Abstract
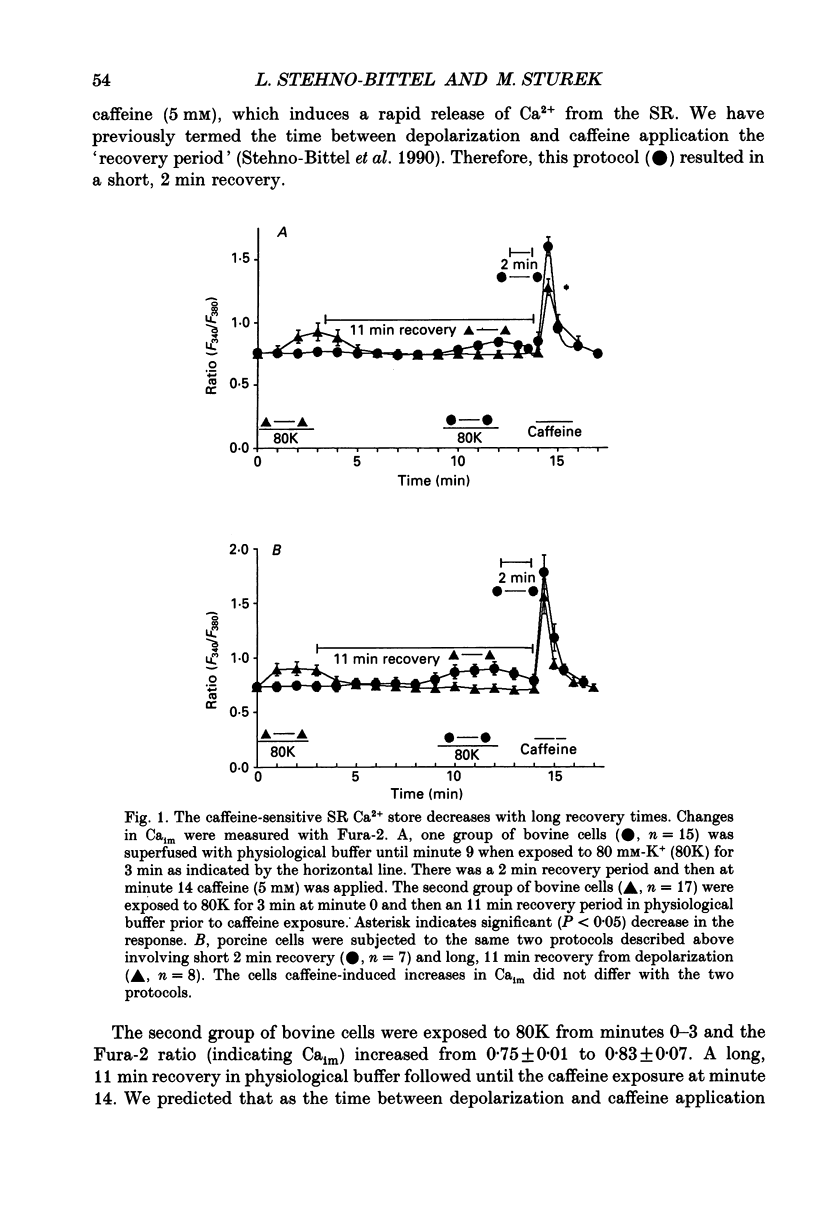
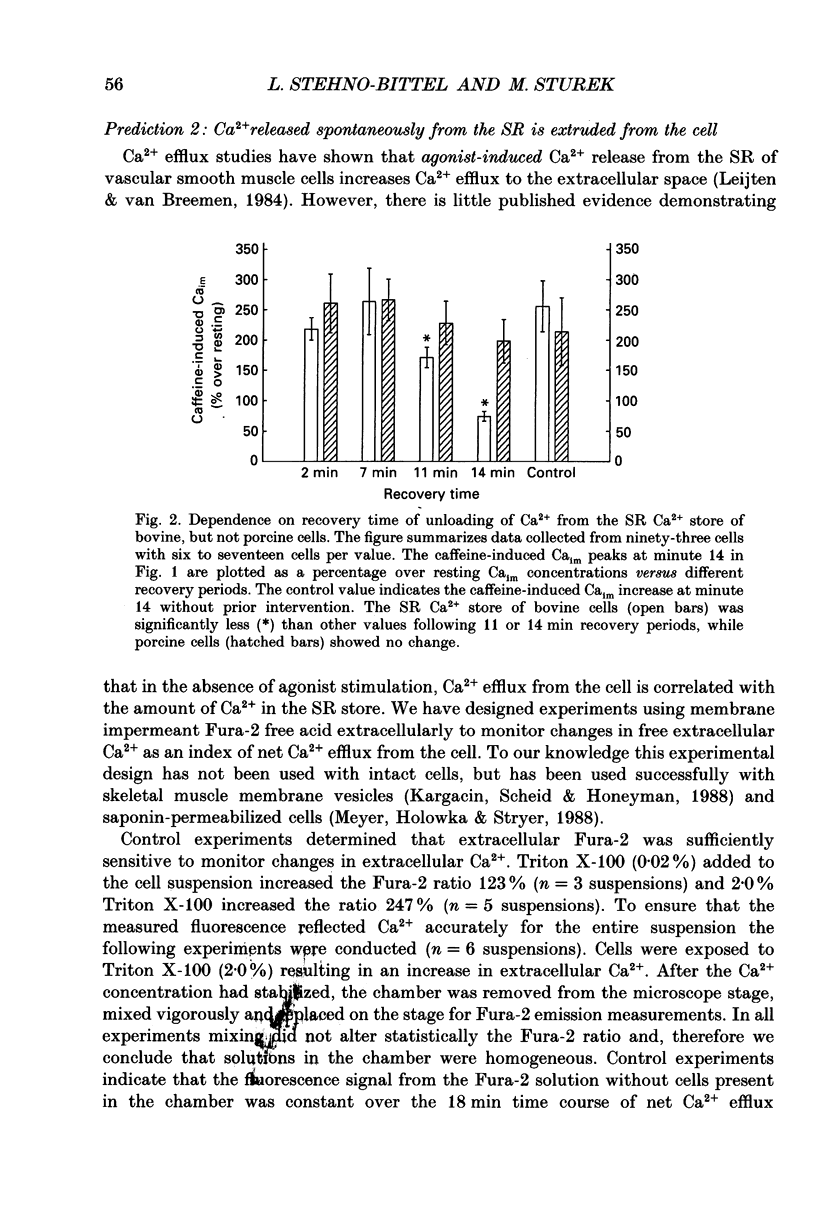
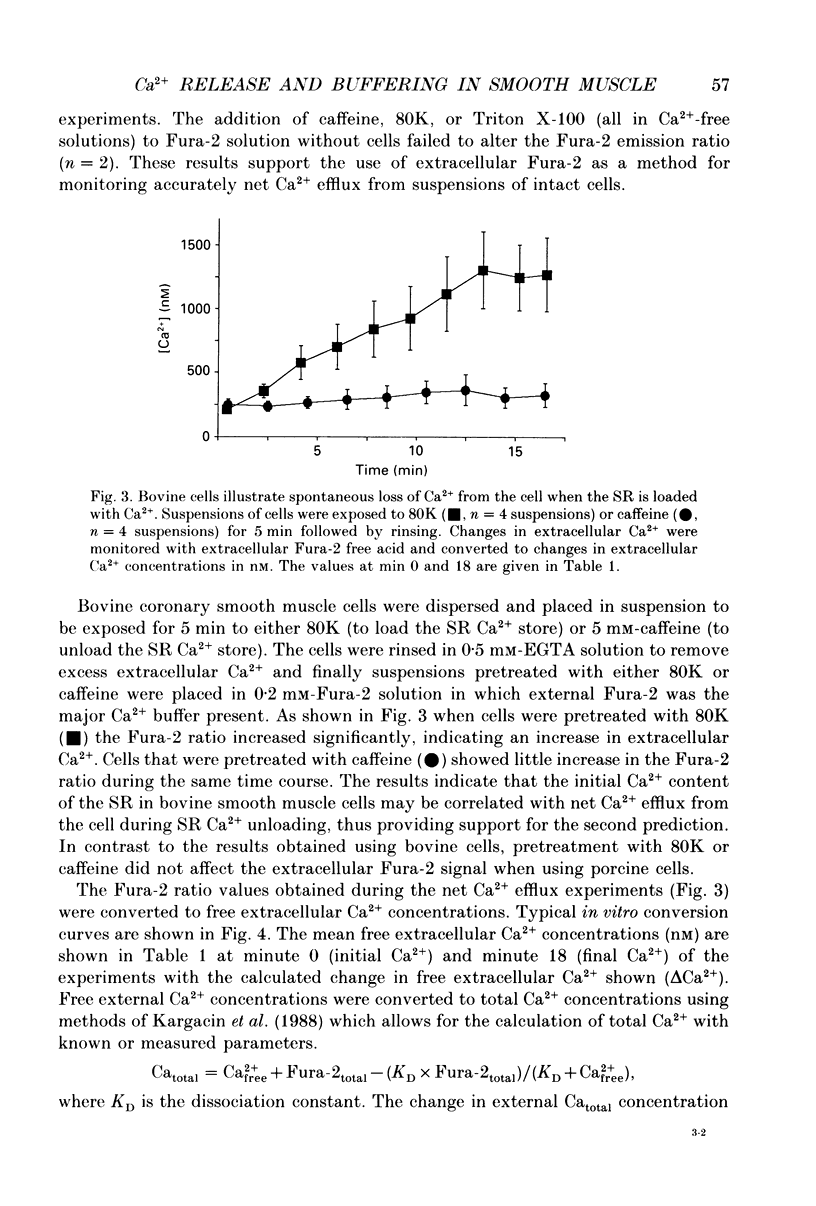
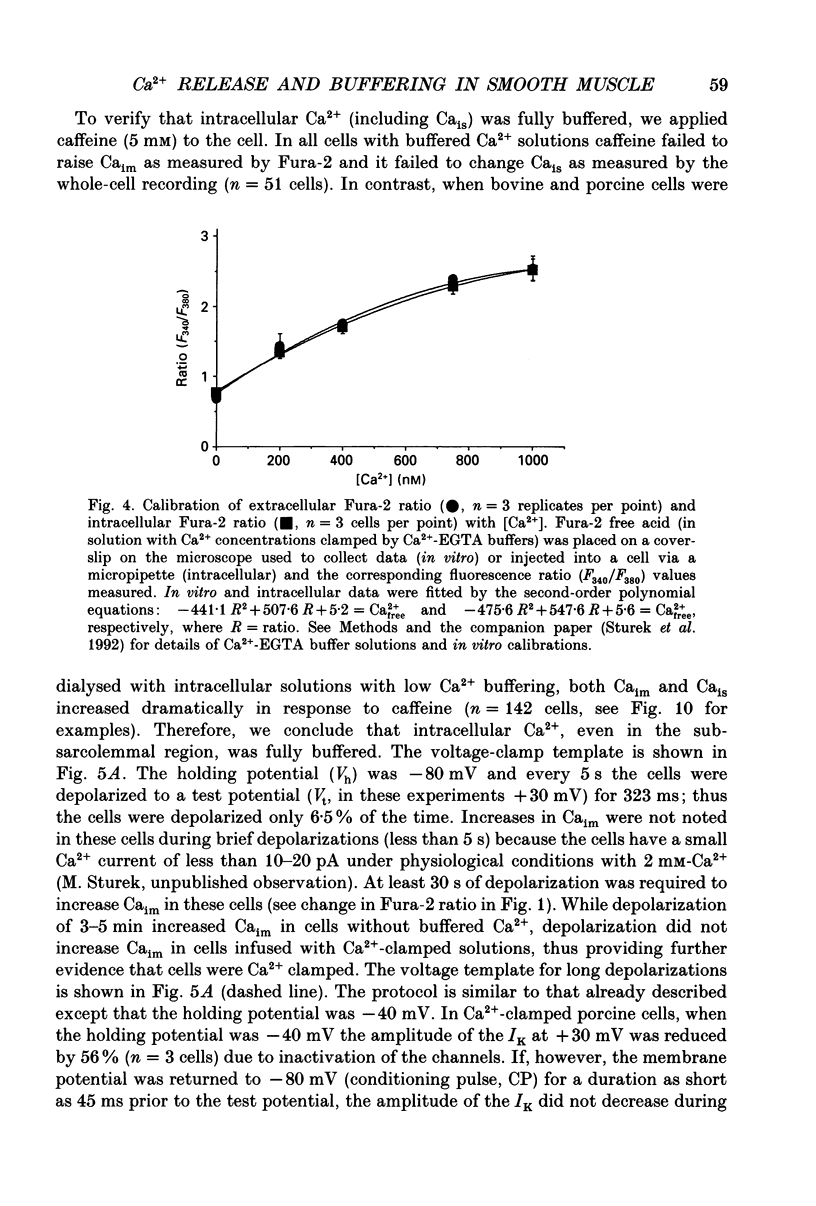
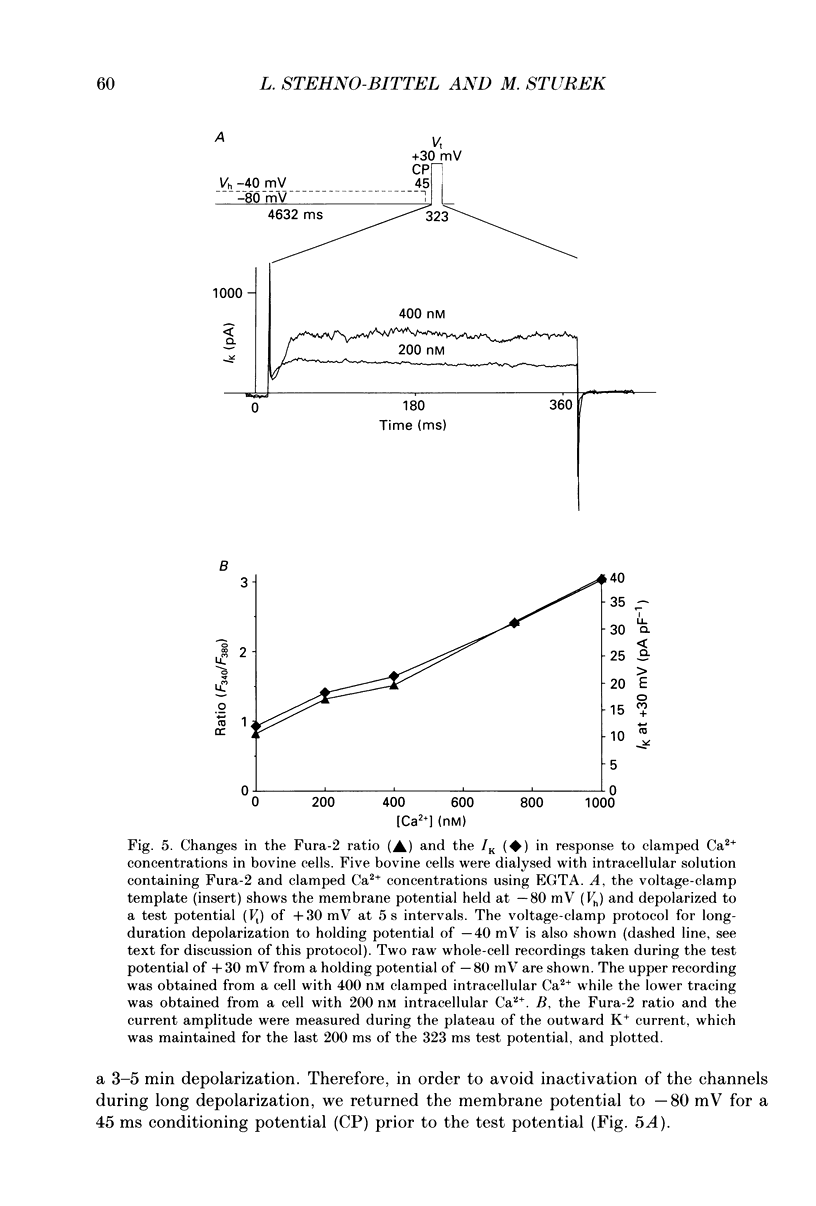
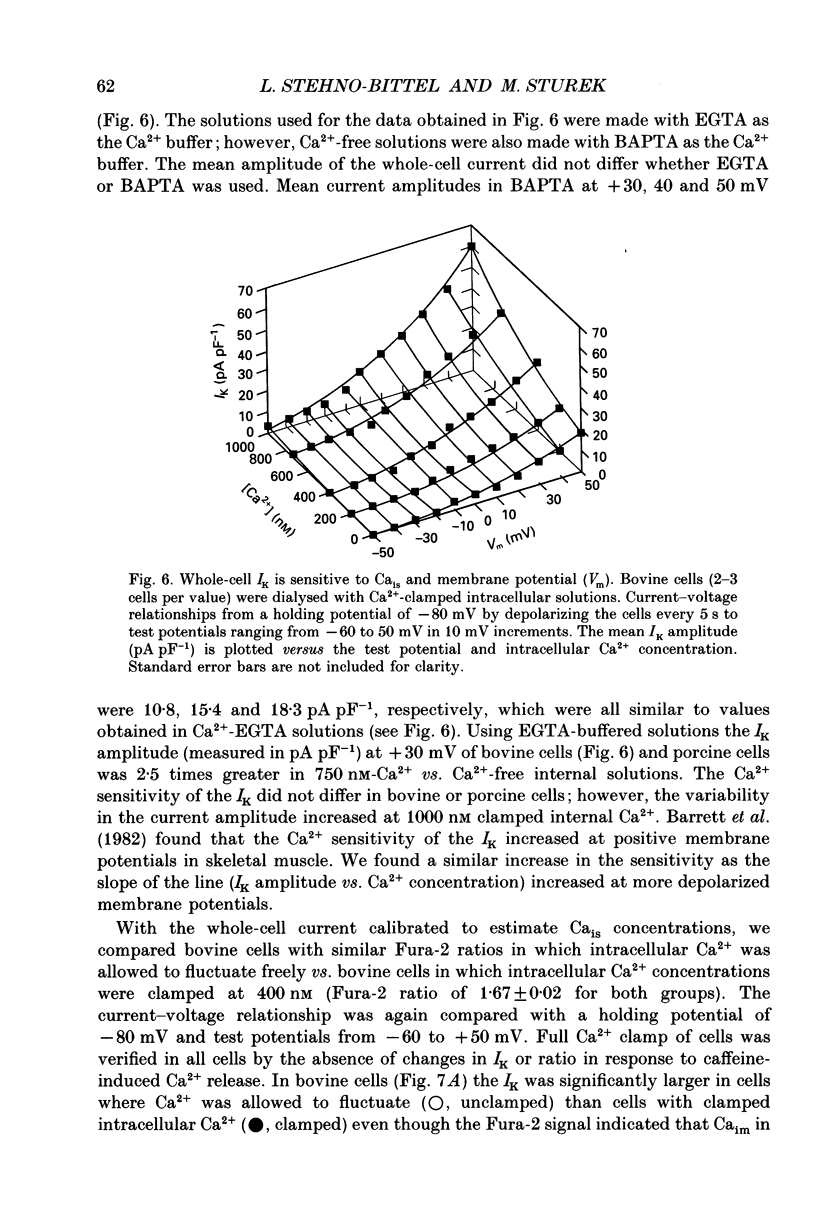
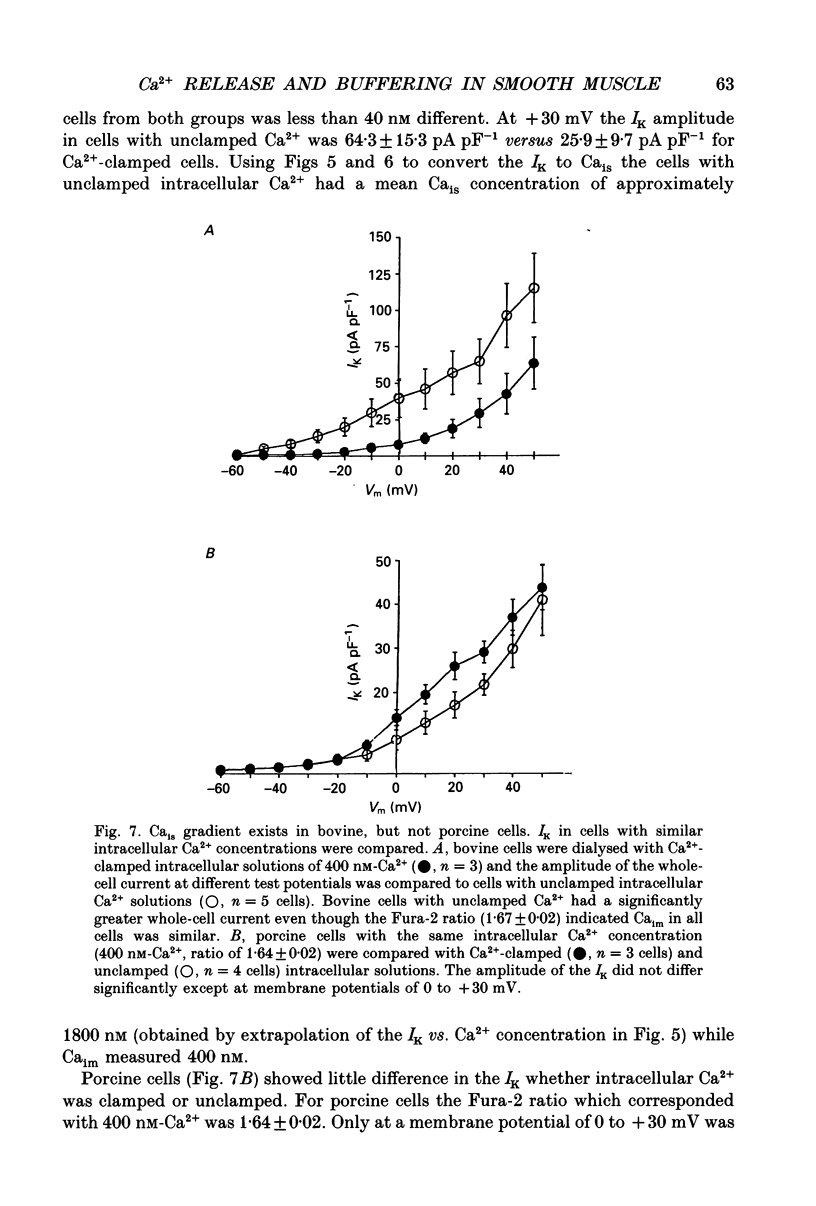
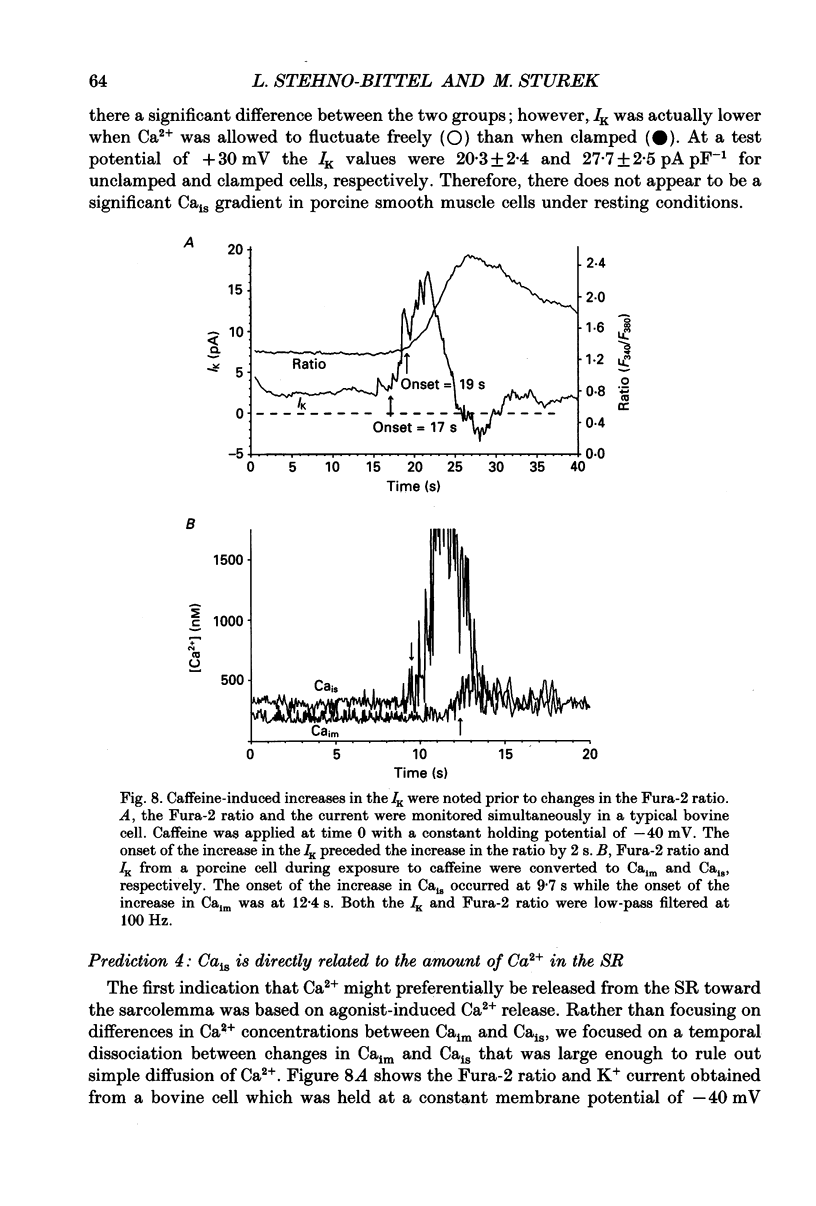
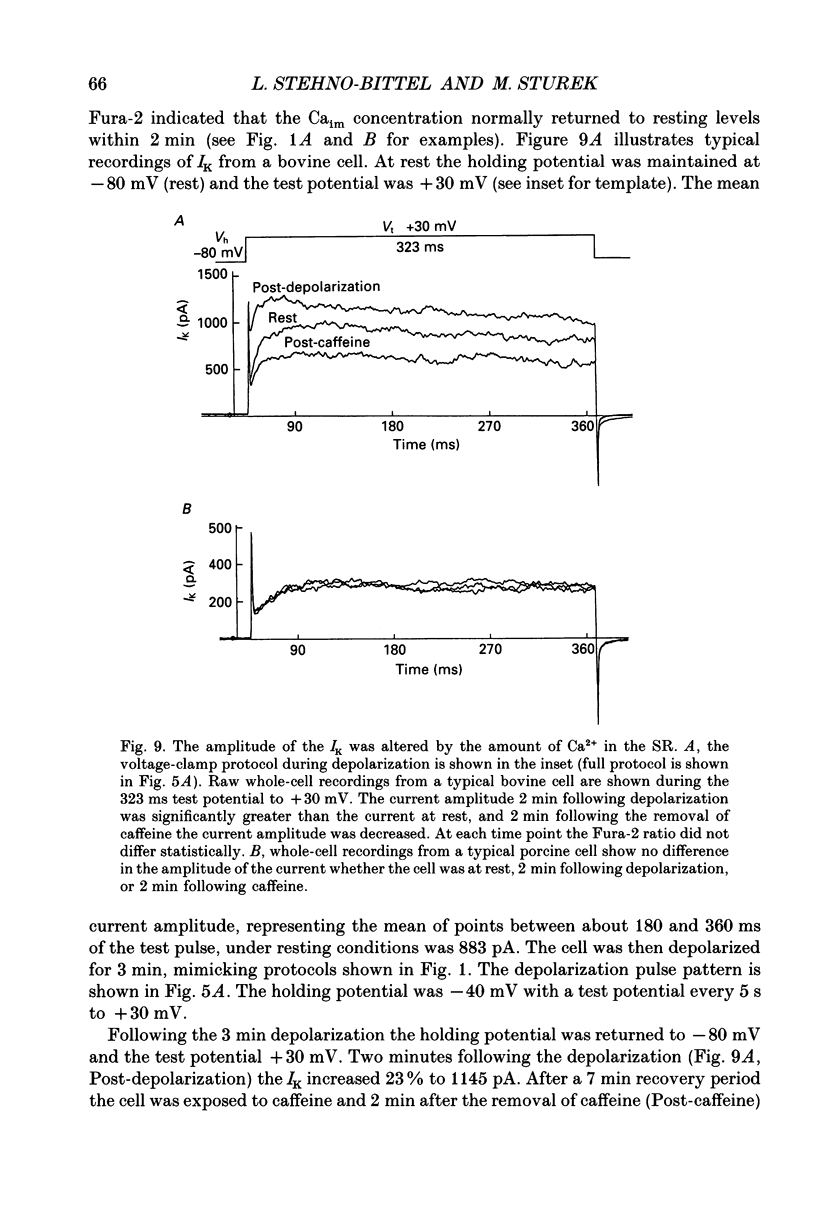
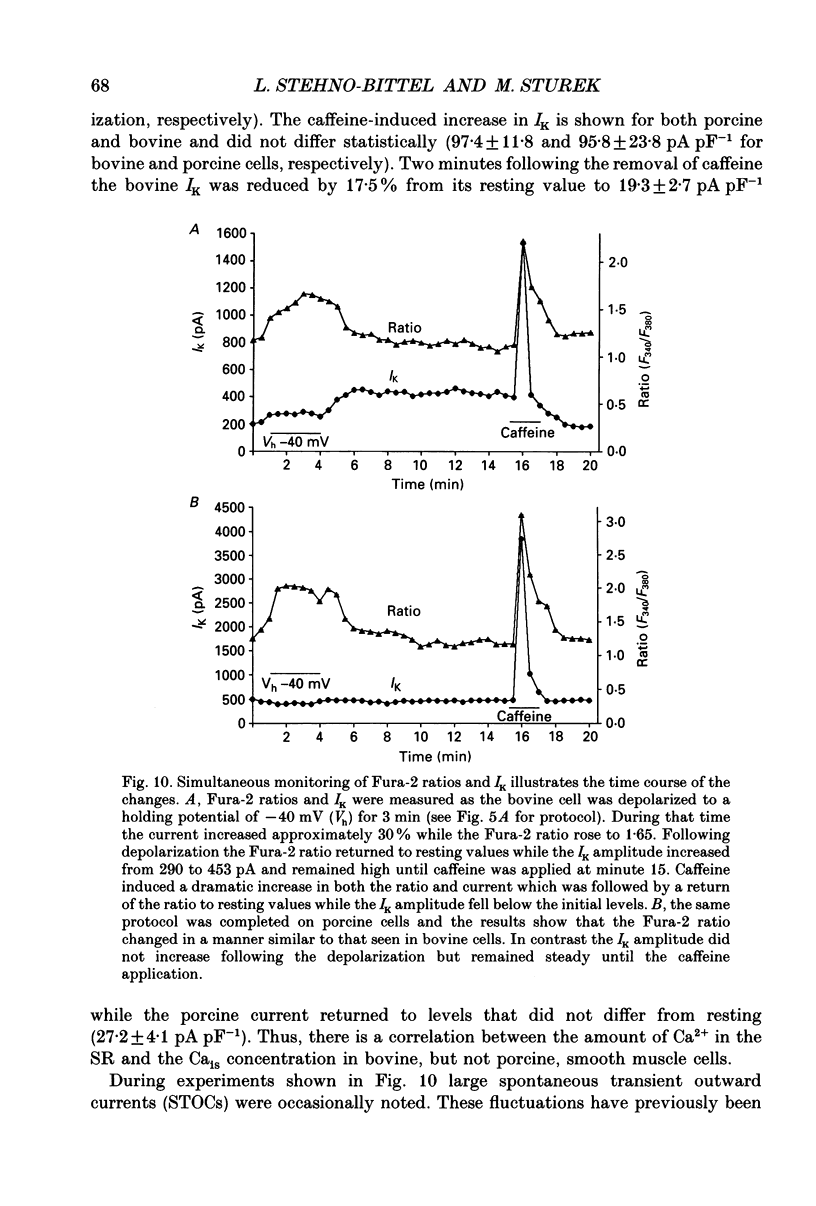
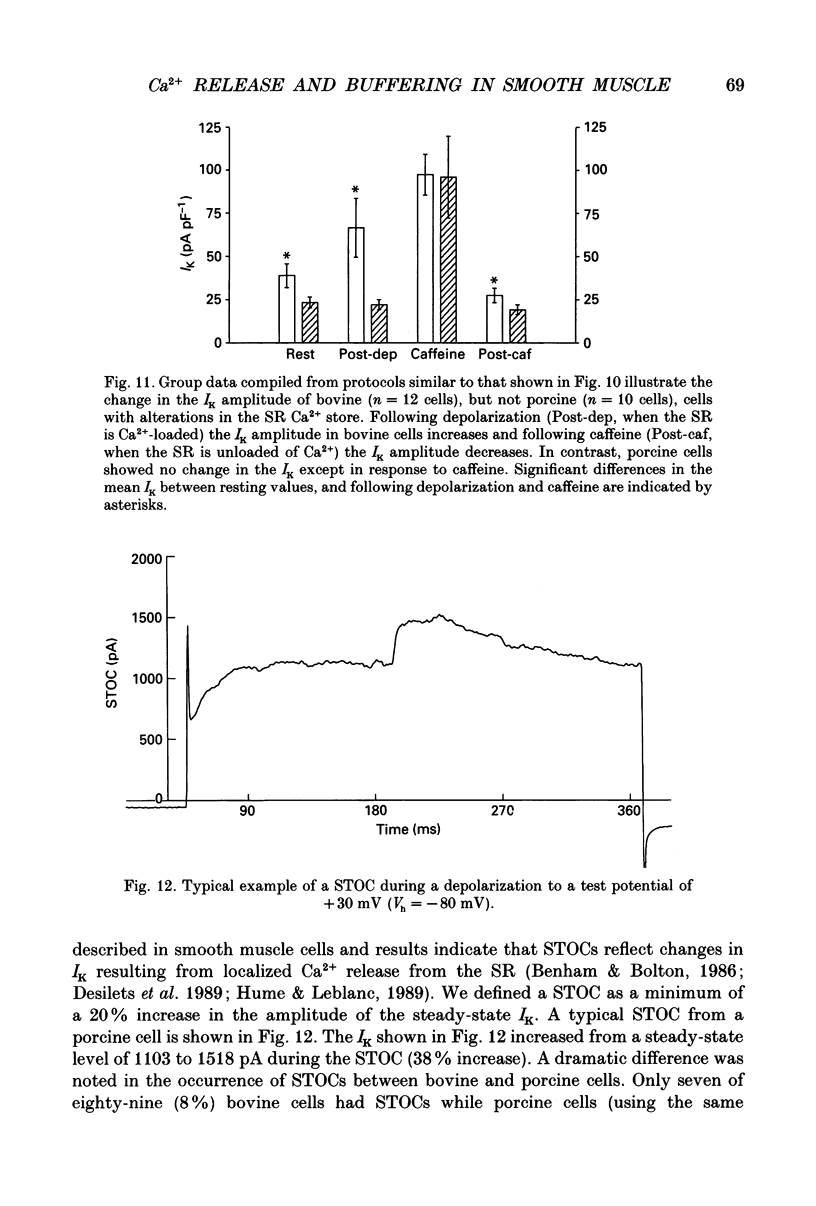
1. We tested the hypothesis that the Ca(2+)-loaded sarcoplasmic reticulum (SR) of coronary artery smooth muscle spontaneously releases Ca2+ preferentially toward the sarcolemma to be extruded from the cell without increasing the average free myoplasmic [Ca2+] (Ca(im)) concentration. 2. The SR of bovine cells was Ca(2+)-loaded by depolarization-induced Ca2+ influx. Release (unloading) of Ca2+ from the SR during recovery from depolarization was determined by Fura-2 microfluorometry of Ca(im). The SR Ca2+ unloading was maximal following a long (14 min) recovery from depolarization, as shown by the 66% decrease in the peak caffeine-induced Ca(im) transient compared to the Ca(im) transient after a short (2 min) recovery. No increase in Ca(im) occurred during the long recovery. No unloading of the SR Ca2+ store was noted in porcine cells. 