Abstract
This study aims to assess the effect of combining virtual walking (VW) therapy with a physical exercise (PE) program compared to PE alone on lower limb strength and muscle activation in people with incomplete spinal cord injury (iSCI). 38 participants performed 3 sessions/week during 6 weeks of Experimental Intervention (EI): VW and PE; or Control intervention (CI): Placebo-VW and PE. Strength and muscle activation of main lower limb muscles were assessed. EI group exhibited a general strength increase after intervention (T2), (16.31–34.72 N), and maintained this improvement up to 1-month-follow-up (T3) for hip abduction and extension movements. The CI group only showed improvements in hip abduction and extension movements (18.34 (7.13) N and 19.98 (9.60) N, respectively). EI group also exhibited an increase of activation in all agonistic muscles in T2 (36.02–20.24 µV), except gastrocnemius. Gastrocnemius and rectus femoris activation as antagonistic decreased during dorsal flexion (− 14.28 (5.61) µV) and hip extension (− 14.78 [6.11] µV), respectively. CI group only showed an activation increase of agonistic muscles of hip abduction and extension (22.16 (9.80) µV and 28.82 (9.14) µV, respectively), without changes in antagonistic activation. VW could enhance the PE effects regarding muscle strength and activation in people with iSCI.
Registration number: NCT04809987.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-86845-8.
Keywords: Spinal cord injury, Virtual walking, Physical exercise, Muscle activation
Subject terms: Health care, Medical research
Introduction
Individuals with spinal cord injury (SCI) often experience sensory and/or motor impairment, with an impact on various functional capacities and hindering the performance of daily activities1.
One of the most invalidating problems is the loss of voluntary movement control. The coordination between agonist and antagonist muscles is particularly affected due to disrupted communication between the brain and the muscles. Normally, the brain sends signals to activate the agonists while inhibiting the antagonists, but when a SCI occurs, this mechanism is impaired. As a result, the coactivation of agonist/antagonist muscles becomes dysfunctional. This lack of proper control leads to muscle spasticity and reduced motor coordination, further limiting mobility. Additionally, the loss of sensory feedback exacerbates the difficulty in coordinating movements, contributing to the overall motor impairment2. This has a profound psychological and socio-economic impact on their life3.
Nevertheless, approximately 60% of people with SCI have incomplete injuries, allowing for spontaneous functional recovery, the extent on which varies based on injury characteristics and individual factors. Previous research indicates that the neuroplasticity of residual corticospinal fibres plays a crucial role in spontaneous functional recovery4,5. This process can be enhanced if the neural recovery mechanisms are properly stimulated. In this way, low-cost strategies such as therapeutic physical exercise programs are usually carried out6, aiming to encourage neuroplasticity and the reorganization of the cortical representation of the muscles7–9 through early, intensive and specific therapies1,3. These therapies are typically focused on the recovery of function, mainly through functional motor stimulation (i.e. exercises focused on specific tasks [i.e. gait, balance, etc.]). However, people with neurological diseases are sometimes unable to achieve the quantity and quality of physical exercise required for a clinically relevant improvement, due to the injury itself. Therefore, previous papers propose to combine physical exercise with external devices such as exoskeletons10 in order to assist the movement. However, a clear disadvantage is their high cost.
Research suggests that, in addition to exercise, nervous reorganization and functional recovery can be influenced by the action of mirror neurons found in motor and premotor areas, as well as other cortical and subcortical regions11–13. These neurons report activation, not only when a movement is executed but also when a purposeful motor action is observed and closely examined14.
Several studies have explored the impact of mirror neuron-based therapy on motor function in populations with central nervous system (CNS) diseases, such as stroke, traumatic brain injury, Parkinson’s disease, or Alzheimer’s disease. Specifically, experimental research analyses the effectiveness of different mirror neuron-based therapies including mirror therapy15, virtual reality therapies16 or Action-Observation therapies17. In general, promising results have been obtained in all these populations.
Given the potential for recovery in incomplete SCI as noted earlier, therapies that activate mirror neurons might boost neuroplasticity to enhance motor function; their effectiveness has been reported in other CNS conditions. But, so far, no previous study has analysed the effect of this therapeutic approach on functional recovery in this population; only the effect on pain has been analysed10,18–20.
This study aims to assess the effect of stimulating mirror neurons by combining virtual walking (VW) therapy with a physical exercise (PE) program compared to PE alone. The evaluation will focus on outcomes related to lower limb muscle activation and maximum strength in individuals with incomplete SCI.
Methods
A single-blinded, two-arm, randomized controlled trial design was employed. This study was approved by the Ethics Committee of the University of Valencia (2425590) and performed in accordance with the latest revision of the Declaration of Helsinki. All participants signed the written informed consent to participate in the study. Moreover, this study was registered at ClinicalTrials.gov (22/05/2021; NCT04809987) and its feasibility was comproved21 before the execution of the full-scale study.
Participants
A number of hospitals and associations participated in the recruitment (Hospital Universitari i Politècnic La Fe [Spain]; Asociación de Personas con Lesión Medular y otras Discapacidades Físicas [Spain]; Associació TetraSport [Spain]; Coordinator of People with Physical Functional Diversity of the Valencian Community [Spain]; University Clinic of Nutrition, Physical Activity and Physiotherapy [Spain]; L’association Sport et Thérapies Neuro-rééducatives Avancées [France] and the Henry Gabrielle Hospital [France]). The inclusion criteria for the participants were: (i) incomplete SCI (ASIA Impairment Scale (AIS) C or D); (ii) cervical, thoracic, or lumbar injury; (iii) six months or more since injury22; (iv) ability to walk with or without aids; and iv. ability to understand instructions (Mini-Mental State Examination > 23 points). All subjects had undergone conventional rehabilitation after their injury at their referral hospital. In order to monitor the amount of physical exercise performed during the six months before and during the intervention, the Spanish version of the International Physical Activity Questionnaire (IPAQ)23 was used. All of them showed similar amount and intensity level of activity was used. Compliance with the inclusion criteria was checked by an SCI-specialist doctor, from the relevant hospital unit. The exclusion criteria were: (i) traumatic pathology affecting the legs; (ii) other alterations of the CNS or Peripheral Nervous System (SNP); (iii) alterations of the vestibular system; and (iv) concomitant diseases.
