Abstract
Alcohol-associated hepatitis, considered a severe form of alcohol-associated liver disease, carries with it multiple negative health outcomes ranging not only to increased hospitalizations but also increased rates of mortality. While the inpatient management remains critical in optimizing clinical outcomes, a shift in focus to the outpatient management of alcohol-associated hepatitis is warranted as a long-term solution to this emerging health pandemic. Here, we review the clinical presentation, diagnosis, and current prognostication scoring systems for alcohol-associated hepatitis. We then offer a multimodal approach to the continued management of alcohol-associated hepatitis in the outpatient setting encompassing not only nutritional optimization, alcohol use disorder treatment, and the medical management of chronic liver disease, but also briefly review the current trend of the use of liver transplantation.
Keywords: Alcohol hepatitis, Severe alcohol hepatitis
Introduction
Alcohol use, both worldwide and within the United States, has steadily increased. The most recent World Health Organization’s Report on Alcohol and Health estimated that 2.3 billion people were active alcohol users in 2016. Furthermore, heavy episodic drinking (≥ 60 g of alcohol in one sitting monthly) and rates of alcohol use disorder (AUD) have also increased over the past decade [1, 2]. Notably in 2014, the estimated lifetime prevalence of AUD was reported to be as high as 29.1% in the United States, while currently the global prevalence overall is 9% and up trending [2, 3]. Adverse effects of harmful alcohol use have led to increased hospitalizations and disease burden while ultimately accounting for 5.3% of all worldwide deaths in 2016 [1]. Unfortunately, it is expected that in the coming decade both alcohol use and its associated negative health outcomes will continue to rise.
Alcohol-associated liver disease (ALD), generally referring to a spectrum of liver injury secondary to alcohol use, can range from steatosis to end-stage cirrhosis [4]. Along this spectrum of ALD is a severe form of steatohepatitis termed alcohol-associated hepatitis (AH). AH is not only associated with increased hospitalizations but also increases short-term complications including a significant mortality rate ranging from 30 to 50% at 3 months [5]. Therefore, given the overall rise in harmful alcohol use and severe complications of ALD including AH, a coordinated effort is needed to address this developing health pandemic. Here, we aim to introduce a framework for the outpatient management of patients recently discharged for AH with the overall goal to improve clinical outcomes and prevent associated complications.
Alcohol-Associated Hepatitis (AH): Clinical Presentation
Alcohol-associated hepatitis (AH) is considered a severe syndrome of ALD. Clinically, AH presents as rapid onset liver dysfunction characterized by jaundice, abdominal pain, malaise, and fever. Specifically, the associated jaundice is expected to have developed acutely and typically within 12 weeks prior to presentation. Right-sided abdominal pain is common and likely the manifestation of alcohol-induced hepatic injury (hepatocyte ballooning and inflammation) presenting as tender hepatomegaly. Furthermore, as AH may be an acute insult occurring in the setting of chronic liver disease, the evidence of and clinical stigmata of cirrhosis (ascites, variceal bleeding, and hepatic encephalopathy) can also be presenting features of AH [6].
AH is generally diagnosed clinically with supportive laboratory findings; specifically, key criteria for diagnosis include the aforementioned clinical features of AH, a history of heavy alcohol use, and the ruling out of other etiologies of acute liver injury such as viral hepatitis, drug-induced liver injury, ischemic hepatitis, and autoimmune hepatitis [7]. The necessity of a liver biopsy to aid in the diagnosis of AH is not currently recommended in the absence of confounding factors or inability to rule out previously discussed etiologies that may hinder a diagnosis of AH. Typical histologic features of AH include but are not limited to steatosis, hepatocellular ballooning, infiltration by neutrophils/lymphocytes, Mallory–Denk bodies, and evidence of fibrosis [5].
Alcohol-Associated Hepatitis (AH): Early Management and Prognostication
Clinically, disease severity of AH is significant both in predicting rates of morbidity and mortality and for consideration of therapeutic interventions. Currently, there are several prognostic scoring systems utilized not only to determine the severity of AH and to predict short-term morbidity/mortality but also to guide treatment. Here, we will briefly discuss a few scoring systems currently used clinically and in research settings.
Maddrey’s Discriminant Function (MDF) can be used to distinguish non-severe from severe AH, with severe AH classified as patients with MDF score ≥ 32, a calculation that is based on the serum prothrombin time and bilirubin at the time of presentation in AH patients [8]. This distinction between severe and non-severe AH is critical as the short-term mortality rate of severe AH has previously been reported to exceed 50% at 28 days without intervention; in comparison, the short-term mortality rate at 28 days for non-severe presentations of AH has been reported to be low 6% [8, 9]. Importantly, MDF was initially introduced in 1978 and derived from a controlled trial assessing the use of a glucocorticoid (prednisolone) for the treatment of AH. As such, another use of MDF aside from stratifying presentations by AH severity is to assist in determining whether or not to treat with prednisolone [8, 9].
The Model for End-Stage Liver Disease (MELD) was initially established to predict the short-term mortality for patients with cirrhosis undergoing a transjugular intrahepatic portosystemic shunt procedure and has subsequently been validated to predict mortality at both 30 and 90 days for AH. The MELD score utilizes serum bilirubin, creatinine, and international normalized ratio (INR) to generate a composite score which can be re-calculated at different time points to serve as a continuous model for re-assessing prognosis. Currently, a MELD score of > 20 defines severe alcohol-associated hepatitis (SAH) [10–12]. Modifications to the MELD score to include serum sodium (MELD-Na) offer better overall predictor of 180-day mortality in patients with ascites, when compared to the original MELD [13]. Thus, the utility of MELD and MELD-Na is to stratify disease severity and for prognostication.
The Glasgow Alcoholic Hepatic Score (GAHS), initially developed to aid in determining which patients presenting with AH would benefit from glucocorticoid (GC) therapy, is currently being used as an adjunct to MDF. The GAHS model utilizes multiple clinical factors including patient’s age, serum bilirubin, urea, prothrombin time, and leukocyte count to generate a composite score. Currently, a score > 9 indicates a poor prognosis and thus GC therapy would be recommended, whereas a score < 9 confers a mortality rate of 13% at 28 days and thus GC is not warranted [14, 15]. The ABIC model (abbreviation denoting incorporated variables: age, serum bilirubin, INR, and creatinine) is utilized to stratify patients into tiers of low, intermediate, or high rate of mortality risk at 3 months and 1-year time points. Compared to the above models, ABIC model’s strength is its ability to offer long-term prognosis for patients. At 3 months, mortality rate for low, intermediate, and high-risk tiers was 0, 30, and 75%, respectively [16].