3. Approximately 80% of the outward K+ current in bovine and porcine cells was sensitive to subsarcolemmal Ca2+ (Ca(is)) concentrations. Whole-cell voltage clamp using pipette solutions with Ca2+ concentrations clamped between 0 and 1000 nM with Ca(2+)-EGTA or Ca(2+)-BAPTA buffers showed increasing K+ currents (normalized for cell membrane surface area) as a function of both membrane potential and Ca(is). Clamping of Ca(im) and Ca(is) was verified by the lack of changes in K+ current and Fura-2 ratio in response to Ca2+ influx, Ca(2+)-free external solution, or caffeine-induced Ca2+ release. At +30 to +50 mV the K+ current amplitude showed a similar sensitivity to Ca2+ as Fura-2. These data indicate that in this experimental preparation Ca(2+)-activated K+ current is a valid estimate of Ca(is). 4. Simultaneous Ca(im) and Ca(is) measurements in bovine cells which were not Ca(2+)-clamped (2 x 10(-4) M-EGTA pipette solution) showed that during the long recovery period the K+ current (reflecting Ca(is)) increased 55%, while Ca(im) did not change. 5. In quiescent bovine cells the Ca(is) was higher than Ca(im), while the higher resting Ca(is) gradient was not apparent in porcine cells. 6. The Ca(is) concentration was directly related to the amount of Ca2+ in the SR in bovine, but not porcine cells. Depletion of the SR in bovine cells by caffeine resulted in a 58% decrease in K+ current compared to the resting K+ current. 7. Caffeine-induced Ca2+ release caused an increase in Ca(is) which preceded the increase in Ca(im) by approximately 2 s.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M., Shuman H., Somlyo A. P., Somlyo A. V. Total cytoplasmic calcium in relaxed and maximally contracted rabbit portal vein smooth muscle. J Physiol. 1984 Dec;357:185–201. doi: 10.1113/jphysiol.1984.sp015496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne N. G., Large W. A. Membrane ionic mechanisms activated by noradrenaline in cells isolated from the rabbit portal vein. J Physiol. 1988 Oct;404:557–573. doi: 10.1113/jphysiol.1988.sp017306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deth R., Casteels R. A study of releasable Ca fractions in smooth muscle cells of the rabbit aorta. J Gen Physiol. 