To calculate the sample size, the G*Power24 software was used. Since there were no previous studies using a similar intervention for assessing functionality from which to extract the estimated effect size, a power or ability of 80% to detect false negatives, an ability to detect a medium effect size (Cohen’s d = 0.5), and a 0.05 probability of type I error, were established. Taking into consideration that participants were to be divided into two groups, and a total of 4 assessments were to be conducted, a total of at least 40 participants were included.
Procedure
During the first visit, potential volunteers signed the informed consent. In order to control the possible confounding factors, a clinical interview was conducted to collect the anthropometric and demographic data (i.e., height, weight, age and sex), and clinical data evaluated by the doctors of the volunteer’s relevant hospital of origin (i.e., Level of injury, Level in AIS, Cause of injury, Comorbidities and Walking aids). Moreover, participants were randomly divided into two groups using the Random Allocation Software25 with a simple randomization method performed by a blinded assistant: Experimental Intervention (EI) group, in which VW and gait-specific physical exercises were administered, and Control Intervention (CI) group, in which placebo VW and the same PE program were administered.
Intervention protocol
A total of 18 sessions (3 sessions per week for 6 weeks) lasting 45 min were performed, based on the criteria established by the American College of Sports Medicine26. The intervention protocol was applied by two trained researchers (NSR and EMG)21.
Depending on the group, the participants performed one of two types of interventions: (i) EI: Virtual Walking (VW) combined with Physical exercise (PE); or (ii) CI: Placebo VW combined with PE. Each part is described below:
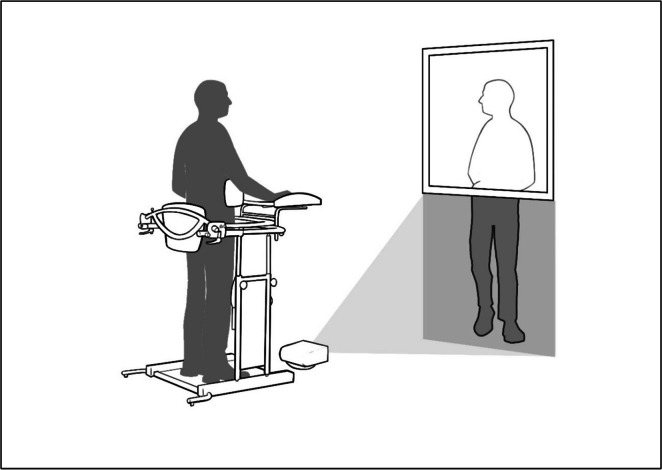
VW: the participant was placed in a standing position (using an ad-hoc designed system) in front of a mirror (which reflected the upper body) and a height-adjustable screen (lower body) on which a legs-in-motion video was projected (Supplementary Material 1). In order to adapt the legs in each case, healthy people with varying constitutions were previously recorded. Thus, there was a visual accommodation of the legs projected to the size of each participant during the first session. Participants were asked to watch and integrate the projected legs into their reflected upper body, creating the illusion of a complete mirror image. This VW program lasted 10 min (Fig. 1).
Placebo VW: the instrumentation was the same as for the VW intervention, based on previous studies applying this protocol aiming at treating neuropathic pain18,19,27. However, the projection consisted of a series of videos not showing any type of human or animal movement; accordingly, brain motor areas were not stimulated14. For CI, participants were asked to pay attention and focus on the landscapes projected in the video, avoiding thinking about anything else. Maintaining the experimental set-up and the positioning of the patient let to maintain the greatest fidelity to the EI protocol, except for the projected video. Therefore, potential differences between groups after the intervention should be attributed to the activity of the mirror neurons produced by VW.
PE: this intervention (Annex 1) was divided into two parts. In the first one, specific gait training was performed, including coordination and balance training. In the second one, strength and stretching training was performed. The protocol was adapted to the physical condition of each participant, considering the level of fatigue perceived. Annex 1 shows the variety of exercises according to functional ability and fatigue. In addition, exercises were adjusted weekly, considering each participant’s progress. This program lasted 30 min.
Fig. 1.
Set-up protocol.
Assessment
Two blinded assessors conducted all tests (SMC and PSA). These researchers were blinded to patient allocation. To ensure even greater blinding, the assessors were not involved in any aspect of the intervention process and had no contact with the intervention team. Assessments were conducted in a separate laboratory, and patients were instructed not to discuss any details of the intervention they received with the assessors. Additionally, all equipment and test procedures were standardized to minimize potential bias or cues that could reveal the group allocation to the evaluators.
Four evaluations were carried out:
T1: before the intervention.
T2: post-intervention (6 weeks from the start of the study).
T3: 4 weeks after the intervention (10 weeks from the start of the study).
T4: 12 weeks after the intervention (18 weeks from the start of the study).
Maximum voluntary isometric strength and muscle activity were both recorded during the following actions: ankle dorsiflexion, knee extension, hip abduction, hip extension, and ankle plantar flexion. Both recordings were started manually by the same operator.
Before starting the test, the researcher explained the movement to the participants. They then performed a familiarization test, and the researcher corrected any possible compensations. The strongest lower limb (according to the AIS scale) was measured.
Participants were asked for three maximum voluntary isometric contractions (MVIC). Each measurement was repeated 3 times (5 s contraction and 5 s rest) and with approximately 2 min of rest between the different muscle group assessments. We manually identified the start and end points. To do this, we used the double threshold method28, which involves comparing the amplitude of the rectified EMG signal to a preset threshold value (i.e., 30 µV), during a present number of consecutive samples exceeding the first threshold (i.e., 30 ms).
For the evaluation, the participants were positioned in lateral decubitus, with their legs in a neutral position retained with a suspension system. This procedure was chosen to allow the recording of muscle activity described in the following section. Annex 2 shows the description of each assessed movement, as well as the instructions given to the participants.
The isometric strength of the lower limb was measured using a load cell (Strength sensor kit, Chronojump Boscosystem®, Barcelona). This sensor was previously validated (ICC 0.75–0.90)29 and used in other strength-impaired population30. For the signal analysis, the central 3 s of each repetition were used. Thus, the maximum peak and the mean strength in these 3 s was selected from each repetition to subsequently obtain their average. Generally, there were no differences between repetitions that exceeded the threshold of 20%. Only in a few cases in which bad positions or compensated movements occurred in any of the contractions, we decided to repeat the attempt. The difference of 20% was computed based on the mean strength (the software provided the data in real-time). We ensured that participants had enough time to recover (1 min)31.
