Abstract
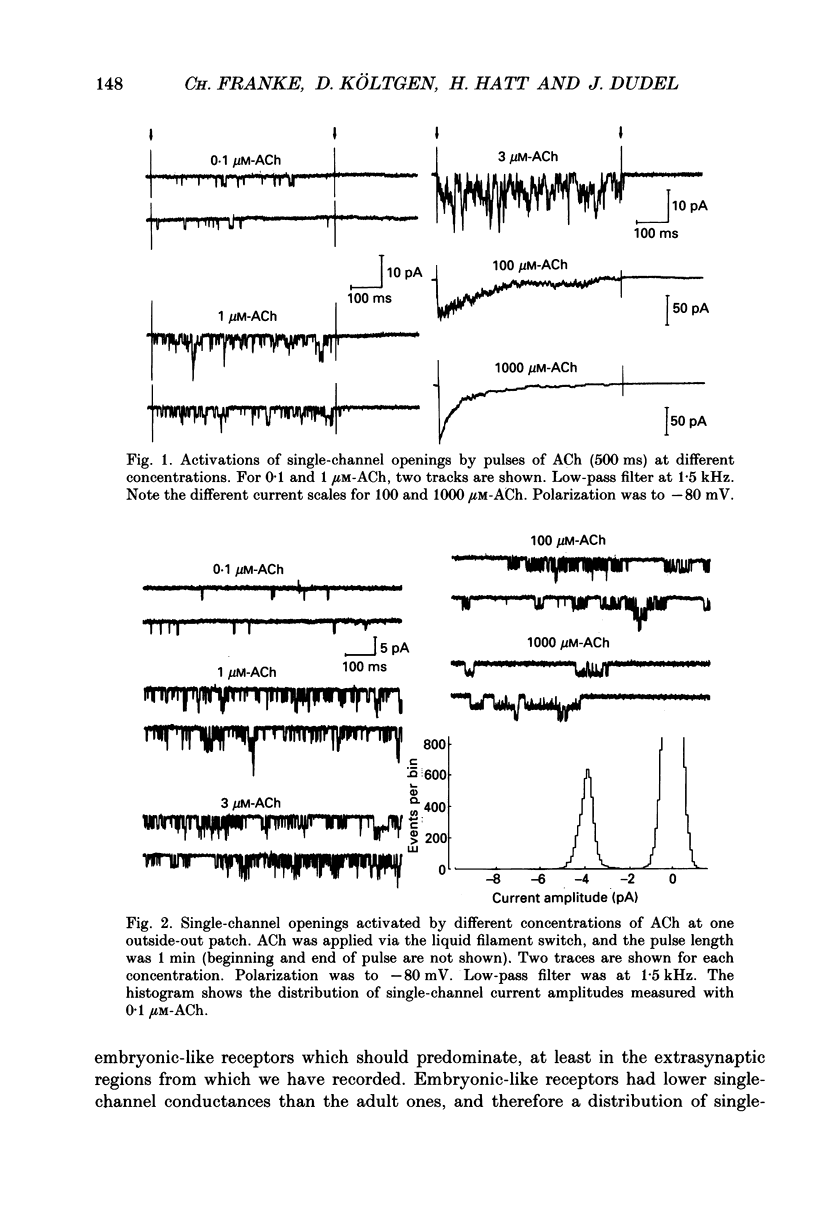