1977 Apr;69(4):401–416. doi: 10.1085/jgp.69.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Désilets M., Driska S. P., Baumgarten C. M. Current fluctuations and oscillations in smooth muscle cells from hog carotid artery. Role of the sarcoplasmic reticulum. Circ Res. 1989 Sep;65(3):708–722. doi: 10.1161/01.res.65.3.708. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Foskett J. K., Gunter-Smith P. J., Melvin J. E., Turner R. J. Physiological localization of an agonist-sensitive pool of Ca2+ in parotid acinar cells. Proc Natl Acad Sci U S A. 1989 Jan;86(1):167–171. doi: 10.1073/pnas.86.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hume J. R., Leblanc N. Macroscopic K+ currents in single smooth muscle cells of the rabbit portal vein. J Physiol. 1989 Jun;413:49–73. doi: 10.1113/jphysiol.1989.sp017641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 1989 Aug;94(2):363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargacin M. E., Scheid C. R., Honeyman T. W. Continuous monitoring of Ca2+ uptake in membrane vesicles with fura-2. Am J Physiol. 1988 Nov;255(5 Pt 1):C694–C698. doi: 10.1152/ajpcell.1988.255.5.C694. [DOI] [PubMed] [Google Scholar]
- Leijten P. A., van Breemen C. The effects of caffeine on the noradrenaline-sensitive calcium store in rabbit aorta. J Physiol. 1984 Dec;357:327–339. doi: 10.1113/jphysiol.1984.sp015502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijten P. A., van Breemen C. The relationship between noradrenaline-induced contraction and 45Ca efflux stimulation in rabbit mesenteric artery. Br J Pharmacol. 1986 Dec;89(4):739–747. doi: 10.1111/j.1476-5381.1986.tb11178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988 Apr 29;240(4852):653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Missiaen L., Declerck I., Droogmans G., Plessers L., De Smedt H., Raeymaekers L., Casteels R. Agonist-dependent Ca2+ and Mn2+ entry dependent on state of filling of Ca2+ stores in aortic smooth muscle cells of the rat. J Physiol. 1990 Aug;427:171–186. doi: 10.1113/jphysiol.1990.sp018166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Suzuki S., Sugi H. Physiological and ultrastructural studies on the mechanism of stretch-induced contractile activation in rabbit cerebral artery smooth muscle. Jpn J Physiol. 1986;36(4):745–760. doi: 10.2170/jjphysiol.36.745. [DOI] [PubMed] [Google Scholar]