For the assessment of muscle activity, the electromyographic (EMG) signal was recorded using the 5-channel BTS 100 FreeEMG system (BTS Bioengineering, Garbagnate Milanese, Milan, Italy). This system was previously used in neurological populations32. Muscle activity of the tibialis anterior (as the main representative of dorsiflexion), rectus femoris (as representative of the quadriceps muscle group during knee extension), gluteus medius (as main representative of hip abduction), biceps femoris (representative of the hamstring muscle group during hip extension), and external gastrocnemius (representative of the triceps sural muscle group during ankle extension) was obtained.
Prior to assessment, body hair was shaved in the intended areas for sensor placement, and 98º alcohol was applied to clean the dermal surface. The sensors were placed following the criteria established by SENIAM33, 1.5–2 cm apart, in parallel with muscle fibres:
Tibialis anterior: the electrodes were placed at 1/3 on the line between the tip of the fibula and the tip of the medial malleolus.
Rectus femoris: the electrodes were placed at 50% on the line from the anterior spina iliaca superior to the superior part of the patella.
Gluteus medius: the electrodes were placed at 50% on the line from the crista iliaca to the trochanter.
Biceps femoris: the electrodes were placed at 50% on the line between the ischial tuberosity and the lateral epicondyle of the tibia.
Lateral Gastrocnemius: electrodes were placed at 1/3 of the line between the head of the fibula and the heel.
Registration of the EMG signal (µV) was performed with the EMG Analyser software (BTS Bioengineering, Italy), using a sample rate of 1000 Hz. The data obtained were subsequently processed using the same software. The raw signal was filtered by a 40–400 Hz bandpass filter, by the 4th order Butterworth filter. In addition, a 50 Hz centred notch filter was used to remove artifacts produced by electrical light from the laboratory itself. The root mean square (RMS) of the central 3 s of each muscle action was used for further analysis.
Electromyographic signal outcome was the amplitude of the mean signal of each repetition (i.e., the average amplitude of each of the contractions) of the agonist muscle and antagonist muscle for each movement.
Statistical analysis
Data analysis was carried out using the SPSS v28 statistical software (IBM Inc., Chicago, USA). The normal distribution of the sample was analysed using the Shapiro-Wilk test, the homoscedasticity of the groups using Levene’s test, and sphericity using Mauchly’s test.
For the inference analysis, a mixed factorial ANOVA was used, with the between-subjects factor “intervention group” with two categories (EI and CI) and with the within-subjects factor “time” with 4 categories (including the 4 evaluations: T1, T2, T3 and T4). The dependent variables used were the peak and mean of the maximum strength (in N) and muscle activation (in µVV) during IMVC. For post-hoc comparisons between assessments, the Šidák correction was used. Significant differences were assumed when the p-value was less than 0.05.
Results
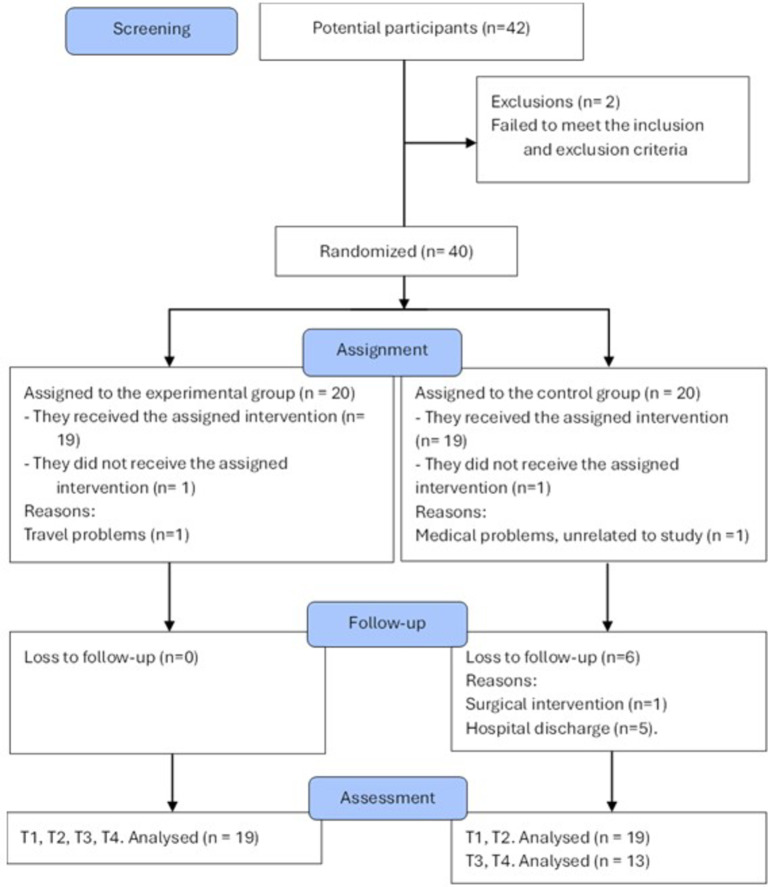
The study included 38 people with incomplete SCI, 19 allocated to EI group and 19 allocated to CI group (Fig. 2). No differences between groups were found in demographic and clinical data (p = 0.08 for “sex” outcome, p = 0.34 for “age” outcome, 0.25 for “time since the injury” outcome, p = 0.56 for “level of injury” outcome p = 0.14 for “type of injury” outcome, and p = 0.50 for “AIS classification” outcome; Table 1). In the EI group, 7 participants presented AIS grade C, while 12 presented AIS grade D; the mean (SD) age was 51.21 (12.80) years old and the mean time since injury was 85.11 (100.33) months. The CI group also presented 7 participants with AIS grade C, while 12 presented AIS grade D; the mean (SD) age was 49.26 (16.56) years old and the mean time since injury was 62.68 (105.15) months.
Fig. 2.
Flow diagram. T1 pre-intervention assessment, T2 post-intervention assessment, T3 1 month follow-up, T4 3 months follow-up.
Table 1.