With several prognostic scoring systems currently available, the accuracy in their ability to predict short-term mortality in AH has been investigated. In one global study spanning 85 tertiary centers and including up to 2581 patients, the diagnostic accuracy of the aforementioned scoring system (MDF, MELD, GAHS, and ABIC) was compared in regard to their ability to predict short-term mortality. The primary endpoints of this study included 28- and 90-day mortality. MELD outperformed all scoring systems at both 28- and 90-day mortality (AUC 0.775 and 0.773, respectively), while MDF was the least accurate (AUC 0.701 and 0.709, respectively) in predicting short-term mortality. This study also assessed MELD-Na which performed nearly identically to MELD at both endpoints (AUC 0.776 at 28-day and 0.773 at 90-day mortality). Overall, this study concluded that the MELD score should be the prognostic scoring system of choice when predicting short-term mortality [17].
Given the potential for detrimental outcomes not only acutely during an episode of AH, but also with subsequent recurrent events, both the inpatient and post-discharge management are critical in preventing negative outcomes. The acute management of AH with GCs has been extensively studied by a host of clinical trials and subsequent meta-analyses, however, results at times have been conflicting and indeterminant. One meta-analysis incorporating 5 randomized clinical trials (RCTs) demonstrated that GCs did significantly improve 28-day survival in patients with SAH [18]. This meta-analysis was followed by the largest RCT titled STOPAH, which initially did not demonstrate a 28-day survival benefit with the use of GCs compared to placebo in SAH; however, on post hoc multivariable analysis factoring for confounding variables, ultimately did reveal a 28-day survival benefit with GC use, notably though, this survival benefit was not seen at 90 days or at 1 year [19]. Subsequently, a large worldwide multicenter retrospective study revealed that the use of GC therapy improved 30-day survival for patients with SAH with a MELD between 25 (HR 0.58, p < 0.001) and 39 (HR 0.57, p < 0.001), further supporting the use of GC therapy in the treatment of SAH [20]. It must be noted that prior to the initiation of GC therapy, it is important to ensure there are no contraindications, most notably an active uncontrolled infection, as these patients may have worse outcomes on GC therapy [21]. Other relative contraindications including an active gastrointestinal bleed, uncontrolled diabetes, and renal insufficiency must also be considered prior to initiating GC therapy [22]. Otherwise, once an infection is ruled out and GCs are started, frequent outpatient monitoring is warranted throughout the remaining course of GC therapy.
Combination therapy, in particular GCs with the addition of N-acetylcysteine (NAC), has also been investigated in the treatment of SAH. Mechanistically, the use of an antioxidant therapy such as NAC is believed to counteract the excess of reactive oxygen species suspected in an inflamed liver during an episode of SAH [23]. In one RCT, directly comparing prednisolone alone to combination therapy consisting of prednisolone with NAC in patients presenting with SAH did reveal a 1-month mortality benefit in those receiving combination therapy (8% vs 24%, p = 0.006). Notably, this survival benefit with combination therapy was not seen at 3- or 6-month follow-up [24]. Furthermore, a meta-analysis incorporating 22 RCTs, also revealed that combination therapy of NAC and GCs at least in the short term (defined as 4 weeks) is associated with a reduced mortality rate [25]. Once GC therapy is initiated, response to therapy should be assessed to determine whether continuation is warranted, this is most commonly done via the Lille score. The Lille Model is utilized to dynamically assess a patient’s response to GC therapy in real time by correlating improvements in serum bilirubin levels. Specifically, this model assesses serum bilirubin levels at day 7 of therapy to determine whether or not GC therapy should be continued. In patients with a Lille score greater than 0.45, the mortality rate is 75% and thus GC therapy discontinuation is recommended as this is considered a lack of response to GC therapy [26]. The use of the Lille Model at day 4 of GC therapy has also been investigated as a predictor of therapy response. In one retrospective study, the Lille Model at day 4 was found to be as accurate as day 7 in predicting mortality in response to GC therapy for patients being treated for SAH. Specifically, the area under the receiver operating characteristic curve (AUROC) comparing day 4 and day 7 to predict 28-day (0.77 vs 0.74 respectively, p = 0.406) and 90-day mortality (0.77 vs 0.75, respectively, p = 0.706) were similar with no statistical difference [27]. Therefore, the Lille score at day 4 may also serve as an appropriate time point to assess the response to GC therapy for patients treated for SAH.
Alcohol-Associated Hepatitis (AH): Short-Term Management Post Discharge
Alcohol Abstinence
The cornerstone for both the short- and long-term management of AH is strict alcohol abstinence, however, in many circumstances this remains one of the most challenging treatment goals to achieve. Prior studies have demonstrated that abstinence remains one of the only independent predictors of long-term survival for patients previously diagnosed with SAH [28]. Alternatively, continued alcohol use has revealed a dose-dependent effect of alcohol consumption on the risk of mortality after GC therapy for AH [29]. Furthermore, alcohol abstinence remains the most important disease modifying behavior for preventing not only recurrence of future AH episodes, but ultimately the progression to end-stage cirrhosis [30]. A retrospective review further revealed that recurrent episodes of SAH in patients due to alcohol relapses were worse in disease severity as defined by worse MDF and MELD scores compared to their previous SAH presentation, and also an overall worse mortality rate at 57.1% [31]. Early intervention defined as residential or outpatient addiction treatment or participation in a mutual support group at least 30 days post discharge for AH was shown to reduce the risk of hospital readmissions, alcohol relapse, and the mortality rate [32]. Therefore, alcohol abstinence is the mainstay of treatment for AH both acutely post discharge after an admission for SAH but also lifelong to prevent further recurrences of SAH and progression of ALD to end-stage cirrhosis. Further discussion regarding a multimodality approach including pharmacotherapy and psychosocial support will be addressed below in the long-term management of alcohol use disorder (AUD).
