreversible myocardial damage, especially in the presence of IMH. The degree of MVO also evolves after reperfusion, and the timing of CMR (5 days, IQR:3–6) was identified as the time point for the emergence and stabilization of MVO in this study26. IMR and MVO provide different temporal and angular insights into coronary microcirculatory function. Thus, microcirculatory dysfunction identified by IMR and MVO suggests different pathophysiologic mechanisms. This discrepancy explains why IMR and MVO were inconsistent in previous studies9. In this study, the measurement time of AMR and MVO were different, and the microvascular function of the culprit vessels may vary over time. This temporal variability could be a potential driving factor for the observed differences between AMR and MVO. However, given the role of MVO as an independent predictor of poor prognosis and LV remodeling6,7, improving its intraoperative predictive accuracy and ensuring timely intervention are critical clinical goals.
In this study, we shift the focus from analyzing intracoronary physiological index and imaging performance of MVO to combining coronary angiography-derived index and clinical data with machine learning algorithms to construct predictive models. Recent advances in artificial intelligence in healthcare have had a significant impact on medical diagnostics, biometrics, and multidimensional data processing. In particular, XGBoost, a machine learning framework, has shown mastery in preventing overfitting and enhancing generalization capabilities. Our main goal is to predict MVO intraoperatively during PPCI and develop predictive models through machine learning to improve diagnostic accuracy and minimize false negatives. The optimized XGBoost algorithm, post hyperparameter tuning, exhibited excellent performance in our classification model, with AUC and accuracy denoted as 0.866 and 0.810, respectively. In addition, we aimed to use the SHAP value to elucidate the contribution of various factors to MVO prediction. Machine learning algorithms are often viewed as black box and can be clarified using SHAP value, which elucidate the impact of individual features on the overall model. The SHAP values indicated that high AMR, high QFR, elevated thrombus burden, and thrombus aspiration were positively associated with MVO prediction, whereas high SBP and high pre-TFG were negatively associated. Some of these factors are consistent with CMD risk factors identified in previous studies8. Moreover, the influence of abnormal physiologic markers in the coronary arteries and emergency room blood pressure readings on MVO provides new ideas for future studies.
Study limitations
Our study has some limitations. Firstly, this study is retrospective and has a small sample size. To address this issue, we plan to include more prospective samples in future research to improve our predictive model. Secondly, the single center design of this study may introduce biases. To reduce this bias, we plan to include independent cohorts from different regions for external validation in subsequent studies, in order to improve the generalizability of the model. Thirdly, the current model relies entirely on structured data in the clinical environment. The future focus of work will be on integrating unstructured data to enrich the learning ability of the model and make it more effective in adapting to the characteristics of different patients. Fourthly, the Velocityhyp in AMR in this study was calculated through software simulation. If adenosine is used simultaneously, it may make the value of hyperemic flow velocity more accurate, which is beneficial for the construction of ML model27,28.
Conclusions
AMR demonstrates predictive capabilities for IS and MVO mass. ML models, integrating AMR with clinical data, have shown efficacy in predicting MVO during PPCI. Further, SHAP values identified AMR as a critical determinant in MVO prediction.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Abbreviations
- MVO
Microvascular obstruction
- CMR
Cardiac magnetic resonance
- AMR
Angio-based microvascular resistance
- ML
Machine learning
- PPCI
Primary percutaneous coronary intervention
- LGE
Late gadolinium enhancement
- STEMI
ST-segment elevation myocardial infarction
- XGBoost
Extreme gradient boosting
- SHAP
Shapley additive explanations
Author contributions
Formal analysis, ZZ, YD, and PX; Methodology, ZZ and QD; Software, XBB, SYQ, XMG and YNX; Supervision, XB; Validation, QD and YG; Writing—original draft, ZZ; Writing—review & editing, LNK and BX. The results were discussed, and the text was evaluated by all authors.
Funding
This research was funded by the following funding: the National Key R&D Program of Ministry of Science and Technology (2022YFA1205904), the Frontier Fundamental Research Program of Jiangsu Province for Leading Technology (BK20222002). Jiangsu Provincial Key Research and Development Foundation (BE2022665). All authors report no conflict of interest.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The data collection for the study was approved by the Medical Ethics Committee of Nanjing Drum Tower Hospital (No.2021-531-02). Participants gave informed consent to participate in the study before taking part.
Footnotes
Zhe Zhang, Yang Dai and Peng Xue are contributed equally to this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qing Dai, Email: daiqing811002@163.com.
Biao Xu, Email: xubiao62@nju.edu.cn.
Lina Kang, Email: kanglina@njglyy.com.