Demographic and clinical data of the included sample.
| Group-ID | Sex | Age | Time since injury (months) | Level of injury | Type | AIS |
|---|---|---|---|---|---|---|
| EI-1 | Male | 62.00 | 88.00 | T5 | Trauma | C |
| EI-2 | Male | 48.00 | 77.00 | T6 | Trauma | C |
| EI-3 | Male | 58.00 | 432.00 | C5-C6 | Trauma | D |
| EI-4 | Male | 50.00 | 99.00 | T12-L1 | Oncology | D |
| EI-5 | Female | 64.00 | 63.00 | C4-C7 | Trauma | D |
| EI-6 | Male | 72.00 | 133.00 | T12-L12 | Trauma | D |
| EI-7 | Male | 46.00 | 64.00 | T6-T7 | Surgical | C |
| EI-8 | Female | 37.00 | 131.00 | T10 | Trauma | C |
| EI-9 | Female | 20.00 | 228.00 | T4-L3 | Congenital | D |
| EI-10 | Male | 48.00 | 61.00 | L2-S1 | Oncology | D |
| EI-11 | Male | 39.00 | 54.00 | L5-S1 | Trauma | D |
| EI-12 | Male | 40.00 | 57.00 | C6-T1 | Surgical | D |
| EI-13 | Male | 46.00 | 41.00 | L3 | Trauma | C |
| EI-14 | Female | 52.00 | 42.00 | L2 | Ischaemia | C |
| EI-15 | Male | 41.00 | 7.00 | C4 | Trauma | D |
| EI-16 | Male | 59.00 | 7.00 | C4 | Surgical | C |
| EI-17 | Male | 59.00 | 14.00 | T8-T10 | Ischaemia | D |
| EI-18 | Male | 66.00 | 7.00 | C4-C5 | Ischaemia | D |
| EI-19 | Male | 66.00 | 12.00 | D10 | Ischaemia | C |
| CI-20 | Male | 35.00 | 17.00 | C3-C6 | Trauma | D |
| CI-21 | Female | 65.00 | 14.00 | C4 | Trauma | D |
| CI-22 | Female | 68.00 | 17.00 | D5 | Surgical | D |
| CI-23 | Female | 42.00 | 68.00 | D12 | Other | C |
| CI-24 | Male | 38.00 | 456.00 | S1 | Congenital | D |
| CI-25 | Female | 70.00 | 34.00 | C4 | Other | D |
| CI-26 | Male | 77.00 | 12.00 | L3-L4 | Surgical | D |
| CI-27 | Male | 40.00 | 14.00 | C4 | Trauma | C |
| CI-28 | Female | 69.00 | 26.00 | C4-C7 | Other | D |
| CI-29 | Male | 28.00 | 13.00 | C1-C2 | Other | C |
| CI-30 | Female | 22.00 | 65.00 | T11-T12 | Trauma | C |
| CI-31 | Male | 43.00 | 18.00 | C5 | Trauma | D |
| CI-32 | Female | 31.00 | 19.00 | T2-T3 | Ischaemia | D |
| CI-33 | Male | 36.00 | 6.00 | C2-C7 | Ischaemia | D |
| CI-34 | Female | 53.00 | 20.00 | C3-C4 | Trauma | D |
| CI-35 | Male | 54.00 | 95.00 | D7 | Trauma | C |
| CI-36 | Male | 60.00 | 91.00 | T12-L1 | Oncology | D |
| CI-37 | Male | 39.00 | 187.00 | C2 and D4 | Trauma | C |
| CI-38 | Female | 66.00 | 19.00 | C4-C6 | Surgical | D |
The normal distribution and the homoscedasticity were accomplished (p > 0.05 in the Shapiro-Wilk and Levene’s tests, respectively). Data also accomplished the Sphericity for all measures (p > 0.05), except for strength and muscle activation of hamstrings and tibialis anterior (p < 0.05 in the Mauchly test).
For didactic proposes, we have selected one of the muscle groups to better display the results (Figs. 3 and 4) obtained over the assessments (the information of all muscle groups is in Tables 2 and 3). Moreover, Supplementary Material 2 shows an example of strength and activation recording.
Fig. 3.
Results of load cell (Newtons) during maximum voluntary isometric contraction for hip extension. T1 pre-intervention assessment, T2 post-intervention assessment, T3 1 month follow-up, T4 3 months follow-up, EI experimental Intervention, CI control intervention.
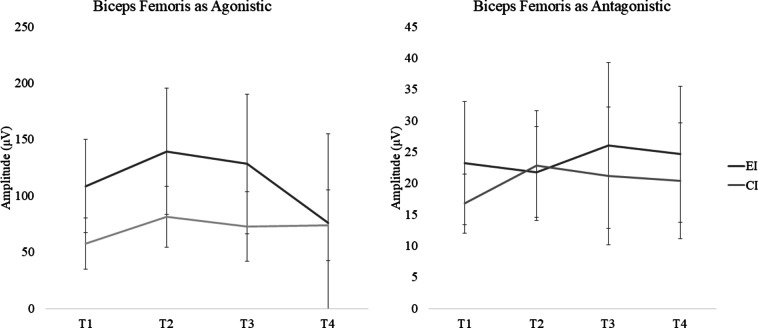
Fig. 4.
Results of muscle activation (µV) during maximum voluntary isometric contraction for biceps femoris. T1 pre-intervention assessment, T2 post-intervention assessment, T3 1 month follow-up, T4 3 months follow-up, EI experimental Intervention, CI control Intervention.
Table 2.
Results of load cell (Newtons) during maximum voluntary isometric contraction.