Nutrition
Both protein-calorie malnutrition along with deficiencies in folate, thiamine, zinc, and vitamin D are commonly present in patients presenting with ALD and SAH; one study demonstrated that protein energy malnutrition was present in every patient (cohort of 271) with SAH [33, 34]. Although alcohol is a source of calories, alcohol itself lacks both macronutrients (protein and fat) and micronutrients (vitamins and minerals), and thus is an insufficient means of nutrition [33]. The etiology of malnutrition in SAH is likely multifactorial and partly secondary to decreased oral intake along with an underlying hypermetabolic/catabolic state. Given malnutrition is a significant risk factor for survival, nutritional evaluations are critical to optimize post-discharge outcomes for patients recently admitted for SAH. Currently, there are several methods that can be utilized to assess for malnutrition in AH as displayed in Fig. 1; important factors to consider when assessing for malnutrition, however, ensure factoring in for hypervolemic states (peripheral edema, ascites, etc.) that may influence a patient’s BMI which is used in several tests evaluating for malnutrition [35]. In regards to outpatient management of malnutrition in AH, the first mainstay of therapy includes alcohol cessation not only because meeting caloric needs is difficult to achieve with continued alcohol use, but also because the improved nutritional status seen with alcohol abstinence can also result in improved immunologic function by way of cellular mediated immunity [36]. Unfortunately, data on the management of outpatient AH are lacking and largely inferred from inpatient nutritional management of patients with cirrhosis. For example, one such extrapolation currently accepted for the management of AH that is known to occur in patients with cirrhosis is the avoidance of fasting periods as prolonged periods without nutritional intake can result in a state of metabolic starvation characterized by decreased glucose oxidation and increased catabolism of protein and fat. In one study, patients with cirrhosis were observed to enter a starvation state after overnight fasting compared to the 2–3 days of fasting necessary to induce this state in the healthy control cohort [37]. Therefore, in order to avoid periods of prolonged fasting in patients with cirrhosis or AH, it is currently recommended that patients have frequent meals supplemented by periodic snacking along with an evening snack prior to bedtime to prevent this starvation state from occurring that may further result in catabolic adverse effects (i.e., loss of muscle mass). The recommended total daily caloric intake should range between 30 and 35 kcal/kg per day along with a protein intake ranging between 1.2 and 1.5 g/kg per day [38, 39]. As previously discussed, evaluation of micronutrients should also be assessed for repletion; for example, repletion of thiamine must always be considered to prevent the development of Wernicke’s encephalopathy. Outpatient nutrition follow-up should also be considered in cases where malnutrition is severe or refractory to prior therapies.
Fig. 1.
Nutritional assessment recommendations for patients presenting with alcohol-related hepatitis
Medical Optimization
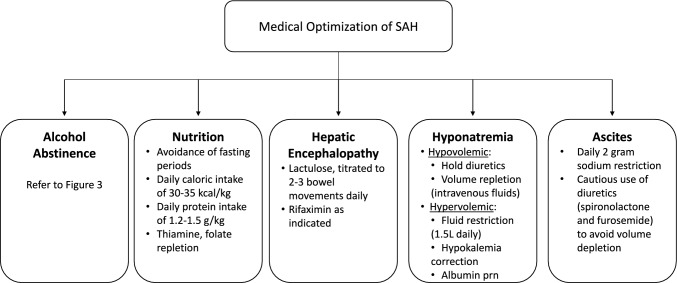
As previously discussed, patients presenting for SAH may also have signs and symptoms related to underlying chronic liver disease and dysfunction, which include but are not limited to hyponatremia, hepatic encephalopathy, ascites, and variceal bleeds. Here, we will briefly discuss the medical management of these complications to optimize clinical outcomes (Fig. 2).
Fig. 2.
Short-term management of patients presenting with severe alcohol-related hepatitis. Key: SAH—severe alcohol-related hepatitis, prn—as needed
Hepatic Encephalopathy
Hepatic encephalopathy (HE), typically characterized as a reversible syndrome of neurologic/brain dysfunction can vary in its clinical presentation from changes in behavior or mild confusion to difficulty in arousability and coma. HE may also be a presenting feature of SAH with impaired liver function [6, 40, 41]. Although the exact pathogenesis of HE is unclear, a likely etiology is the excess of circulating toxins such as ammonia and glutamine crossing the blood–brain barrier resulting in impairment. Known precipitants of HE include electrolyte disturbances, infections, gastrointestinal bleeding, and sedative medications. For episodes of overt hepatic encephalopathy, typically diagnosed clinically and staged in severity by tools such as the West Haven criteria may require a hospitalization [41]. Therefore, mainstay therapy includes not only identifying and reversing known precipitants but also facilitating the reduction of excess toxins by medical therapy [42]. Lactulose is generally the first choice in management and titrated to 2–3 bowel movements daily [41]. After the overt HE episode has resolved, secondary prophylaxis with rifaximin is warranted and should be prescribed to prevent recurrent episodes [41].
Hyponatremia
Hyponatremia, defined as a serum sodium less than 135 mEq/L, is a commonly seen abnormality in patients with liver disease including SAH [42, 43]. Treatment is dependent on characterizing the fluid status of the patient as the management of hypervolemic from hypovolemic hyponatremia differs. Furthermore, hyponatremia in patients with SAH and cirrhosis is typically chronic and does not typically warrant emergent therapy. In cases where hypovolemic hyponatremia is suspected, discontinuation of diuretic therapy along with volume expansion with intravenous isotonic fluids should be considered. More commonly, hypervolemic hyponatremia is encountered in this patient population. Treatment of hypervolemic hyponatremia entails fluid restriction, typically less than 1.5L daily, while considering intravascular volume expansion with albumin administration, hypokalemia correction, and the temporary discontinuation of diuretics [44].
Ascites
Fluid accumulation within the peritoneal cavity, ascites, is a common complication of end-stage liver disease and often seen in SAH. All patients with new onset ascites should have an abdominal paracentesis for diagnostic fluid analysis. In regard to the management of ascites, if alcohol use is suspected to be contributing to a patient’s underlying liver injury, as is the case for those with SAH, alcohol abstinence should be practiced. Current guidelines including sodium restriction to less than 2 g daily, along with the use of diuretics, typically with spironolactone and furosemide should be utilized. As discussed above, once serum sodium levels fall to less than 125 mmol/L, fluid restriction should also be considered not only for the management of hyponatremia but also to optimize fluid status [45].
