Abstract
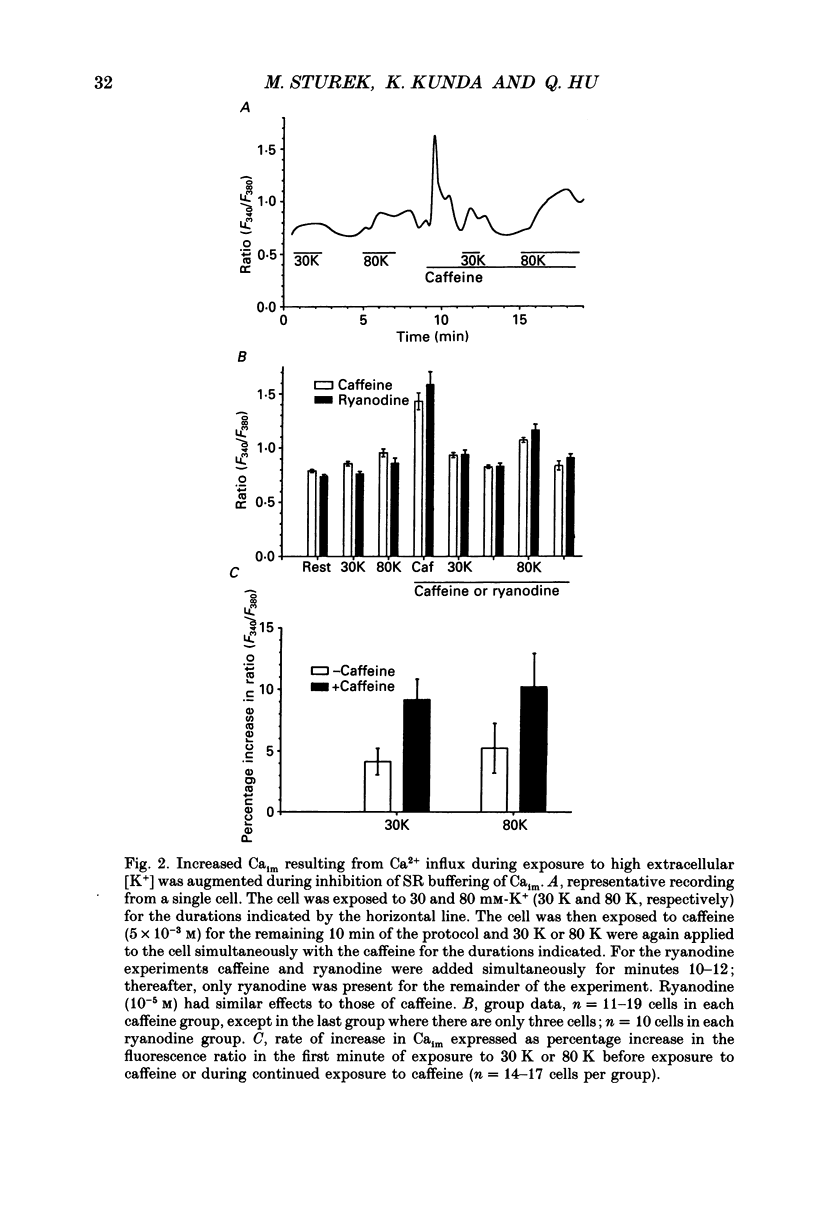
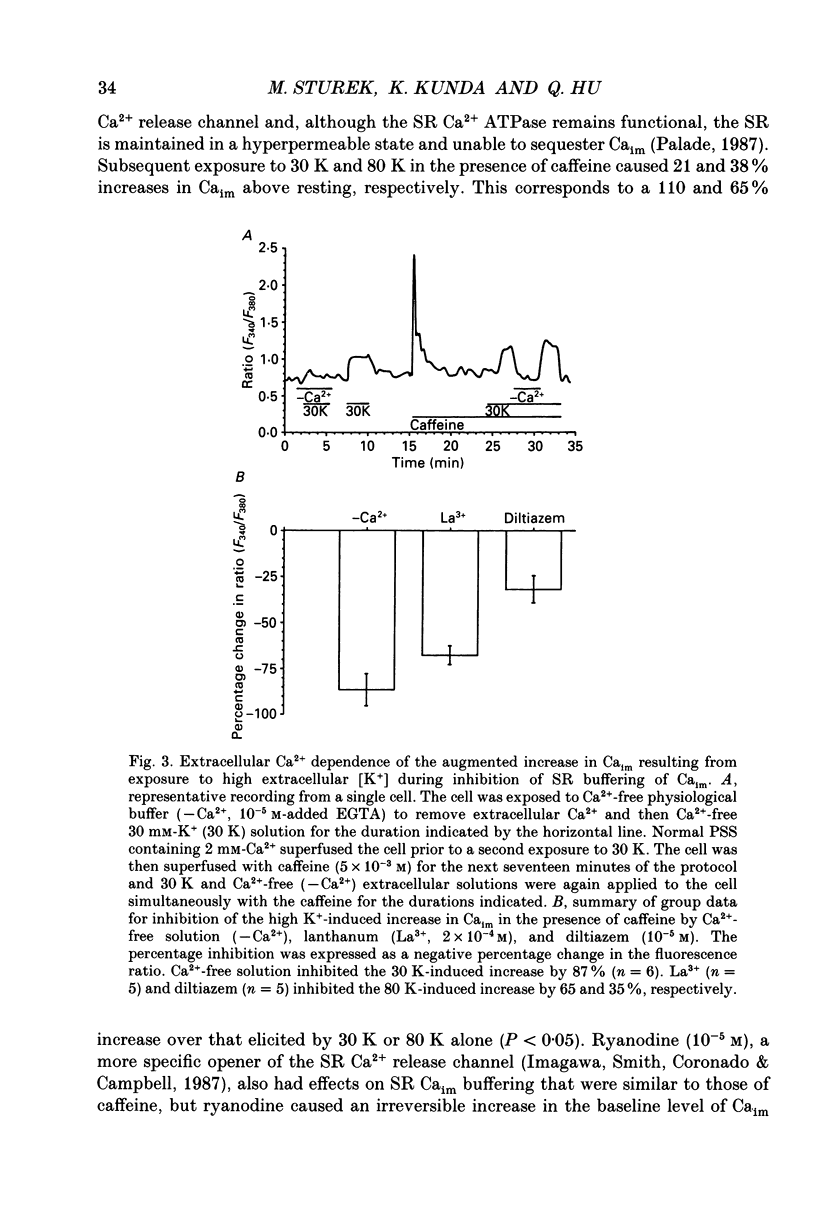
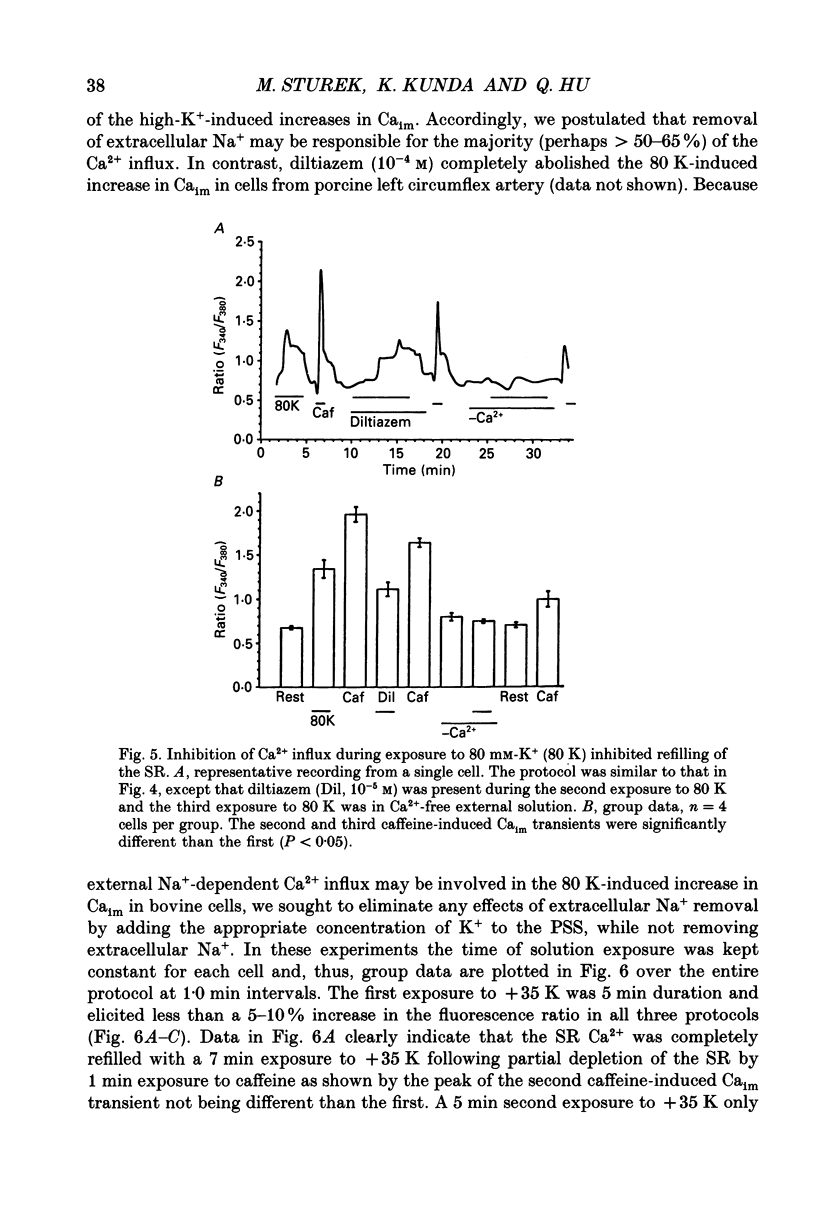
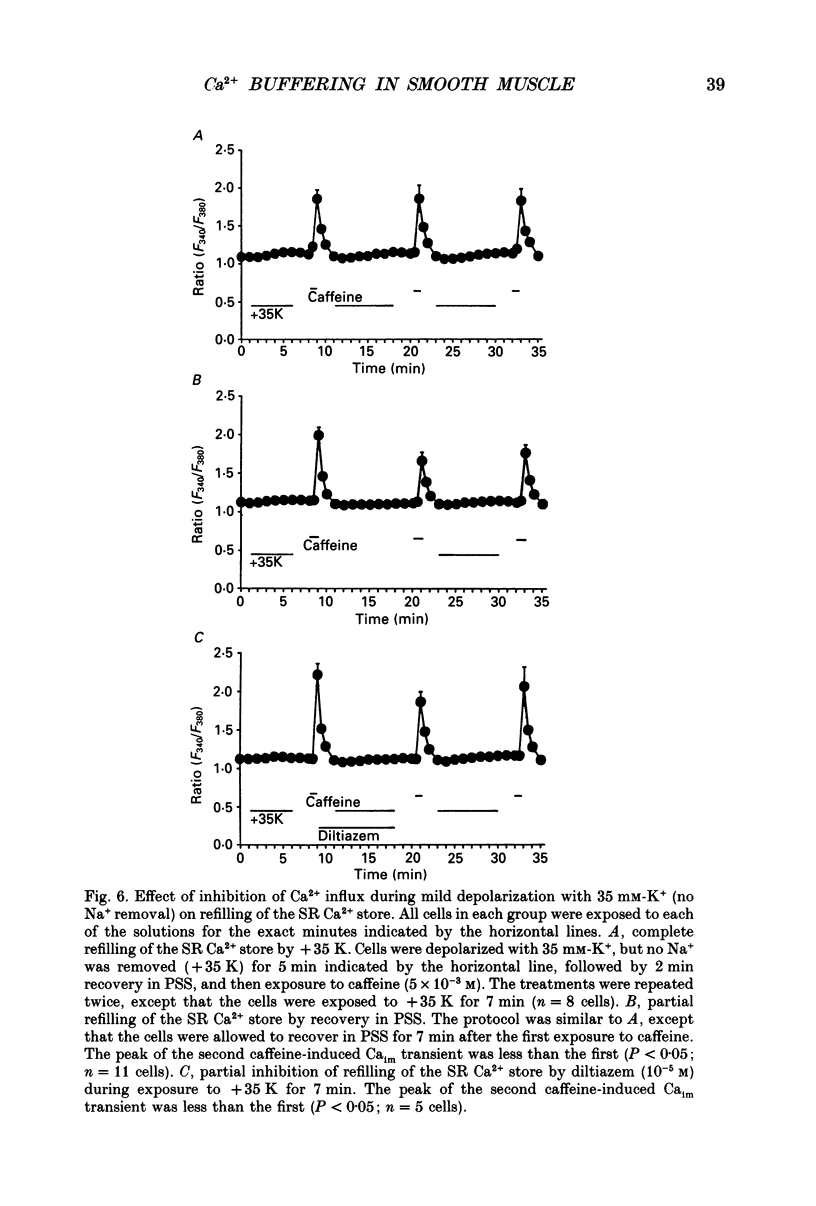
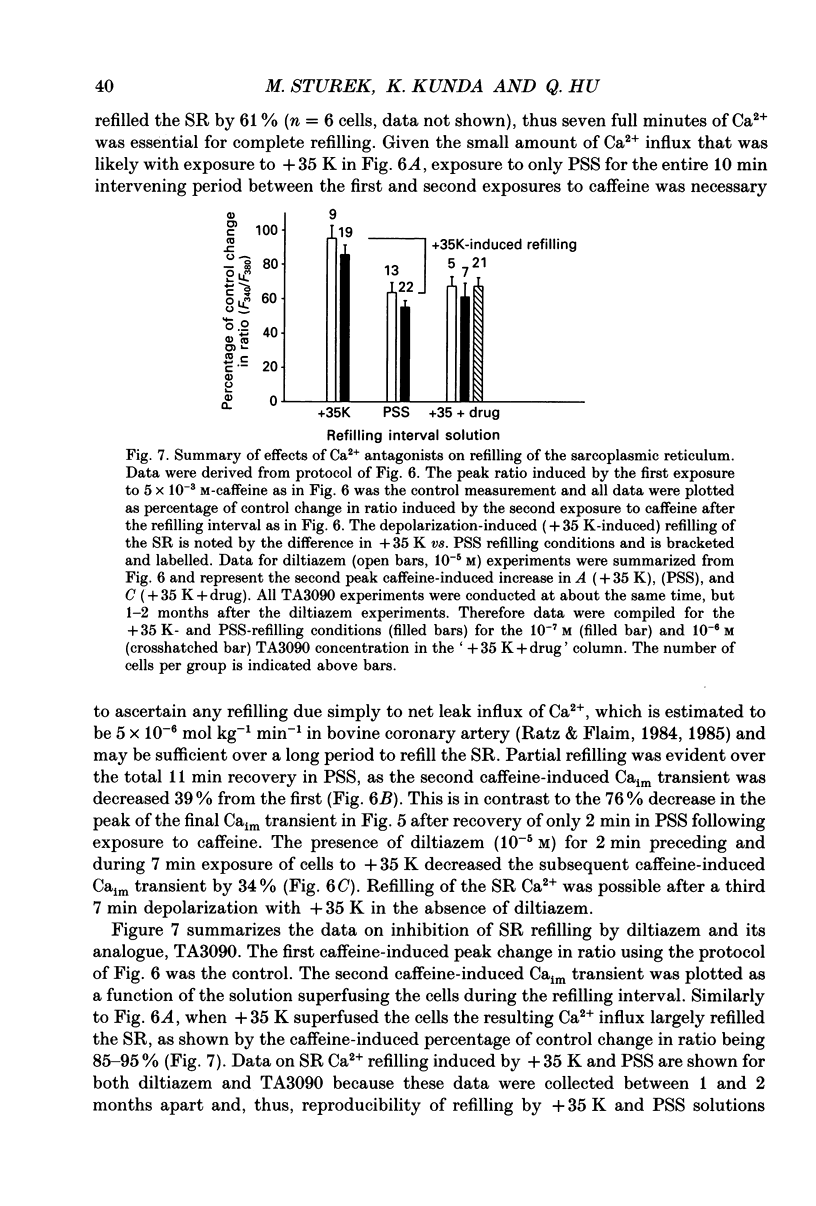
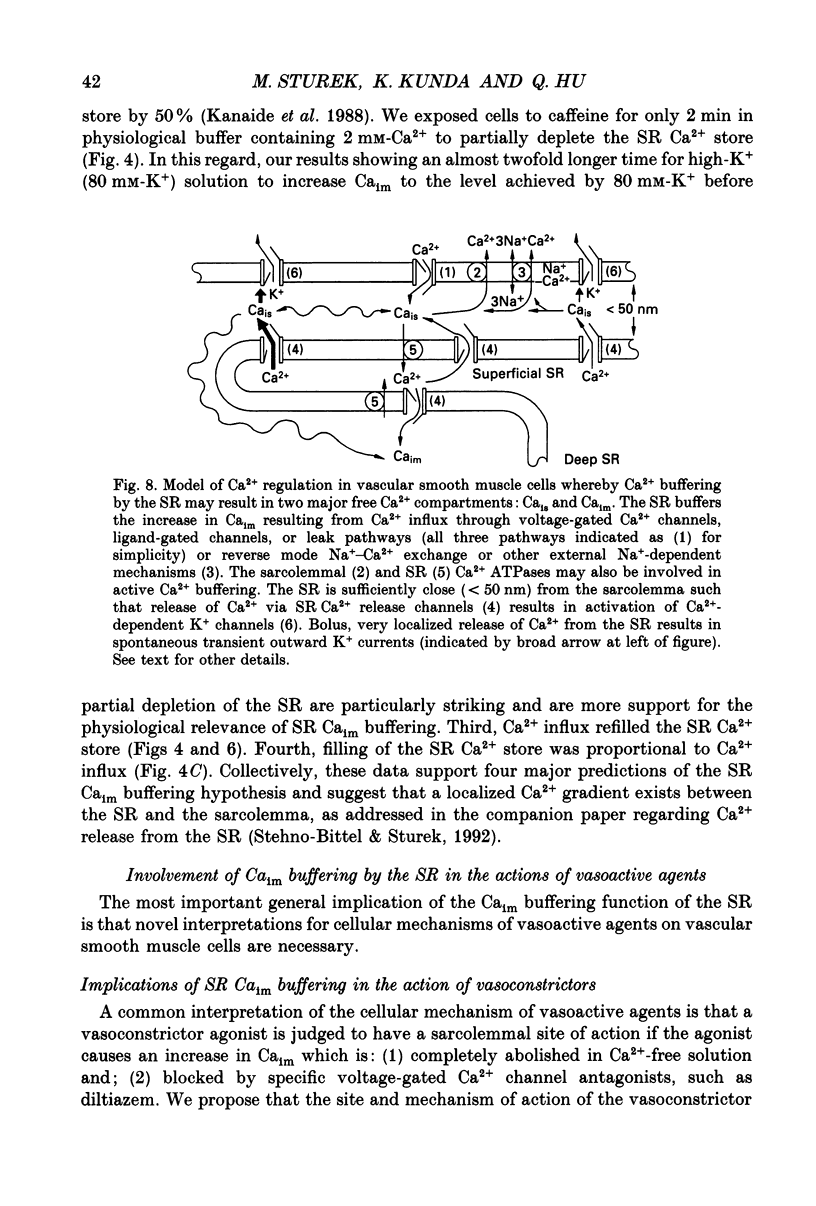
1. We tested the hypothesis that the sarcoplasmic reticulum (SR) buffers (attenuates) the increase in averaged myoplasmic free [Ca2+] (Ca(im)) resulting from Ca2+ influx. 2. Fura-2 measurements of Ca(im) were obtained in single smooth muscle cells freshly dispersed from bovine coronary artery. 3. Caffeine (5 x 10(-3) M) elicited a transient increase in Ca(im) and depleted the SR Ca2+ store. In the continued presence of caffeine or 10(-5) M-ryanodine SR buffering of Ca(im) was inhibited. Subsequent exposure to high extracellular [K+] (greater than 30 mM, equimolar Na+ removal) elicited a 2-fold more rapid and 2-fold greater peak increase in Ca(im) than high K+ elicited when SR buffering of Ca(im) was normal. The augmented increase in Ca(im) was inhibited 35% by 10(-5) M-diltiazem, 65% by 2 x 10(-4) M-LaCl3, and 87% in Ca(2+)-free external solution. 4. When Ca(im) buffering capacity was increased by partially depleting the SR with a transient (1 min) exposure to caffeine, subsequent exposure to 80 nM-K+ solution increased Ca(im) almost 2-fold more slowly than 80 mM-K+ before depletion of Ca2+ from the SR. However, the influxing Ca2+ was sequestered by the SR and refilled it, as evident by the subsequent caffeine-induced Ca(im) transient being identical