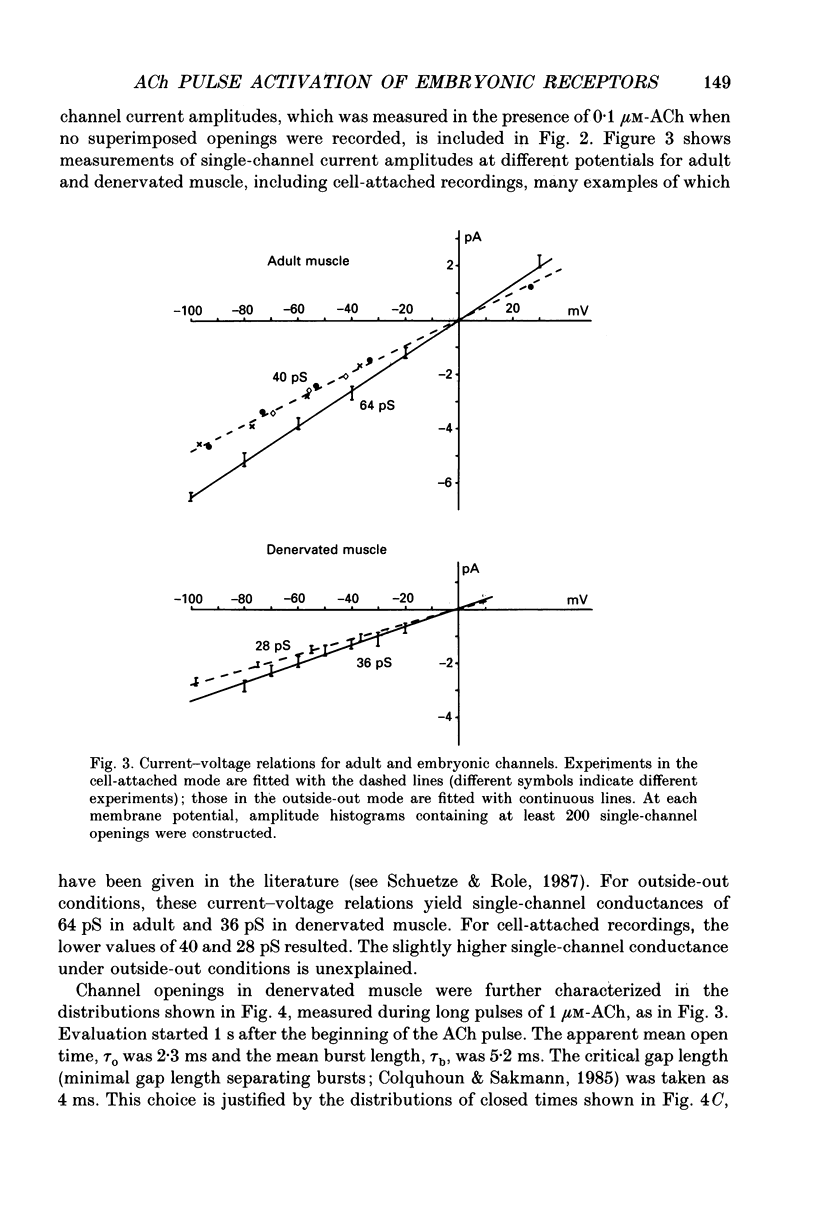