Screening for Gastroesophageal Varices
Optimal timing for pursuing screening of gastroesophageal varices in patients with SAH has not been determined. Further, utilization of elastography as suggested by the recent BAVENO guidelines to assess for liver stiffness is not applicable to patients who are early in their recovery from SAH, as elastography readings are often not reliable [46, 47]. We suggest pursuing variceal screening within the first 3–6 months of diagnosis among patients with platelet count < 150,000, but further studies are needed to validate these suggestions [48].
Alcohol-Associated Hepatitis (AH): Long-Term Management and Follow-Up
Alcohol Use Disorder
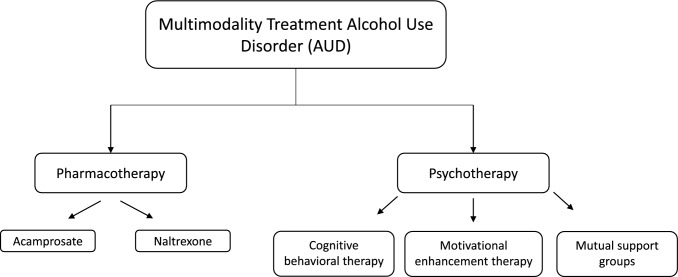
As previously discussed, continued alcohol use after a presentation for AH is associated with worse long-term outcomes including recurrent episodes of AH and increased rates of mortality [29, 31]. Therefore, the long-term goal after any admission for SAH should be complete abstinence from alcohol as this remains one of the single most important factors for survival. Despite this known benefit, abstinence remains one of the most challenging therapeutic goals to achieve partly due to the underlying difficulties of treating addiction. Therefore, care of patients subsequently discharged for SAH must include long-term plans of addressing alcohol use and possible addiction. Here, we will discuss screening for AUD and the therapeutic interventions both pharmacotherapy and behavioral therapy currently available (Fig. 3).
Fig. 3.
Multimodality treatment of alcohol use disorder
Screening for AUD
Screening for AUD should be completed for all patients recently admitted for SAH as the prevalence of alcohol misuse is rising. In one retrospective study following a cohort of 93,612 patients with cirrhosis and suspected ALD, 41% of the cohort met criteria for AUD. Notably, this study also revealed that patients who received either behavioral and/or pharmacotherapy for AUD had a significant reduction in future liver decompensations and an improved long-term all-cause mortality further highlighting the need for aggressive screening for AUD and treatment [49].
There are multiple screening tools that can be used to assess for the level of alcohol use and screen for AUD. In particular, the National Alcohol Abuse and Alcoholism (NIAAA) recommends a single question to assess for binge drinking among men and women; if positive, then the completion of the AUD Identification Test (AUDIT) screening tool is recommended [50]. A truncated version of this AUDIT self-reported questionnaire termed AUDIT-C can also be administered with a reported sensitivity of 80% in identifying alcohol misuse [51]. If either screening tool is positive and concerning for alcohol misuse, then a formal evaluation should be pursued to diagnose AUD. Interventions, including the referral for addiction specialist, or the initiation of pharmacotherapy, should be considered if either the AUDIT-C or complete AUDIT screening tools indicate alcohol misuse (AUDIT-C score > 4 or AUDIT score > 8) [52].
Pharmacotherapy
Although there are currently 3 medications (acamprosate, disulfiram, and naltrexone) approved by the U.S. Food and Drug Administration to treat AUD, studies examining their use in ALD and AH are limited. Acamprosate, thought to both increase the activity of the GABAergic system along with decreasing the activity of glutamate by antagonizing N-methyl-D-aspartate (NMDA) receptors, has been used therapeutically to reduce cravings and thus alcohol intake [53]. In fact, a meta-analysis comprising of 24 RCTs demonstrated that acamprosate was not only able to reduce the risk of any subsequent alcohol drinking (RR 0.86, 95% CI 0.81–0.91) but also increase the duration of abstinence thus supporting its continued use to maintain abstinence after detoxification [54]. Given acamprosate does not undergo hepatic metabolism, it is generally considered safe for use in patients with liver disease, however, prolonged use has been noted to result in an increased risk of encephalopathy given its antagonism of NMDA receptors, and thus should be used cautiously in patients with a history of hepatic encephalopathy [55]. Disulfiram, an inhibitor of acetaldehyde dehydrogenase, is currently prescribed as a deterrent to alcohol use as it induces symptoms of nausea, vomiting, tachycardia, hypotension, and facial flushing when co-ingested with alcohol. Most importantly, however, disulfiram is contraindicated in patients with cirrhosis given reported cases of drug-induced liver failure and hepatitis further complicated by need for liver transplant and in some cases death [56]. Naltrexone, an antagonist of both mu and kappa-opioid receptors, clinically is thought to inhibit the alcohol-induced release of dopamine thus reducing its reward effect to ultimately diminish the drive for alcohol use and relapse [57]. On meta-analysis, naltrexone has been shown to significantly reduce the rate of alcohol relapse with short-term administration (less than 12 weeks) [58]. Although liver injury and overall hepatoxicity secondary to naltrexone are rare, naltrexone use in patients with severe liver dysfunction/decompensated cirrhosis requires close monitoring [59].
Behavioral Therapy
As part of the multimodal approach in the management of AUD, behavioral therapy as directed by an addiction specialist is recommended. In regard to different behavioral therapies, typical treatment may include a combination of individual talk therapy, cognitive behavioral therapy, motivational interviewing, peer-led mutual aid fellowships (e.g., Alcoholic Anonymous), or inpatient rehabilitation/detox programs. Although there is limited evidence to suggest which type of behavioral therapy is superior to others in the treatment of AUD, what is currently agreed upon is that a multimodal approach in conjunction with the guidance of addiction medicine specialist may offer the best approach to obtaining alcohol abstinence [52].