to the first. Increasing extracellular [K+] (thus, increasing depolarization and Na+ removal) caused proportional increases in Ca(im) and the subsequent caffeine-induced Ca(im) transients were proportionally larger, indicating a graded filling of the SR by Ca2+ influx. 5. Diltiazem (10(-5) M) inhibited the refilling of the SR achieved by 80 mM-K+, by 26%. Refilling was inhibited 76% by 80 mM-K+, Ca(2+)-free solution, indicating the fraction of refilling dependent on influx of Ca2+ through voltage-gated Ca2+ channels, leak channels, and other influx pathways. Mild depolarization with 35 mM-K+ (no Na+ removal) often caused no increase in Ca(im), but influx through voltage-gated Ca2+ channels occurred because the SR Ca2+ store was refilled. Also, 10(-5) M-diltiazem or 10(-6) M-TA3090 inhibited the refilling to levels attributable only to leak influx of Ca2+. 6. All data support our hypothesis that the SR significantly attenuates the amount of Ca2+ influx that accumulates to increase Ca(im).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







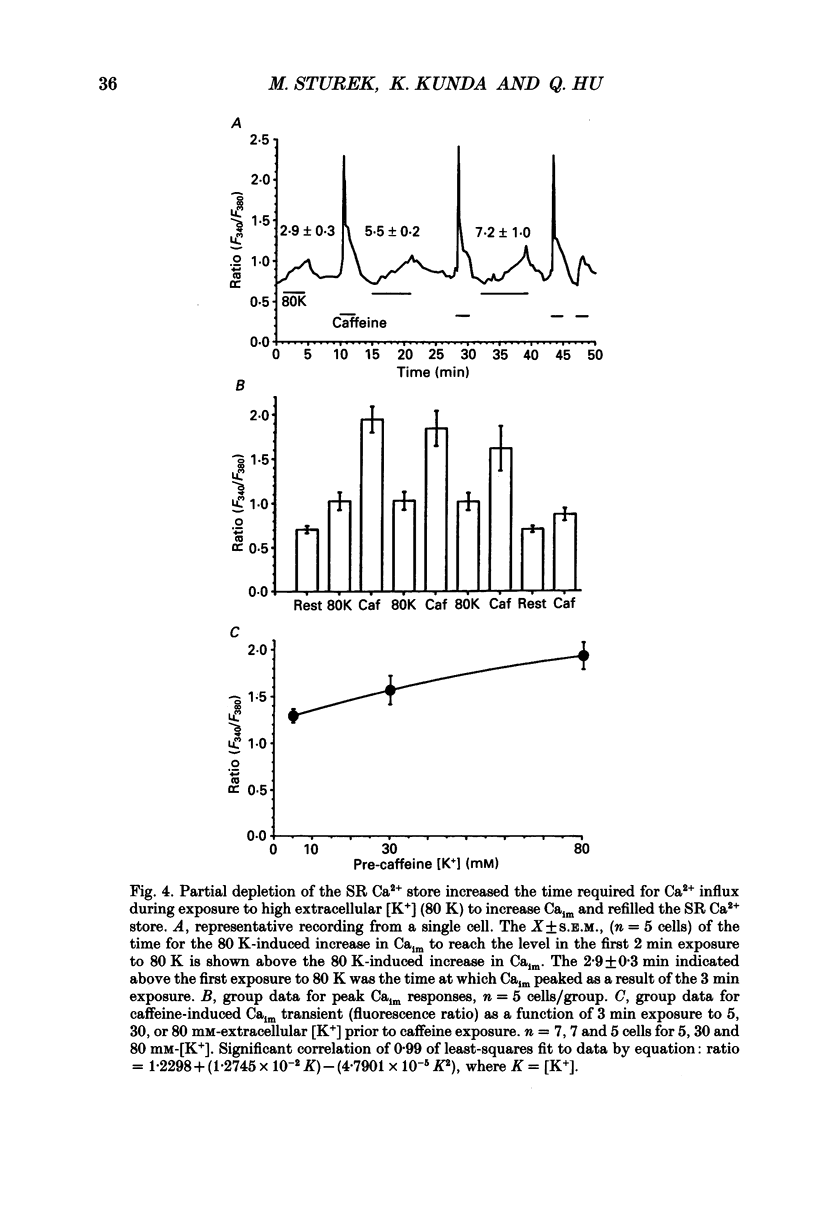








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Sturek M., Puga A., Hermsmeyer K. Calcium channels in muscle cells isolated from rat mesenteric arteries: modulation by dihydropyridine drugs. Circ Res. 1986 Aug;59(2):229–235. doi: 10.1161/01.res.59.2.229. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley A. B., Morgan K. G. Alterations in cytoplasmic calcium sensitivity during porcine coronary artery contractions as detected by aequorin. J Physiol. 