- Nishimura J., Khalil R. A., van Breemen C. Agonist-induced vascular tone. Hypertension. 1989 Jun;13(6 Pt 2):835–844. doi: 10.1161/01.hyp.13.6.835. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987 Apr;252(4 Pt 1):C401–C410. doi: 10.1152/ajpcell.1987.252.4.C401. [DOI] [PubMed] [Google Scholar]
- Pallotta B. S., Hepler J. R., Oglesby S. A., Harden T. K. A comparison of calcium-activated potassium channel currents in cell-attached and excised patches. J Gen Physiol. 1987 Jun;89(6):985–997. doi: 10.1085/jgp.89.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold C. M. Desensitization of swine arterial smooth muscle to transplasmalemmal Ca2+ influx. J Physiol. 1989 Sep;416:273–290. doi: 10.1113/jphysiol.1989.sp017760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J., Akaike N., Tomoike H., Kanaide H., Nakamura M. Ca-activated K channel in cultured smooth muscle cells of rat aortic media. Am J Physiol. 1988 Sep;255(3 Pt 2):H410–H418. doi: 10.1152/ajpheart.1988.255.3.H410. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Shuman H., Sloane B., Scarpa A. Electron probe analysis of calcium compartments in cryo sections of smooth and striated muscles. Ann N Y Acad Sci. 1978 Apr 28;307:523–544. doi: 10.1111/j.1749-6632.1978.tb41980.x. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L., Laughlin M. H., Sturek M. Exercise training alters Ca release from coronary smooth muscle sarcoplasmic reticulum. Am J Physiol. 1990 Aug;259(2 Pt 2):H643–H647. doi: 10.1152/ajpheart.1990.259.2.H643. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L., Laughlin M. H., Sturek M. Exercise training depletes sarcoplasmic reticulum calcium in coronary smooth muscle. J Appl Physiol (1985) 1991 Nov;71(5):1764–1773. doi: 10.1152/jappl.1991.71.5.1764. [DOI] [PubMed] [Google Scholar]
- Sturek M., Kunda K., Hu Q. Sarcoplasmic reticulum buffering of myoplasmic calcium in bovine coronary artery smooth muscle. J Physiol. 1992;451:25–48. doi: 10.1113/jphysiol.1992.sp019152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi H., Daimon T. Translocation of intracellularly stored calcium during the contraction-relaxation cycle in guinea pig taenia coli. Nature. 1977 Sep 29;269(5627):436–438. doi: 10.1038/269436a0. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Sturek M., Miller R. J. Measurement of neuronal Ca2+ transients using simultaneous microfluorimetry and electrophysiology. Pflugers Arch. 1988 Jul;412(1-2):216–223. doi: 10.1007/BF00583753. [DOI] [PubMed] [Google Scholar]
- Wagner-Mann C., Bowman L., Sturek M. Primary action of endothelin on Ca release in bovine coronary artery smooth muscle cells. Am J Physiol. 1991 Apr;260(4 Pt 1):C763–C770. doi: 10.1152/ajpcell.1991.260.4.C763. [DOI] [PubMed] [Google Scholar]
- Wagner-Mann C., Sturek M. Endothelin mediates Ca influx and release in porcine coronary smooth muscle cells. Am J Physiol. 1991 Apr;260(4 Pt 1):C771–C777. doi: 10.1152/ajpcell.1991.260.4.C771. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. An apical-membrane chloride channel in human tracheal epithelium. Science. 1986 Jun 27;232(4758):1648–1650. doi: 10.1126/science.2424085. [DOI] [PubMed] [Google Scholar]
- Wilde D. W., Lee K. S. Outward potassium currents in freshly isolated smooth muscle cell of dog coronary arteries. Circ Res. 1989 Dec;65(6):1718–1734. doi: 10.1161/01.res.65.6.1718. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]