References
- 1.Heusch, G. & Gersh, B. J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur. Heart J.38 (11), 774–784. 10.1093/eurheartj/ehw224 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Stone, G. W. et al. Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials. J. Am. Coll. Cardiol.67 (14), 1674–1683. 10.1016/j.jacc.2016.01.069 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Durante, A. et al. Identification of high-risk patients after ST-Segment-Elevation myocardial infarction: comparison between angiographic and magnetic resonance parameters. Circ. Cardiovasc. Imaging. 10 (6), e005841. 10.1161/CIRCIMAGING.116.005841 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Bulluck, H., Dharmakumar, R., Arai, A. E., Berry, C. & Hausenloy, D. J. Cardiovascular magnetic resonance in Acute ST-Segment-Elevation myocardial infarction: recent advances, controversies, and future directions. Circulation137 (18), 1949–1964. 10.1161/CIRCULATIONAHA.117.030693 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sezer, M. et al. Coronary microvascular Injury in Reperfused Acute myocardial infarction: a View from an integrative perspective. J. Am. Heart Assoc.7 (21), e009949. 10.1161/JAHA.118.009949 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kali, A. et al. Persistent microvascular obstruction after myocardial infarction culminates in the confluence of Ferric Iron Oxide Crystals, Proinflammatory Burden, and adverse remodeling. Circ. Cardiovasc. Imaging. 9 (11), e004996. 10.1161/CIRCIMAGING.115.004996 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Waha, S. et al. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: an individual patient data pooled analysis from seven randomized trials. Eur. Heart J.38 (47), 3502–3510. 10.1093/eurheartj/ehx414 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Padro, T. et al. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on ‘coronary microvascular dysfunction in cardiovascular disease’. Cardiovasc. Res.116 (4), 741–755. 10.1093/cvr/cvaa003 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Maria, G. L. et al. Index of Microcirculatory Resistance as a Tool to characterize microvascular obstruction and to predict infarct size regression in patients with STEMI undergoing primary PCI. J. Am. Coll. Cardiol. Img. 12 (5), 837–848. 10.1016/j.jcmg.2018.02.018 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Scarsini, R. et al. Coronary microvascular dysfunction assessed by pressure wire and CMR after STEMI predicts long-term outcomes. J. Am. Coll. Cardiol. Img. 14 (10), 1948–1959. 10.1016/j.jcmg.2021.02.023 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Fan, Y. et al. In vivo validation of a Novel Computational Approach to assess Microcirculatory Resistance based on a single angiographic view. J. Pers. Med.12 (11), 1798. 10.3390/jpm12111798 (2022). Published 2022 Oct 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu, S. et al. Diagnostic accuracy of quantitative flow ratio for assessment of coronary stenosis significance from a single angiographic view: a novel method based on bifurcation fractal law. Catheter Cardiovasc. Interv. 97 (Suppl 2), 1040–1047. 10.1002/ccd.29592 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Schulz-Menger, J. et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance – 2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J Cardiovasc Magn Reson. ;22(1):19. Published 2020 Mar 12. (2020). 10.1186/s12968-020-00610-6 [DOI] [PMC free article] [PubMed]
- 14.Degenhardt, F., Seifert, S. & Szymczak, S. Evaluation of variable selection methods for random forests and omics data sets. Brief. Bioinform. 20 (2), 492–503. 10.1093/bib/bbx124 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, T. & Guestrin, C. XGBoost: A Scalable Tree Boosting System, Proc. 22nd ACM SIGKDD Int. Conf. Knowledge Discovery and Data Mining, pp. 785–794, [Online]. (2016). Available: https://arxiv.org/abs/1603.02754
- 16.Lundberg, S. M. & Lee, S-I. A unified approach to interpreting model predictions. Adv. Neural Inf. Process. Syst. :4765–4774. (2017).
- 17.Borlotti, A. et al. Acute Microvascular Impairment Post-reperfused STEMI is reversible and has additional clinical predictive value: a CMR OxAMI Study. J. Am. Coll. Cardiol. Img. 12 (9), 1783–1793. 10.1016/j.jcmg.2018.10.028 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamirani, Y. S., Wong, A., Kramer, C. M. & Salerno, M. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: a systematic review and meta-analysis. J. Am. Coll. Cardiol. Img. 7 (9), 940–952. 10.1016/j.jcmg.2014.06.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fearon, W. F. et al. Novel index for invasively assessing the coronary microcirculation [published correction appears in Circulation. ;108(25):3165]. Circulation. 2003;107(25):3129–3132. (2003). 10.1161/01.CIR.0000080700.98607.D1 [DOI] [PubMed]
- 20.Payne, A. R. et al. Microvascular Resistance predicts myocardial salvage and infarct characteristics in ST-Elevation myocardial infarction. J. Am. Heart Assoc.1 (4), e002246. 10.1161/JAHA.112.002246 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konijnenberg, L. S. F. et al. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc. Res.116 (4), 787–805. 10.1093/cvr/cvz301 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Echavarría-Pinto, M., Collet, C., Escaned, J., Piek, J. J. & Serruys, P. W. State of the art: pressure wire and coronary functional assessment. EuroIntervention13 (6), 666–679. 10.4244/EIJ-D-17-00503 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Eftekhari, A. et al. Prognostic value of microvascular resistance and its association to fractional flow reserve: a DEFINE-FLOW substudy. Open. Heart. 9 (1), e001981. 10.1136/openhrt-2022-001981 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boerhout, C. K. M. et al. Prognostic value of structural and functional coronary microvascular dysfunction in patients with non-obstructive coronary artery disease; from the multicentre international ILIAS registry. EuroIntervention18 (9), 719–728. 10.4244/EIJ-D-22-00043 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuculi, F. et al. Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST-segment elevation myocardial infarction [published correction appears in J Am Coll Cardiol. ;65(8):866. Choudhury, Robin C [Corrected to Choudhury, Robin P]]. J Am Coll Cardiol. 2014;64(18):1894–1904. (2015). 10.1016/j.jacc.2014.07.987 [DOI] [PubMed]
- 26.Niccoli, G., Scalone, G., Lerman, A. & Crea, F. Coronary microvascular obstruction in acute myocardial infarction. Eur. Heart J.37 (13), 1024–1033. 10.1093/eurheartj/ehv484 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Kest, M. et al. Angiography-based coronary microvascular assessment with and without intracoronary pressure measurements: a systematic review. Clin. Res. Cardiol.113 (12), 1609–1621. 10.1007/s00392-023-02338-6 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tar, B. et al. Hyperemic contrast velocity assessment improves accuracy of the image-based fractional flow reserve calculation. Cardiol. J.28 (1), 163–165. 10.5603/CJ.a2020.0144 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.