1987 Apr;385:437–448. doi: 10.1113/jphysiol.1987.sp016500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981 Aug;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFeo T. T., Morgan K. G. Calcium-force coupling mechanisms during vasodilator-induced relaxation of ferret aorta. J Physiol. 1989 May;412:123–133. doi: 10.1113/jphysiol.1989.sp017607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Désilets M., Driska S. P., Baumgarten C. M. Current fluctuations and oscillations in smooth muscle cells from hog carotid artery. Role of the sarcoplasmic reticulum. Circ Res. 1989 Sep;65(3):708–722. doi: 10.1161/01.res.65.3.708. [DOI] [PubMed] [Google Scholar]
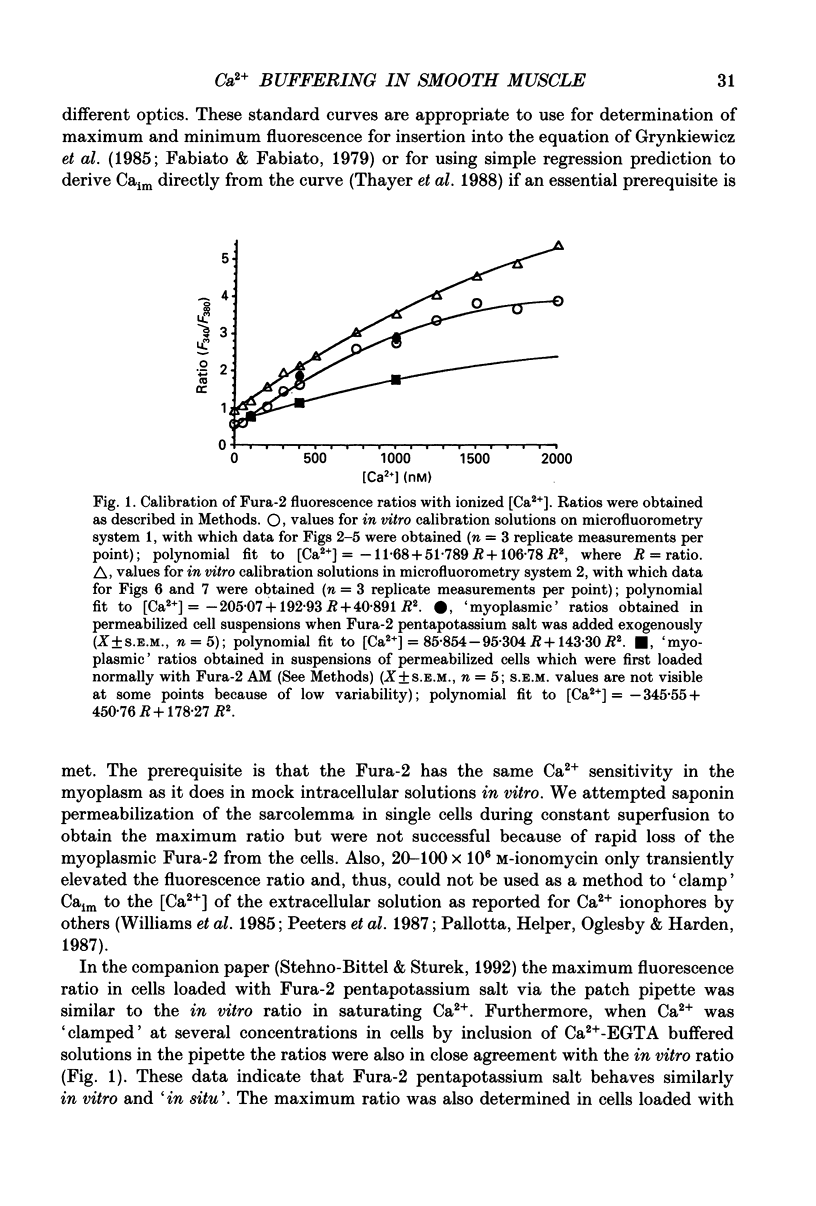
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Contribution of two types of calcium channels to membrane conductance of single myocytes from guinea-pig coronary artery. J Physiol. 1990 Jul;426:19–42. doi: 10.1113/jphysiol.1990.sp018125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind T., Miller R., Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol Rev. 1986 Dec;38(4):321–416. [PubMed] [Google Scholar]
- Goldman W. F., Wier W. G., Blaustein M. P. Effects of activation on distribution of Ca2+ in single arterial smooth muscle cells. Determination with fura-2 digital imaging microscopy. Circ Res. 1989 May;64(5):1019–1029. doi: 10.1161/01.res.64.5.1019. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hermsmeyer K., Sturek M., Rusch N. J. Nitrendipine inhibition of calcium current in rat vascular muscle cells. J Cardiovasc Pharmacol. 1988;12 (Suppl 5):S100–S103. [PubMed] [Google Scholar]