Long-Term Fibrosis
ALD contributes significantly to a substantial proportion of cirrhosis burden, with majority of SAH patients often presenting with underlying cirrhosis, as noted on explant evaluation of patients undergoing early liver transplantation for SAH [60]. In AH patients, the extent of fibrosis serves as a prognostic indicator for short-term mortality. Liver biopsy remains the gold standard for fibrosis evaluation and diagnosis of cirrhosis, as it provides a comprehensive assessment of the location and quantity of fibrotic tissue. In a single-center retrospective study [5], with the objective of evaluating the impact of fibrosis on survival in AH patients, a liver biopsy taken from patients with AH within 48 h of admission showed that 118 out of 121 patients had either extensive fibrosis, bridging fibrosis, or cirrhosis. The findings support the correlation between the presence of fibrosis or cirrhosis in AH patients to poor outcomes.
Despite being the gold standard, liver biopsy is typically not the preferred method to diagnose liver fibrosis due to its invasive nature, high cost, and potential complications. The need to use reliable non-invasive test (NIT) alternatives have become a necessity in the current day and age. Current NIT options include serologic markers and liver stiffness measurement (LSM). However, detecting fibrosis in AH poses a significant challenge, as acute hepatitis is frequently associated with false-positive results in serologic and LSM tests [46]. Furthermore, the initial clinical presentation of SAH and serological findings may often resemble those observed in end-stage liver disease. The subsequent section will present the NIT methods used to evaluate fibrosis in chronic ALD. Additional extrapolation will be necessary in order to derive data related to patients recovering from a recent episode of AH.
Next to liver biopsy, magnetic resonance elastography (MRE) emerges as the most precise LSM for detecting early stages of liver fibrosis due to any cause of chronic liver disease. In a recent meta-analysis, it outperformed transient elastography (TE) in detecting advanced fibrosis (AUC 0.94 vs. 0.83, respectively, p 0.001) [46]. It is marginally superior to TE in cases of advanced fibrosis (METAVIR F3-F4). MRE utilization also remains uncommon due to its elevated cost and limited accessibility. Specific data on the utilization of MRE in detecting fibrosis solely due to ALD are still limited.
Although TE remains the most extensively studied LSM for patients with chronic ALD, there are still no optimal cutoff values for TE to diagnose or exclude advanced fibrosis in ALD patients. In a single-center retrospective study of 118 patients enrolled in an alcohol withdrawal program to evaluate the efficacy of utilizing TE to assess liver fibrosis in patients with alcohol use, TE demonstrated a strong correlation with liver biopsy and transjugular hepatic venous pressure gradient, with a negative predictive value of 92 and 93% in ruling out severe fibrosis (≥ F3) and cirrhosis, respectively. Additionally, elasticity cut-offs for ALD-related fibrosis are often higher in patients with active or recent sobriety compared to other disease etiologies, suggested at ≥ 11.7, ≥ 15.2, and ≥ 21.2 kPa for F2, F3, and F4, respectively in this study [61]. In a real-world multicenter study to validate the Baveno VI threshold for excluding and diagnosing compensated advanced chronic liver disease (cACLD) using TE, a cutoff at < 8 kPa for excluding cACLD (sensitivity 93%) and > 12 kPa (91% sensitivity and 92% specificity) for diagnosing cACLD (AUC 0.87; 95% CI 0.86–0.88; p < 0.001) was concluded for ALD patients who were likely sober for longer periods compared to prior patients [62]. Hence, the duration of sobriety should be considered when assessing LSM, with ideal duration of at least six to nine or more months of sobriety suggested before LSM assessment for lower cut-offs. At centers where TE is not available, alternative LSM methods like point-shear wave elastography and 2D-shear wave elastography should be utilized, which still have growing data on chronic liver disease [46].
Serum markers were studied in a multicenter prospective study of ALD patients to compare the accuracy of the Enhanced Liver Fibrosis (ELF), FibroTest, and TE in the detection of advanced liver fibrosis (Kleiner stage ≥ F3), where liver biopsy was used as a reference. ELF (AUROC 0.92, 95% CI, 0.89–0.96) and FibroTest (AUROC 0.88, 95% CI, 0.84–0.92) showed comparable diagnostic accuracies that did not differ significantly from those of TE. However, when compared to FIB-4 (AUROC 0.85, 95% CI, 0.80–0.90) or APRI (AUROC 0.80, 95% CI, 0.74–0.86), ELF and FibroTest demonstrated superior accuracy. Furthermore, the study concluded that ELF < 10.5 or FibroTest < 0.58 can rule out advanced fibrosis in ALD patients [63]. While non-patented tests such as FIB-4 and APRI may be readily assimilated in to electronic medical record and offer ease of use to all providers, patented options offer better accuracy when available.
A retrospective cohort study was conducted to assess the utility of measuring INR and platelet count to predict cirrhosis in ALD. Ultrasound and CT scans were used as references. Pre-sobriety platelet count < 70,000 cells/mm3 ruled in cirrhosis (positive LR 6.8, 95% CI: 3.4, 14), while count ˃ 200,000 ruled out cirrhosis (negative LR 0.18, 95% CI: 0.10, 0.35). In patients presenting with SAH, the study noted that presenting platelet count and INR carried a high likelihood ratio to predict cirrhosis (LR: 6.5, 95% CI: 4.3, 11.0), but needs further validation [64].
Given the high incidence of baseline cirrhosis in patients with SAH, the impact of cirrhosis on patient mortality, it is reasonable to presume that all cases of SAH may have background cirrhosis at presentation. Long-term follow-up should be arranged accordingly, including bi-annual HCC surveillance, and monitoring for or managing portal hypertensive complications, while awaiting fibrosis assessment after recovery from SAH.
Liver Transplant Evaluation
Liver transplantation (LT) for AH is rapidly increasing at a higher rate than all the other indications of LT. According to the United Network for Organ Sharing (UNOS), LT for AH patients has increased five folds from 2014 to 2019, and the number of centers performing LT for AH has increased from 14 to 47 in 2019 [65]. One significant reason for this substantial increase is the expansion of the selection criteria for early LT evaluation for SAH patients presenting with their initial decompensation.
The most recent revision to AASLD guidelines recommends early consideration for liver transplantation, regardless of the duration of sobriety among ALD patients. Centers used to reserve LT for patients who had been abstinent for six months. However, it has been extended to include patients with less than six months of sobriety due to improving understanding of AUD, lack of studies correlating fixed periods of sobriety as the only marker to gauge post-LT alcohol relapse, and success shown with early transplantation across multiple studies [52, 65]. Early LT evaluation is now recommended, particularly for SAH patients not responding to corticosteroid therapy.