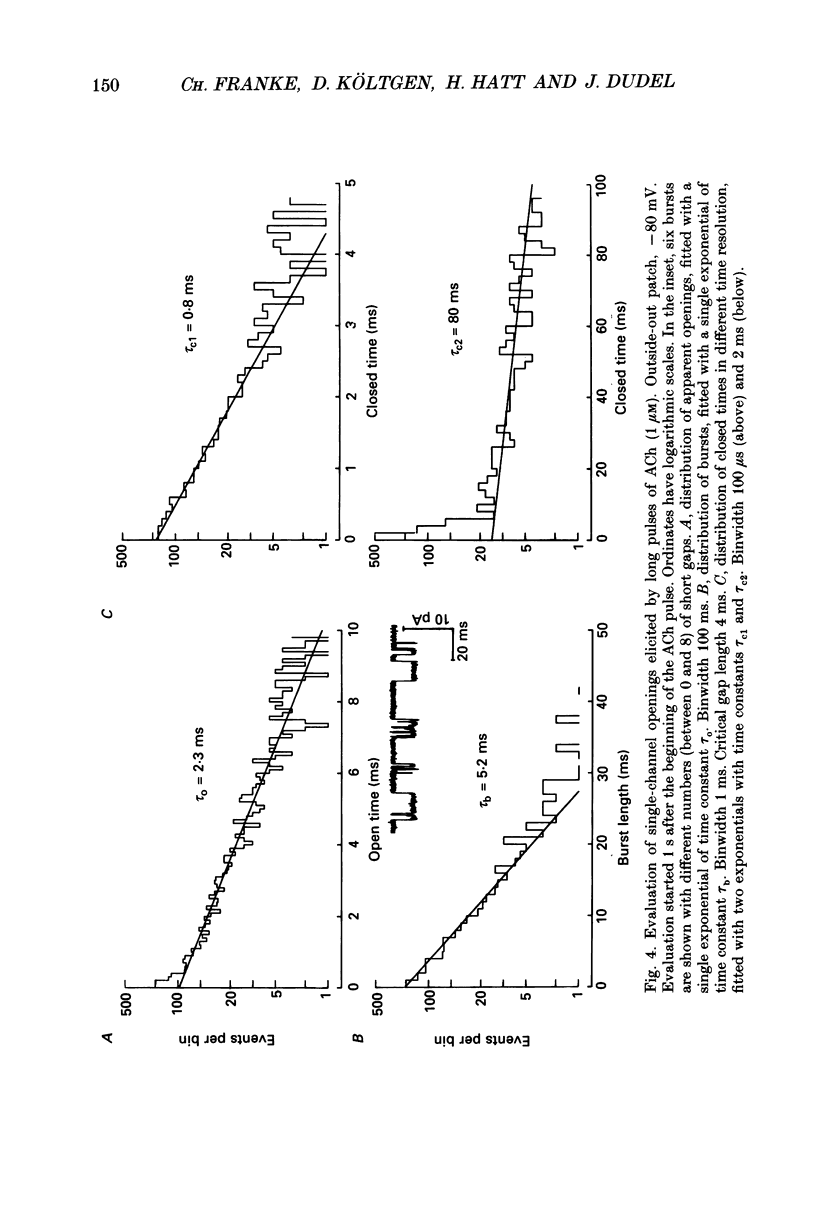
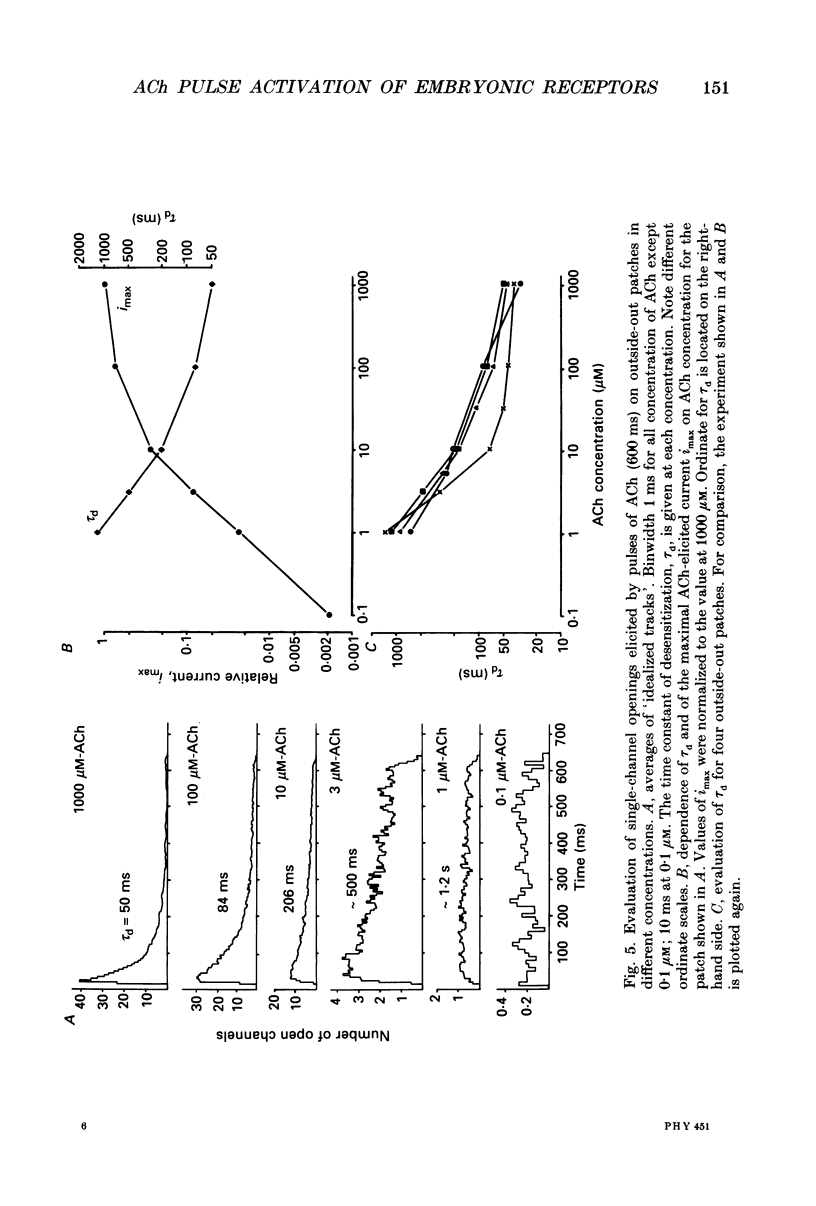