- Hill J. L., Gettes L. S. Effect of acute coronary artery occlusion on local myocardial extracellular K+ activity in swine. Circulation. 1980 Apr;61(4):768–778. doi: 10.1161/01.cir.61.4.768. [DOI] [PubMed] [Google Scholar]
- Hisayama T., Takayanagi I. Ryanodine: its possible mechanism of action in the caffeine-sensitive calcium store of smooth muscle. Pflugers Arch. 1988 Sep;412(4):376–381. doi: 10.1007/BF01907555. [DOI] [PubMed] [Google Scholar]
- Hume J. R., Leblanc N. Macroscopic K+ currents in single smooth muscle cells of the rabbit portal vein. J Physiol. 1989 Jun;413:49–73. doi: 10.1113/jphysiol.1989.sp017641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa T., Smith J. S., Coronado R., Campbell K. P. Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel. J Biol Chem. 1987 Dec 5;262(34):16636–16643. [PubMed] [Google Scholar]
- Inoue Y., Oike M., Nakao K., Kitamura K., Kuriyama H. Endothelin augments unitary calcium channel currents on the smooth muscle cell membrane of guinea-pig portal vein. J Physiol. 1990 Apr;423:171–191. doi: 10.1113/jphysiol.1990.sp018017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaide H., Kobayashi S., Nishimura J., Hasegawa M., Shogakiuchi Y., Matsumoto T., Nakamura M. Quin2 microfluorometry and effects of verapamil and diltiazem on calcium release from rat aorta smooth muscle cells in primary culture. Circ Res. 1988 Jul;63(1):16–26. doi: 10.1161/01.res.63.1.16. [DOI] [PubMed] [Google Scholar]