The chances of recovering from SAH with medical management were often quoted as a reason to hold off on LT, but data suggest that recovery may be considerably lower than suspected. In a multicenter retrospective study, SAH patients who were denied for LT were estimated to have 3.8% (95% CI, 0–9.1%), 7.3% (95% CI, 1.9–12.7%), and 10% (95% CI, 4.5–15.4%) probability for their liver disease to recompensate in 3 months, 6 months, and 1 year, respectively [66]. Meanwhile, in a single-center retrospective study of ALD patients with MELD > 25 and < 90 days of abstinence who were evaluated for LT, 35% of ALD patients who did not undergo LT experienced spontaneous recovery (SR), with SR defined as improvement in MELD score < 21. Factors associated with SR were younger age (< 44) (OR, 0.92; p = 0.004), lower INR (OR; 0.31; p = 0.03), and lower peak MELD < 34 (OR, 0.83; p = 0.02). Among the 35% of patients who had SR, only 20.6% reached a compensated state with MELD ≤ 15 and no therapy for HE or ascites [67]. We therefore advocate for early LT for SAH who qualify due to a significant impact on overall survival and improvement in quality of life, while understanding that LT remains a limited resource. In a multicenter prospective study, SAH patients who underwent early LT evaluation and were listed for LT had better 2-year survival (70.6%) in comparison to non-listed matched SAH control patients (18.2%) [68]. In the same study, the 2-year survival for SAH patients who had early LT was 89·7% (95% CI 79·6–95·0). In a separate study reviewing UNOS data for LT outcomes for AH, the 1- and 5-year graft survival for LT in AH patients was 91.7 and 81.9%, respectively, and the five-year patient survival was 84.8%, supporting longevity of LT as a treatment option for SAH [65].
Although LT for AH has significantly increased, the majority of AH patients who undergo LT evaluation (60–75%) are denied for transplantation [69, 70]. In a single-center retrospective chart review of inpatient LT referrals for AH, 82 patients were evaluated for LT, out of which 62 were denied. Reasons for denial were primarily psychosocial factors rather than medical comorbidities. The main reasons for LT denial were lack of insight in to alcohol use disorder, insufficient social support, and the inability to maintain a therapeutic relationship with the transplant team [69]. Some demographic factors can affect transplant listings as well as outcomes. In a retrospective comparison between LT from ALD cirrhosis and AH, a higher percentage of the recipients were of Asian descent and recipients were often younger in age. Hispanics showed better post-LT graft and patient survival (p < 0.05), while graft rejection was more likely in patients with diabetes (25%) and of male gender (8%). Hispanics were less likely to be waitlisted and transplanted, while women had higher likelihood of listing [71]. These factors highlight some of the disparities transplant recipients face with access to liver transplantation, and extends to the ALD population as well.
Conclusion
The rise in ALD along with one of its severe manifestations, alcohol-associated hepatitis, has increased over the past few decades resulting not only in increased rates of hospitalizations and mortality but has also progressed to being the leading indication for liver transplantation in the United States. In an effort to optimize clinical outcomes, a coordinated outpatient multimodal approach is warranted. Early outpatient management of patients recently discharged for alcohol-associated hepatitis should focus on nutrition and the medical management of manifestations of chronic liver disease, all critical in preventing future negative health outcomes. Concomitant initiation of treatment for AUD to prevent relapse of alcohol use should be initiated with goal of long-term sobriety being the goal, best accomplished through collaborating with behavioral medicine providers. A long-term sequelae of ALD and commonly a co-existing presentation of patients with AH is advanced hepatic fibrosis, if not cirrhosis. Patient who do not respond to medical therapy should be evaluated for liver transplantation, as liver transplantation offers excellent long-term solution for patients with ALD and AH. We further recommend that early liver transplantation be considered when clinically appropriate along with the utilization of the aforementioned pillars of AH management including that of AUD treatment to optimize post-transplant outcomes. Quite simply, to manage a multifactorial disease such as ALD and AH, a multimodal approach with a coordinated effort from multiple providers is necessary.
Author’s contribution
Role in the Study: Study concept and design (SD, AS); acquisition of data (SD, MM); analysis and interpretation of data (SD, MM); drafting of the manuscript (SD, MM); critical revision of the manuscript for important intellectual content (SS, AS); statistical analysis (n/a); obtained funding (not applicable); administrative, technical, or material support (SD, MM, AS); study supervision (AS).
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global status report on alcohol and health 2018. https://www.who.int/publications/i/item/9789241565639.