| EI group | CI group | |||||||
|---|---|---|---|---|---|---|---|---|
| T1 (19) | T2 (19) | T3(19) | T4(19) | T1 (19) | T2 (19) | T3 (13) | T4 (13) | |
| Dorsal flexion—Peak | 58.17 (54.50) | 76.64 (56.69)* | 71.80 (53.85) | 77.56 (58.45) | 35.37 (34.43) | 43.67 (47.02) | 43.56 (49.42) | 51.83 (2.87) |
| Dorsal flexion—Mean | 46.95 (45.97) | 63.27 (50.78)* | 59.16 (47.07) | 71.81 (59.11)* | 39.23 (41.72) | 49.36 (49.91) | 46.44 (51.33) | 48.68 (57.78) |
| Dorsal flexion—strength gain (%) compared with T1 | 24.01–37.76% | 52.95% | ||||||
| Knee extension—Peak | 94.59 (64.03) | 121.22 (72.80)* | 108.99 (70.55) | 116.26 (98.29) | 88.11 (51.83) | 99.47 (59.31) | 91.26 (59.32) | 90.38 (55.26) |
| Knee extension—Mean | 87.15 (58.81) | 111.87 (67.95)* | 101.40 (66.18) | 109.30 (94.30) | 78.96 (47.58) | 83.33 (58.44) | 83.89 (52.26) | 83.45 (49.42) |
| Knee extension—strength gain (%) from T1 | 28.15–28.36% | |||||||
| Hip abduction—Peak | 71.56 (50.72) | 106.29 (69.28)* | 88.77 (57.58)* | 78.20 (51.11) | 53.36 (41.74) | 70.83 (42.81)* | 69.57 (54.45) | 69.39 (53.78) |
| Hip abduction—Mean | 68.39 (46.68) | 94.17 (63.77)* | 82.08 (54.80) | 69.06 (44.57) | 44.94 (33.77) | 63.29 (39.32)* | 60.40 (47.94) | 63.38 (49.90) |
| Hip abduction—strength gain (%) from T1 | 37.70 − 48.53% | 24.05% | 32.74–40.83% | |||||
| Hip extension—peak | 73.12 (74.23) | 105.72 (74.17)* | 95.41 (79.89)* | 89.85 (86.90) | 62.16 (35.97) | 82.14 (62.26)* | 88.00 (61.87) | 69.28 (41.05) |
| Hip extension—Mean | 72.88 (68.30) | 96.67 (76.19)* | 89.55 (77.19) | 84.33 (81.83) | 56.48 (33.75) | 73.21 (56.49) | 79.12 (55.53) | 60.55 (36.78) |
| Hip extension—strength gain (%) from T1 | 32.64–44.58% | 30.48% | 32.14% | |||||
| Plantar flexion—Peak | 68.17 (72.93) | 94.09 (85.21)* | 81.71 (77.15) | 89.05 (86.40) | 53.42 (45.53) | 66.94 (85.22) | 68.15 (60.50) | 65.78 (63.67) |
| Plantar flexion—Mean | 62.13 (64.80) | 88.05 (96.66)* | 72.37 (74.92) | 79.42 (82.74) | 47.54 (42.87) | 59.11 (47.45) | 62.65 (55.71) | 56.35 (51.81) |
| Plantar flexion—strength gain (%) from T1 | 38.02–41.72% | |||||||
T1 pre-intervention assessment, T2 post-intervention assessment, T3 1 month follow-up, T4 3 months follow-up, EI experimental intervention, CI control intervention.
*p < 0.05 with T1. Results are expressed as mean (SD).
Table 3.
Results of muscle activation (µV) during maximum voluntary isometric contraction.
| EI group | CI group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 (16) | T2 (16) | T3 (15) | T4 (11) | T1 (13) | T2 (13) | T3 (9) | T4 (5) | ||
| Dorsal flexion | Tibialis anterior (ag) | 73.15 (58.44) | 109.17 (161.06)* | 97.88 (137.51) | 110.20 (143.97) | 56.38 (56.20) | 84.85 (64.37) | 69.10 (2.10) | 75.39 (64.69) |
| Strength gain (%) | 49.24% | ||||||||
| Gastrocnemius (atg) | 33.86 (51.67) | 19.58 (30.40)* | 17.66 (25.79)* | 22.74 (30.76) | 13.57 (9.29) | 14.35 (9.87) | 18.50 (13.69) | 21.54 (12.85) | |
| Strength gain (%) | − 42.17 | − 47.84% | |||||||
| Knee extension | Rectus femoris (ag) | 108.57 (100.76) | 139.42 (94.03)* | 128.27 (110.00) | 75.83 (61.13) | 57.76 (6.19) | 81.51 (49.72) | 72.88 (28.66) | 73.80 (31.95) |
| Strength gain (%) | 28.41% | ||||||||
| Biceps femoris | 23.22 (19.68) | 21.80 (14.46) | 26.06 (26.46) | 24.65 (21.70) | 16.79 (9.47) | 22.83 (17.47) | 21.17 (21.98) | 20.39 (18.46) | |
| Hip abduction | Gluteus maximum (ag) | 52.46 (59.30) | 73.46 (68.35)* | 67.89 (77.29) | 57.82 (65.37) | 47.15 (69.37) | 69.31 (85.02)* | 40.55 (53.04) | 24.12 (21.00) |
| Strength gain (%) | 40.03% | 47.00% | |||||||
| Hip extension | Biceps femoris (ag) | 79.68 (82.88) | 99.92 (112.06)* | 105.48 (123.85) | 99.90 (138.02) | 65.54 (45.52) | 94.36 (59.06)* | 82.65 (61.20) | 81.63 (62.88) |
| Strength gain (%) | 25.40% | 57.70% | |||||||
| Rectus femoris (atg) | 39.20 (49.90) | 24.42 (28.21)* | 22.81 (4.71) | 30.25 (34.81) | 42.35 (45.58) | 39.28 (39.50) | 36.30 (30.13) | 13.12 (10.78) | |
| Strength gain (%) | − 37.70% | ||||||||
| Plantar flexion | Gastrocnemius (ag) | 34.89 (36.87) | 51.79 (78.48) | 39.65 (52.55) | 31.87 (48.87) | 28.71 (17.05) | 35.35 (34.84) | 30.01 (23.97) | 51.47 (38.89) |
| Tibialis anterior (atg) | 23.16 (19.75) | 18.45 (18.16) | 16.95 (18.07) | 21.45 (21.81) | 37.21 (33.50) | 37.35 (32.01) | 44.76 (58.58) | 58.59 (43.35) | |
T1 pre-intervention assessment, T2 post-intervention assessment, T3 one month follow-up, T4 three months follow-up, Ag agonist, Atg antagonist.
*p < 0.05 with T1. Results are expressed as mean (SD).
Table 2 displays the maximum and mean strength results for the evaluated movements during MVIC, including dorsal flexion, knee extension, hip abduction, hip extension, and plantar flexion. Following the intervention, the EI group exhibited a significant overall strength increase for all movements after the intervention (T2), with an increment ranging from 16.31 N to 34.72 N. In contrast, the CI group only demonstrated a significant strength improvement in hip abduction and hip extension movements, showing an increase of 18.34 (7.13) N and 19.98 (9.60) N, respectively.
The EI group maintained significant strength improvements in hip abduction and hip extension movements at T3, four weeks post-intervention, showing an increase of 17.20 (6.33) N and 22.29 (8.59) N from T1, respectively. On the contrary, the CI group did not sustain the improvements during the 4-week follow-up. Neither group maintained the improvements for any movements three months after the conclusion of the intervention.