- Leblanc N., Hume J. R. Sodium current-induced release of calcium from cardiac sarcoplasmic reticulum. Science. 1990 Apr 20;248(4953):372–376. doi: 10.1126/science.2158146. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Niggli E., Hadley R. W. Sodium-calcium exchange in excitable cells: fuzzy space. Science. 1990 Apr 20;248(4953):283–283. doi: 10.1126/science.2326638. [DOI] [PubMed] [Google Scholar]
- Magliola L., Jones A. W. Depolarization-stimulated 42K+ efflux in rat aorta is calcium- and cellular volume-dependent. Circ Res. 1987 Jul;61(1):1–11. doi: 10.1161/01.res.61.1.1. [DOI] [PubMed] [Google Scholar]
- Missiaen L., Declerck I., Droogmans G., Plessers L., De Smedt H., Raeymaekers L., Casteels R. Agonist-dependent Ca2+ and Mn2+ entry dependent on state of filling of Ca2+ stores in aortic smooth muscle cells of the rat. J Physiol. 1990 Aug;427:171–186. doi: 10.1113/jphysiol.1990.sp018166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y., Kimura J. Sodium-calcium exchange current. Dependence on internal Ca and Na and competitive binding of external Na and Ca. J Gen Physiol. 1989 Jun;93(6):1129–1145. doi: 10.1085/jgp.93.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J., Khalil R. A., van Breemen C. Agonist-induced vascular tone. Hypertension. 1989 Jun;13(6 Pt 2):835–844. doi: 10.1161/01.hyp.13.6.835. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987 Apr;252(4 Pt 1):C401–C410. doi: 10.1152/ajpcell.1987.252.4.C401. [DOI] [PubMed] [Google Scholar]