- 2.Glantz MD, Bharat C, Degenhardt L et al. The epidemiology of alcohol use disorders cross-nationally: Findings from the World Mental Health Surveys. Addict. Behav. 2020;102:106128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant BF, Goldstein RB, Saha TD et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015;72:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Shea RS, Dasarathy S, McCullough AJ. Practice Guideline Committee of the American Association for the Study of Liver Diseases, & Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatol. Baltim. Md 2010;51:307–328. [DOI] [PubMed] [Google Scholar]
- 5.Altamirano J, Miquel R, Katoonizadeh A et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology 2014;146:e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levitsky J, Mailliard ME. Diagnosis and therapy of alcoholic liver disease. Semin. Liver Dis. 2004;24:233–247. [DOI] [PubMed] [Google Scholar]
- 7.Bataller R, Arab JP, Shah VH. Alcohol-Associated Hepatitis. N. Engl. J. Med. 2022;387:2436–2448. [DOI] [PubMed] [Google Scholar]
- 8.Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Mezey E, White RI. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978;75:193–199. [PubMed] [Google Scholar]
- 9.Bennett K, Enki DG, Thursz M, Cramp ME, Dhanda AD. Systematic review with meta-analysis: high mortality in patients with non-severe alcoholic hepatitis. Aliment. Pharmacol. Ther. 2019;50:249–257. [DOI] [PubMed] [Google Scholar]
- 10.Dunn W, Jamil LH, Brown LS et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatol. Baltim. Md 2005;41:353–358. [DOI] [PubMed] [Google Scholar]
- 11.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatol. Baltim. Md 2000;31:864–871. [DOI] [PubMed] [Google Scholar]
- 12.Srikureja W, Kyulo NL, Runyon BA, Hu K-Q. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J. Hepatol. 2005;42:700–706. [DOI] [PubMed] [Google Scholar]
- 13.Vaa BE, Asrani SK, Dunn W, Kamath PS, Shah VH. Influence of serum sodium on MELD-based survival prediction in alcoholic hepatitis. Mayo Clin. Proc. 2011;86:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forrest EH, Morris AJ, Stewart S et al. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut 2007;56:1743–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandahl TD, Jepsen P, Ott P, Vilstrup H. Validation of prognostic scores for clinical use in patients with alcoholic hepatitis. Scand. J. Gastroenterol. 2011;46:1127–1132. [DOI] [PubMed] [Google Scholar]
- 16.Dominguez M, Rincon D, Abraldes JG et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am. J. Gastroenterol. 2008;103:2747–2756. [DOI] [PubMed] [Google Scholar]
- 17.Morales-Arráez D, Ventura-Cots M, Altamirano J et al. The MELD Score Is Superior to the Maddrey Discriminant Function Score to Predict Short-Term Mortality in Alcohol-Associated Hepatitis: A Global Study. Am. J. Gastroenterol. 2022;117:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathurin P, O'Grady J, Carithers RL et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011;60:255–260. [DOI] [PubMed] [Google Scholar]
- 19.Thursz, M. R., Forrest, E. H., Ryder, S., & STOPAH investigators. Prednisolone or Pentoxifylline for Alcoholic Hepatitis. N. Engl. J. Med.373, 282–283 (2015). [DOI] [PubMed]
- 20.Arab JP, Diaz LA, Baeza N et al. Identification of optimal therapeutic window for steroid use in severe alcohol-associated hepatitis: A worldwide study. J. Hepatol. 2021;75:1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vergis N, Atkinson SR, Knapp S et al. In Patients With Severe Alcoholic Hepatitis, Prednisolone Increases Susceptibility to Infection and Infection-Related Mortality, and Is Associated With High Circulating Levels of Bacterial DNA. Gastroenterology 2017;152:1068-1077.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am. J. Gastroenterol. 2018;113:175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirano T, Kaplowitz N, Tsukamoto H, Kamimura S, Fernandez-Checa JC. Hepatic mitochondrial glutathione depletion and progression of experimental alcoholic liver disease in rats. Hepatol. Baltim. Md 1992;16:1423–1427. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen-Khac E, Thevenot T, Piquet MA et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N. Engl. J. Med. 2011;365:1781–1789. [DOI] [PubMed] [Google Scholar]
- 25.Singh S, Murad MH, Chandar AK et al. Comparative Effectiveness of Pharmacological Interventions for Severe Alcoholic Hepatitis: A Systematic Review and Network Meta-analysis. Gastroenterology 2015;149:958-970.e12. [DOI] [PubMed] [Google Scholar]
- 26.Louvet A, Naveau S, Abdelnour M et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatol. Baltim. Md 2007;45:1348–1354. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Saenz-de-Sicilia M, Duvoor C, Altamirano J et al. A Day-4 Lille Model Predicts Response to Corticosteroids and Mortality in Severe Alcoholic Hepatitis. Am. J. Gastroenterol. 2017;112:306–315. [DOI] [PubMed] [Google Scholar]
- 28.Potts JR, Goubet S, Heneghan MA, Verma S. Determinants of long-term outcome in severe alcoholic hepatitis. Aliment. Pharmacol. Ther. 2013;38:584–595. [DOI] [PubMed] [Google Scholar]
- 29.Louvet A, Labreuche J, Artru F et al. Main drivers of outcome differ between short term and long term in severe alcoholic hepatitis: A prospective study. Hepatol. Baltim. Md 2017;66:1464–1473. [DOI] [PubMed] [Google Scholar]
- 30.Pessione F, Ramond MJ, Peters L et al. Five-year survival predictive factors in patients with excessive alcohol intake and cirrhosis. Effect of alcoholic hepatitis, smoking and abstinence. Liver Int. Off. J. Int. Assoc. Study Liver 2003;23:45–53. [DOI] [PubMed] [Google Scholar]
- 31.Potts JR, Howard MR, Verma S. Recurrent severe alcoholic hepatitis: clinical characteristics and outcomes. Eur. J. Gastroenterol. Hepatol. 2013;25:659–664. [DOI] [PubMed] [Google Scholar]
- 32.Peeraphatdit TB, Kamath PS, Karpyak VM et al. Alcohol Rehabilitation Within 30 Days of Hospital Discharge Is Associated With Reduced Readmission, Relapse, and Death in Patients With Alcoholic Hepatitis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020;18:477-485.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barve S, Chen S-Y, Kirpich I, Watson WH, Mcclain C. Development, Prevention, and Treatment of Alcohol-Induced Organ Injury: The Role of Nutrition. Alcohol Res. Curr. Rev. 2017;38:289–302. [PMC free article] [PubMed] [Google Scholar]
- 34.Mendenhall CL, Moritz TE, Roselle GA et al. Protein energy malnutrition in severe alcoholic hepatitis: diagnosis and response to treatment. The VA Cooperative Study Group #275. JPEN J. Parenter. Enteral Nutr. 1995;19:258–265. [DOI] [PubMed] [Google Scholar]