Table 3 displays the outcomes of muscle activation of the agonist and antagonist of each isometric contraction, assessed through electromyography. Overall, the EI group exhibited a significant increase in muscle activation, ranging from 36.02 (15.75) µV to 20.24 (8.55) µV, in all agonistic muscles (i.e., tibialis anterior, rectus femoris, gluteus maximus, and biceps femoris), except for plantar flexion (gastrocnemius). This increase was observed immediately after the EI intervention (T2). Contrary, in the CI group, only agonistic muscles for hip abduction and hip extension (gluteus maximus and biceps femoris) exhibited an increase in activation immediately after intervention (T2), with values of 22.16 (9.80) µV and 28.82 (9.14) µV, respectively. No differences were observed in either group at the 4- and 12-week follow-ups (T3 and T4) for any of the assessed movements.
With regard to the antagonistic muscles, in the EI group, the gastrocnemius during dorsal flexion (− 14.28 (5.61) µV) and the rectus femoris during hip extension (− 14.78 (6.11]) µV) displayed a significant decrease in muscle activation at the end of the intervention (T2). This reduction in activation for the gastrocnemius was sustained for up to 4 weeks after the intervention (T3, − 17.99 (6.08)), but not after 12 weeks (T4). Conversely, no differences were identified in the activation of antagonistic muscles in the CI group for any assessment.
Discussion
This study evaluated the effects of a VW combined with PE intervention (i.e., EI) compared to a placebo VW combined with the same PE protocol (i.e., CI) on muscle strength and activation of the agonistic and antagonistic muscle groups during isometric lower limb contractions. The EI group showed significant overall strength improvements across all the assessed muscle groups immediately after the intervention (T2), maintaining the improvements for hip abductors and hip extensors until four weeks post-intervention (T3). Indeed, these muscle groups (hip abductors and hip extensors) were the only to increase their isometric strength immediately after the intervention (T2) in participants allocated to the CI group. But contrary to EI, these improvements were not maintained 4 weeks after the end of the intervention (T3). For the rest of the muscle groups, no significant improvements were achieved in the CI group either at T2 or T3. At 12 weeks follow-up assessment (T4), the improvements were not maintained for any group.
Regarding muscle activation assessed by EMG, the EI group exhibited significant increased agonistic muscle activation during MVIC (except for the gastrocnemius during isometric plantar flexion) immediately after intervention (T2), while the CI group showed significant increases only for the gluteus maximum and biceps femoris during hip abduction and hip extension, respectively. None of the groups maintained these improvements at 4 or 12 weeks after intervention. Furthermore, EI showed significant decreases in antagonistic muscle activation, particularly in the gastrocnemius during dorsal flexion and in the rectus femoris during hip extension, the first being sustained up to four weeks post-intervention. However, CI did not achieve a significant reduction of antagonistic muscle activation after the intervention.
This study represents the first investigation addressing the effects of VW combined with PE on motor capacities despite that it is well known that incomplete SCI patients have an inherent capacity for recovery, or at the very least, for the recruitment of synergistic muscles, ultimately fostering enhanced functional outcomes4,5. Our findings underscore the importance of researching the effects of interventions in addition to PE which may maximize the functional capacity potential in this population; they further highlight the need for more comprehensive and personalized rehabilitation strategies to optimize functional outcomes and improve quality of life.
During PE, there is repeated and sustained activation of this corticospinal pathway7,8,34. This can lead to neuromuscular adaptations, such as more efficient and coordinated recruitment of motor units, increased coordination between agonist and antagonist muscles, and enhancement in force-generating capacity35. However, the CI group which carried out PE only achieved improvements in hip movement (i.e., abduction and extension), both in muscle strength and activation. This may be a consequence of the volume and dosage of the exercise program that was adapted to their level of fatigue36. In this line, there are studies37 that suggest that solely engaging in adapted sports may not suffice to attain the threshold of physical activity recommended by the World Health Organization (WHO) for optimal health. Despite consistent participation in PE, individuals often fall short of meeting these guidelines. Thus, it becomes imperative to complement participation in physical exercise programs with corticospinal facilitation techniques. Our study serves to underline the potential utility of such facilitation, even in cases of partial denervation (i.e., incomplete SCI). While previous research has demonstrated its efficacy on motor function in various pathologies, including neurological disorders15–17, its application in incomplete SCI has so far remained relatively unexplored.
The improvements obtained in strength in the EI group (increases between 28% and 48% in T2 concerning T1), as opposed to the control group, may be linked to the facilitation of the corticospinal pathway4,5 and involvement of mirror neuron activation11–13, resulting in neuromuscular adaptations that enhance motor system efficiency and force-generating capacity38.
Mirror neurons fire both when an individual performs an action and when he or she observes someone else performing the same action. It has been demonstrated that observing movements activates similar motor areas in the brain as those activated during actual movement execution11–13. Therefore, visualization or observation of movements may activate the corticospinal pathway similarly to actual movement execution itself, facilitating motor learning and improving muscle strength. Thus, when an individual observes an action with a given objective performed by another individual, as was noted in the VW protocol, the neurons that represent this action are activated in the motor and premotor cortex of the observer, transforming the information received visually into knowledge39–41. This ability to activate through observation is especially relevant in the case of CNS injury since the neuronal interruption does not allow certain motor tasks to be performed, as in incomplete SCI. Adding this VW protocol to the PE program may explain why the EI group showed greater strength for all assessed muscle actions immediately after finishing the intervention (T2) compared to the CI group, and further maintained the effects over 4 weeks for those muscle actions that improved in the CI group (i.e., hip extension and abduction for T3).
The ability to generate force is intricately linked to EMG activity42, providing valuable insights into neuromuscular function during muscle actions. This is why this study presents EMG recordings, offering information about the agonistic-antagonistic muscle activity involved in force production. Specifically, an increased EMG amplitude of agonistic muscle and a decreased EMG amplitude in antagonistic muscles correlate with higher force output, indicating greater motor unit recruitment and activation42. In both groups, results showed an amplitude increment for those agonistic muscles in which an improvement in strength was obtained. Therefore, a greater activation of the agonist muscles has led to an improvement in strength.
The antagonist muscles were also assessed since agonist-antagonist synergy becomes difficult in people with neurological diseases. None of the results for antagonist muscle activation during MVIC changed after the CI. Conversely, both the rectus femoris during isometric hip extension and the gastrocnemius during isometric dorsal flexion reduced their activation after the EI (T2). In the latter case, these results were sustained up to 4 weeks after the intervention (T3). These results suggested that VW would partially improve the coordination between agonistic and antagonistic muscles through corticospinal pathway activation, facilitating motor learning, and allowing the production of a higher force in the analysed movements.