- Palade P. Drug-induced Ca2+ release from isolated sarcoplasmic reticulum. I. Use of pyrophosphate to study caffeine-induced Ca2+ release. J Biol Chem. 1987 May 5;262(13):6135–6141. [PubMed] [Google Scholar]
- Pallotta B. S., Hepler J. R., Oglesby S. A., Harden T. K. A comparison of calcium-activated potassium channel currents in cell-attached and excised patches. J Gen Physiol. 1987 Jun;89(6):985–997. doi: 10.1085/jgp.89.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters G. A., Hlady V., Bridge J. H., Barry W. H. Simultaneous measurement of calcium transients and motion in cultured heart cells. Am J Physiol. 1987 Dec;253(6 Pt 2):H1400–H1408. doi: 10.1152/ajpheart.1987.253.6.H1400. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Steinhardt R., Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986 Aug 22;233(4766):886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Ratz P. H., Flaim S. F. Acetylcholine- and 5-hydroxytryptamine-stimulated contraction and calcium uptake in bovine coronary arteries: evidence for two populations of receptor-operated calcium channels. J Pharmacol Exp Ther. 1985 Sep;234(3):641–647. [PubMed] [Google Scholar]
- Ratz P. H., Flaim S. F. Mechanism of 5-HT contraction in isolated bovine ventricular coronary arteries. Evidence for transient receptor-operated calcium influx channels. Circ Res. 1984 Feb;54(2):135–143. doi: 10.1161/01.res.54.2.135. [DOI] [PubMed] [Google Scholar]