- 35.McClain CJ, Rios CD, Condon S, Marsano LS. Malnutrition and Alcohol-Associated Hepatitis. Clin. Liver Dis. 2021;25:557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirsch S, de la Maza MP, Gattas V et al. Nutritional support in alcoholic cirrhotic patients improves host defenses. J. Am. Coll. Nutr. 1999;18:434–441. [DOI] [PubMed] [Google Scholar]
- 37.Owen OE, Trapp VE, Reichard GA et al. Nature and quantity of fuels consumed in patients with alcoholic cirrhosis. J. Clin. Invest. 1983;72:1821–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu & European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol.70, 172–193 (2019). [DOI] [PMC free article] [PubMed]
- 39.Plauth M, Bernal W, Dasarathy S et al. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. Edinb. Scotl. 2019;38:485–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferenci P. Hepatic encephalopathy. Gastroenterol. Rep. 2017;5:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vilstrup H, Amodio P, Bajaj J et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatol. Baltim. Md 2014;60:715–735. [DOI] [PubMed] [Google Scholar]
- 42.Mansour D, McPherson S. Management of decompensated cirrhosis. Clin. Med. Lond. Engl. 2018;18:s60–s65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adrogué HJ, Madias NE. Hyponatremia. N. Engl. J. Med. 2000;342:1581–1589. [DOI] [PubMed] [Google Scholar]
- 44.Rondon-Berrios H, Velez JCQ. Hyponatremia in Cirrhosis. Clin. Liver Dis. 2022;26:149–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Runyon, B. A. & AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatol. Baltim. Md49, 2087–2107 (2009). [DOI] [PubMed]
- 46.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, Clinical Practice Guideline Panel, Chair:, EASL Governing Board representative:, & Panel members: EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J. Hepatol.75, 659–689 (2021). [DOI] [PubMed]
- 47.de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J. Hepatol. 2022;76:959–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatol. Baltim. Md 2017;65:310–335. [DOI] [PubMed] [Google Scholar]
- 49.Rogal S, Youk A, Zhang H et al. Impact of Alcohol Use Disorder Treatment on Clinical Outcomes Among Patients With Cirrhosis. Hepatol. Baltim. Md 2020;71:2080–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addict. Abingdon Engl. 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 51.O’Connor EA, Perdue LA, Senger CA et al. Screening and Behavioral Counseling Interventions to Reduce Unhealthy Alcohol Use in Adolescents and Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018;320:1910–1928. [DOI] [PubMed] [Google Scholar]
- 52.Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatol. Baltim. Md 2020;71:306–333. [DOI] [PubMed] [Google Scholar]
- 53.De Witte P, Littleton J, Parot P, Koob G. Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action. CNS Drugs 2005;19:517–537. [DOI] [PubMed] [Google Scholar]
- 54.Rösner S, Hackl-Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M. Acamprosate for alcohol dependence. Cochrane Database Syst. Rev. CD004332 (2010) 10.1002/14651858.CD004332.pub2. [DOI] [PMC free article] [PubMed]
- 55.Leggio L, Lee MR. Treatment of Alcohol Use Disorder in Patients with Alcoholic Liver Disease. Am. J. Med. 2017;130:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Saf. 1999;20:427–435. [DOI] [PubMed] [Google Scholar]
- 57.Sinclair JD. Evidence about the use of naltrexone and for different ways of using it in the treatment of alcoholism. Alcohol Alcohol. Oxf. Oxfs. 2001;36:2–10. [DOI] [PubMed] [Google Scholar]
- 58.Bouza C, Angeles M, Muñoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addict. Abingdon Engl. 2004;99:811–828. [DOI] [PubMed] [Google Scholar]
- 59.Bertolotti M, Ferrari A, Vitale G et al. Effect of liver cirrhosis on the systemic availability of naltrexone in humans. J. Hepatol. 1997;27:505–511. [DOI] [PubMed] [Google Scholar]
- 60.Choi G, Shetty A. The prevalence of cirrhosis in patients transplanted for severe alcohol-associated hepatitis—clarification essential. Dig. Med. Res. 2022;5:54. [Google Scholar]
- 61.Salavrakos M, Piessevaux H, Komuta M, Lanthier N, Stärkel P. Fibroscan Reliably Rules Out Advanced Liver Fibrosis and Significant Portal Hypertension in Alcoholic Patients. J. Clin. Gastroenterol. 2019;53:772–778. [DOI] [PubMed] [Google Scholar]
- 62.Bertolotti M, Ferrari A, Vitale G et al. Refining the Baveno VI elastography criteria for the definition of compensated advanced chronic liver disease. J. Hepatol. 2021;74:1109–1116. [DOI] [PubMed] [Google Scholar]
- 63.Thiele M, Madsen BS, Hansen JF, Detlefsen S, Antonsen S, Krag A. Accuracy of the Enhanced Liver Fibrosis Test vs FibroTest, Elastography, and Indirect Markers in Detection of Advanced Fibrosis in Patients With Alcoholic Liver Disease. Gastroenterology 2018;154:1369–1379. [DOI] [PubMed] [Google Scholar]
- 64.Murali AR, Attar BM, Katz A, Kotwal V, Clarke PM. Utility of Platelet Count for Predicting Cirrhosis in Alcoholic Liver Disease: Model for Identifying Cirrhosis in a US Population. J. Gen. Intern. Med. 2015;30:1112–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cotter TG, Sandikci B, Paul S. Liver transplantation for alcoholic hepatitis in the United States: Excellent outcomes with profound temporal and geographic variation in frequency. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2021;21:1039–1055. [DOI] [PubMed] [Google Scholar]
- 66.Hsu CC, Dodge JL, Weinberg E et al. Multicentered study of patient outcomes after declined for early liver transplantation in severe alcohol-associated hepatitis. Hepatol. Baltim. Md 2023;77:1253–1262. [DOI] [PubMed] [Google Scholar]
- 67.Musto J, Stanfield D, Ley D, Lucey MR, Eickhoff J, Rice JP. Recovery and outcomes of patients denied early liver transplantation for severe alcohol-associated hepatitis. Hepatol. Baltim. Md 2022;75:104–114. [DOI] [PubMed] [Google Scholar]
- 68.Louvet A, Labreuche J, Moreno C et al. Early liver transplantation for severe alcohol-related hepatitis not responding to medical treatment: a prospective controlled study. Lancet Gastroenterol. Hepatol. 2022;7:416–425. [DOI] [PubMed] [Google Scholar]
- 69.Choi G, Benhammou JN, Yum JJ et al. Barriers for Liver Transplant in Patients with Alcohol-Related Hepatitis. J. Clin. Exp. Hepatol. 2022;12:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee BP, Mehta N, Platt L et al. Outcomes of Early Liver Transplantation for Patients With Severe Alcoholic Hepatitis. Gastroenterology 2018;155:422-430.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Samuel S, Choubey A, Koizumi N et al. Demographic inequities exist and influence transplant outcomes in liver transplantation for acute alcohol-associated hepatitis. HPB 2023;25:845–854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.