Regrettably, the results of this study cannot be directly compared with previous research in this population, as existing studies assessing virtual reality programs—only partially similar to ours—have not measured isometric strength, relying instead on clinical functional movement tests43,44. Nevertheless, studies in other populations, such as stroke and Parkinson’s disease, have explored combined virtual reality interventions with physical exercise45–47. Consistent with our findings, they reported strength improvements exclusively in groups receiving combination therapy, underscoring the enhanced efficacy of integrating these approaches.
It should be noted that all the discussed improvements have been achieved through a simple experimental set-up in terms of space and economic investment. This emphasizes the potential for translating these findings into practical and at-home applications, thereby empowering individuals to enhance their strength and functional abilities within the comfort of their environment. Further research should focus on optimizing the protocol to make it even more accessible and user-friendly, targeting specific populations such as those with mobility impairments. It would also be valuable to conduct longer-term studies to assess sustained benefits and to explore integration into rehabilitation programs. Once these adaptations are made and tested, the next logical step would be to develop affordable, easy-to-use kits or mobile applications to guide users in their own homes. This could revolutionize personal health maintenance, enabling a wide range of individuals to maintain or improve their physical capabilities without requiring specialized facilities or significant financial investment.
However, this study presents some methodological limitations that should be considered when interpreting the results: (i) Although not unusual when researching with people presenting spinal cord injury, the sample showed high heterogeneity, so maybe type II errors should not be discarded; (ii) Therapists could not be blinded due to the nature of the intervention; (iii) It was also not possible to completely blind the volunteers since the PE part was to be performed actively; (iv) there were two people who did not complete the treatment, and therefore the proposed sample size was not reached; (v) ain the follow-up assessments (T3 and T4) some volunteers dropped out of the study (for reasons unrelated to the study) and therefore, the adequate sample size was not achieved in these assessments; and (vi) EMG activity of adductor muscles was not recorded because the assessment position for the adductors made contact between EMG sensors impossible.
Conclusion
Based on the results, this study evidenced that activation of the mirror neuron system through VW could enhance the effects of PE regarding muscle strength and activation in people with incomplete SCI. Moreover, the addition of VW can prolong the effect of the treatment after it has ended.
This highlights the potential need for continued treatment to preserve long-term benefits, suggesting that while the EI approach shows promise, ongoing interventions may be necessary to ensure lasting outcomes in muscle strength and activation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors thank to Dra. Carmen Grao. Unidad de Lesión Medular Hospital Universitari i Politècnic La Fe, 46026 Valencia, Spain. In addition, we also thank a number of hospitals and associations: (Hospital Universitari i Politècnic La Fe [Spain]; Asociación de Personas con Lesión Medular y otras Discapacidades Físicas [Spain]; Associació TetraSport [Spain]; Coordinator of People with Physical Functional Diversity of the Valencian Community [Spain]; University Clinic of Nutrition, Physical Activity and Physiotherapy [Spain]; L’association Sport et Thérapies Neuro-rééducatives Avancées [France] and the Henry Gabrielle Hospital [France]).
Abbreviations
- SCI
Spinal cord injury
- VW
Virtual walking
- PE
Physical exercise
- EI
Experimental intervention
- CI
Control intervention
Author contributions
Conceptualization: PSAInvestigation: All authorsWrote the main document: All authorsAll authors reviewed the manuscript.
Funding
This work was supported by Grants from the Spanish Government [PID2021-125694OB-I00; co-financed by EU FEDER funds]; Generalitat Valenciana, Conselleria d’Innovació, Universitats, Ciència i Societat [CIAICO/2021/215]; and from Universitat de València [INV19-01-13-07].
Data availability
Datasets generated during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Ethics Committee of the University of Valencia (2425590) and performed by the latest revision of the Declaration of Helsinki. All participants signed the written informed consent to participate in the study. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alashram, A. R., Padua, E., Hammash, A. K., Lombardo, M. & Annino, G. Effectiveness of virtual reality on balance ability in individuals with incomplete spinal cord injury: a systematic review. J. Clin. Neurosci.72, 322–327 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Hayes, H. B., Chvatal, S. A., French, M. A., Ting, L. H. & Trumbower, R. D. Neuromuscular constraints on muscle coordination during overground walking in persons with chronic incomplete spinal cord injury. Clin. Neurophysiol.125, 2024–2035 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morawietz, C. & Moffat, F. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch. Phys. Med. Rehabil.94, 2297–2308 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Fawcett, J. W. et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord.45, 190–205 (2007). [DOI] [PubMed] [Google Scholar]
- 5.de Araújo, A. V. L. et al. Effectiveness of anodal transcranial direct current stimulation to improve muscle strength and motor functionality after incomplete spinal cord injury: a systematic review and meta-analysis. Spinal Cord.10.1038/s41393-020-0438-2 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Hornby, T. G. et al. Clinical practice guideline to improve locomotor function following chronic stroke, incomplete spinal cord injury, and brain injury. J. Neurol. Phys. Ther.44, 1 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Freund, P. et al. Disability, atrophy and cortical reorganization following spinal cord injury. Brain134, 1610–1622 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nardone, R. et al. Functional brain reorganization after spinal cord injury: systematic review of animal and human studies. Brain Res.1504, 58–73 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Houle, J. D., Morris, K., Skinner, R. D., Garcia-Rill, E. & Peterson, C. A. Effects of fetal spinal cord tissue transplants and cycling exercise on the soleus muscle in spinalized rats. Muscle Nerve22, 846–856 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Roosink, M., Robitaille, N., Jackson, P. L., Bouyer, L. J. & Mercier, C. Interactive virtual feedback improves gait motor imagery after spinal cord injury: an exploratory study. Restor. Neurol. Neurosci.34, 227–235 . [DOI] [PMC free article] [PubMed]