- Rembold C. M. Desensitization of swine arterial smooth muscle to transplasmalemmal Ca2+ influx. J Physiol. 1989 Sep;416:273–290. doi: 10.1113/jphysiol.1989.sp017760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold C. M., Murphy R. A. Myoplasmic [Ca2+] determines myosin phosphorylation in agonist-stimulated swine arterial smooth muscle. Circ Res. 1988 Sep;63(3):593–603. doi: 10.1161/01.res.63.3.593. [DOI] [PubMed] [Google Scholar]
- Scanlon M., Williams D. A., Fay F. S. A Ca2+-insensitive form of fura-2 associated with polymorphonuclear leukocytes. Assessment and accurate Ca2+ measurement. J Biol Chem. 1987 May 5;262(13):6308–6312. [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L., Laughlin M. H., Sturek M. Exercise training alters Ca release from coronary smooth muscle sarcoplasmic reticulum. Am J Physiol. 1990 Aug;259(2 Pt 2):H643–H647. doi: 10.1152/ajpheart.1990.259.2.H643. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L., Sturek M. Spontaneous sarcoplasmic reticulum calcium release and extrusion from bovine, not porcine, coronary artery smooth muscle. J Physiol. 1992;451:49–78. doi: 10.1113/jphysiol.1992.sp019153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumimoto K., Kuriyama H. Mobilization of free Ca2+ measured during contraction-relaxation cycles in smooth muscle cells of the porcine coronary artery using quin2. Pflugers Arch. 1986 Feb;406(2):173–180. doi: 10.1007/BF00586679. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. A., Sturek M., Miller R. J. Measurement of neuronal Ca2+ transients using simultaneous microfluorimetry and electrophysiology. Pflugers Arch. 1988 Jul;412(1-2):216–223. doi: 10.1007/BF00583753. [DOI] [PubMed] [Google Scholar]