- 11.Jeannerod, M. The hand and the object: the role of posterior parietal cortex in forming motor representations. Can. J. Physiol. Pharmacol.72, 535–541 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Dushanova, J. & Donoghue, J. Neurons in primary motor cortex engaged during action observation. Eur. J. Neurosci.31, 386–398 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vigneswaran, G., Philipp, R., Lemon, R. N. & Kraskov, A. M1 corticospinal mirror neurons and their role in movement suppression during action observation. Curr. Biol.23, 236–243 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraskov, A. et al. Corticospinal mirror neurons. Philos. Trans. R. Soc. Lond. B Biol. Sci.369, 20130174 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi, D., Sterba, A., Khatter, H. & Pandian, J. Mirror therapy in stroke rehabilitation: current perspectives. Ther. Clin. Risk Manag.16, 75–85 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maggio, M. G. et al. Effects of robotic neurorehabilitation through lokomat plus virtual reality on cognitive function in patients with traumatic brain injury: a retrospective case-control study. Int. J. Neurosci.130, 117–123 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Abbruzzese, G., Marchese, R., Avanzino, L. & Pelosin, E. Rehabilitation for Parkinson’s disease: current outlook and future challenges. Parkinson. Relat. Disord.22 (Suppl 1), S60–S64 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Moseley, G. L. Using visual illusion to reduce at-level neuropathic pain in paraplegia. Pain130, 294–298 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Soler, M. D. et al. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain133, 2565–2577 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan, M. & Richardson, E. J. Effects of virtual walking treatment on spinal cord injury-related neuropathic pain: pilot results and trends related to location of pain and at-level neuronal hypersensitivity. Am. J. Phys. Med. Rehabil.95, 1 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Mollà-Casanova, S. et al. Effectiveness of virtual-walking intervention combined with exercise on improving pain and function in incomplete spinal cord injury: a feasibility study. Spinal Cord. Ser. Cases10, 64 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richard-Denis, A., Chatta, R., Thompson, C. & Mac-Thiong, J. M. Patterns and predictors of functional recovery from the subacute to the chronic phase following a traumatic spinal cord injury: a prospective study. Spinal Cord.58, 43–52 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Roman-Viñas, B. et al. International physical activity questionnaire: reliability and validity in a Spanish population. Eur. J. Sport Sci.10, 297–304 (2010). [Google Scholar]
- 24.Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods39, 175–191 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Saghaei, M. Random allocation software for parallel group randomized trials. BMC Med. Res. Methodol.4, 26 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson, B. ACSM’s guidelines for exercise testing and prescription 9th Ed. 2014. J. Can. Chiropr. Assoc.58, 328 (2014). [Google Scholar]
- 27.Kumru, H. et al. The effects of transcranial direct current stimulation with visual illusion in neuropathic pain due to spinal cord injury: an evoked potentials and quantitative thermal testing study. Eur. J. Pain17, 55–66 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Drapała, J., Brzostowski, K., Szpala, A. & Rutkowska-Kucharska, A. Two stage EMG onset detection method. Arch. Control Sci.22, 1 (2012). [Google Scholar]
- 29.Gaudet, J. & Handrigan, G. Assessing the validity and reliability of a low-cost microcontroller-based load cell amplifier for measuring lower limb and upper limb muscular force. Sensors20, 4999 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buendía-Romero, Á. et al. A sensitive and practical evaluation to detect lower-limb strength changes in geriatrics: the isometric knee extension test. Appl. Sci.13, 2946 (2023). [Google Scholar]
- 31.Norasi, H., Koenig, J. & Mirka, G. A. Development and Assessment of a Method to Estimate the Value of a Maximum Voluntary Isometric Contraction Electromyogram from Submaximal Electromyographic Data. 10.1123/jab.2021-0229 (2022). [DOI] [PubMed]
- 32.Pasini Neto, H. et al. Effect of posture-control insoles on function in children with cerebral palsy: Randomized controlled clinical trial. BMC Musculoskelet. Disord.13, 193 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermens, H. J. et al. European recommendations for surface electromyography. Roessingh Res. Dev.8, 13–54 (1999). [Google Scholar]
- 34.Houlé, J. D. & Côté, M. P. Axon regeneration and exercise-dependent plasticity after spinal cord injury. Ann. N. Y. Acad. Sci.1279, 154–163 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edgerton, V. R., Kim, S. J., Ichiyama, R. M., Gerasimenko, Y. P. & Roy, R. R. Rehabilitative therapies after spinal cord injury. J. Neurotrauma23, 560–570 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Bayles, M. P. ACSM’s Exercise Testing and Prescription (Lippincott Williams & Wilkins, 2023).
- 37.Ferri-Caruana, A. et al. Accelerometer assessment of physical activity in individuals with paraplegia who do and do not participate in physical exercise. J. Spinal Cord. Med.43, 234–240 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serra-Añó, P. et al. Effects of resistance training on strength, pain and shoulder functionality in paraplegics. Spinal Cord.50, 827–831 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Fadiga, L., Fogassi, L., Pavesi, G. & Rizzolatti, G. Motor facilitation during action observation: a magnetic stimulation study. J. Neurophysiol.73, 2608–2611 (1995). [DOI] [PubMed] [Google Scholar]
- 40.Maeda, F., Kleiner-Fisman, G. & Pascual-Leone, A. Motor facilitation while observing hand actions: specificity of the effect and role of observer’s orientation. J. Neurophysiol.87, 1329–1335 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Patuzzo, S., Fiaschi, A. & Manganotti, P. Modulation of motor cortex excitability in the left hemisphere during action observation: a single- and paired-pulse transcranial magnetic stimulation study of self- and non-self-action observation. Neuropsychologia41, 1272–1278 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Naik, G. R. Computational Intelligence in Electromyography Analysis: A Perspective on Current Applications and Future Challenges (BoD—Books on Demand, 2012).
- 43.Villiger, M. et al. Virtual reality–augmented neurorehabilitation improves motor function and reduces neuropathic pain in patients with incomplete spinal cord injury. Neurorehabil. Neural Repair.27, 675–683 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Villiger, M. et al. Virtual reality rehabilitation system for neuropathic pain and motor dysfunction in spinal cord injury patients. In 2011 International Conference on Virtual Rehabilitation 1–4. 10.1109/ICVR.2011.5971865 (IEEE, 2011).
- 45.Liao, Y. Y., Yang, Y. R., Wu, Y. R. & Wang, R. Y. Virtual reality-based Wii fit training in improving muscle strength, sensory integration ability, and walking abilities in patients with Parkinson’s disease: a randomized control trial. Int. J. Gerontol.9, 190–195 (2015). [Google Scholar]
- 46.Lin, R. C. et al. Effectiveness of early rehabilitation combined with virtual reality training on muscle strength, mood state, and functional status in patients with acute stroke: a randomized controlled trial. Worldviews Evid.-Based Nurs.17, 158–167 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Beyaert, C., Vasa, R. & Frykberg, G. E. Gait post-stroke: pathophysiology and rehabilitation strategies. Clin. Neurophysiol.45, 335–355 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets generated during the current study are not publicly available, but are available from the corresponding author on reasonable request.