- Van Breemen C. Calcium requirement for activation of intact aortic smooth muscle. J Physiol. 1977 Nov;272(2):317–329. doi: 10.1113/jphysiol.1977.sp012046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk A. M., Laird J. D. Characterization of single isolated vascular smooth muscle cells from bovine coronary artery. Blood Vessels. 1984;21(6):267–278. doi: 10.1159/000158529. [DOI] [PubMed] [Google Scholar]
- Wagner-Mann C., Bowman L., Sturek M. Comparison of endothelin- and caffeine-induced calcium mobilization in coronary artery smooth muscle cells. Prog Clin Biol Res. 1990;327:517–524. [PubMed] [Google Scholar]
- Wagner-Mann C., Bowman L., Sturek M. Primary action of endothelin on Ca release in bovine coronary artery smooth muscle cells. Am J Physiol. 1991 Apr;260(4 Pt 1):C763–C770. doi: 10.1152/ajpcell.1991.260.4.C763. [DOI] [PubMed] [Google Scholar]
- Wagner-Mann C., Sturek M. Endothelin mediates Ca influx and release in porcine coronary smooth muscle cells. Am J Physiol. 1991 Apr;260(4 Pt 1):C771–C777. doi: 10.1152/ajpcell.1991.260.4.C771. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Saida K., Yamamoto H., Hwang K., Twort C. Vascular smooth muscle sarcoplasmic reticulum. Function and mechanisms of Ca2+ release. Ann N Y Acad Sci. 1988;522:60–73. doi: 10.1111/j.1749-6632.1988.tb33344.x. [DOI] [PubMed] [Google Scholar]