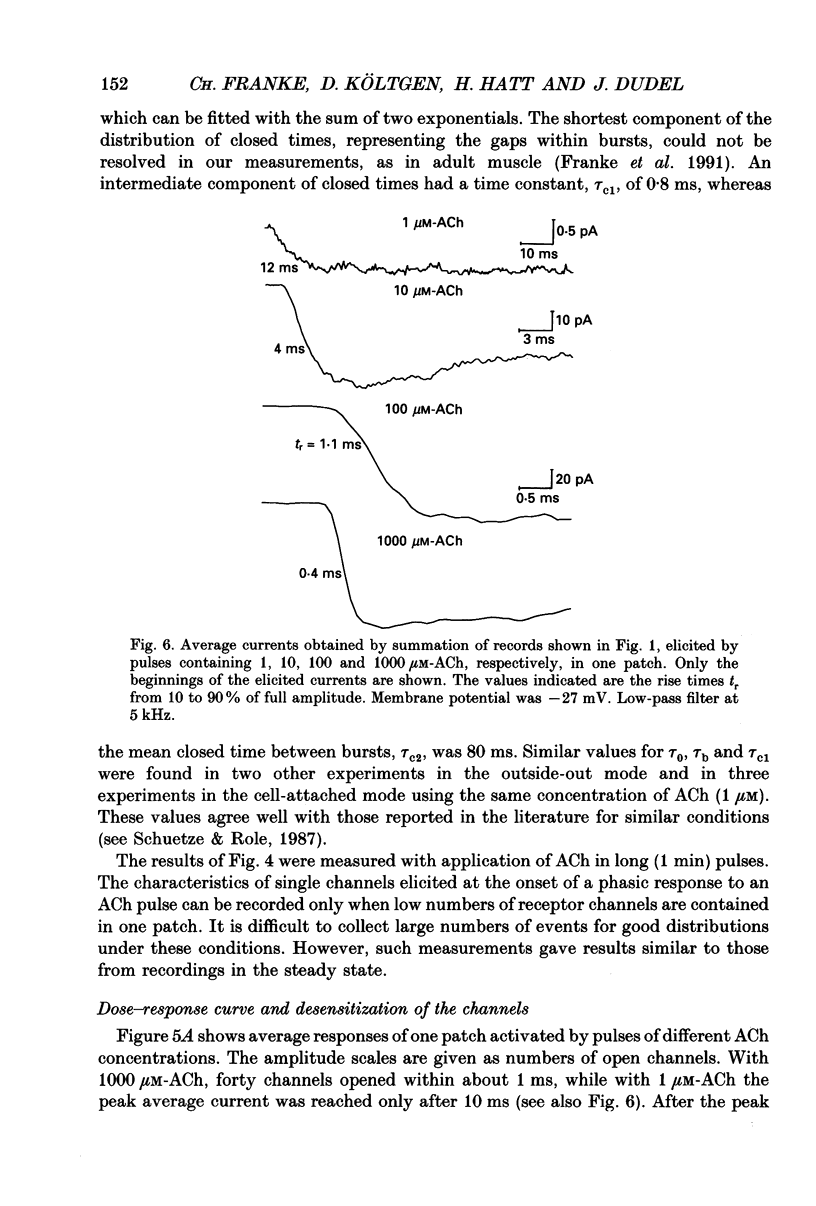
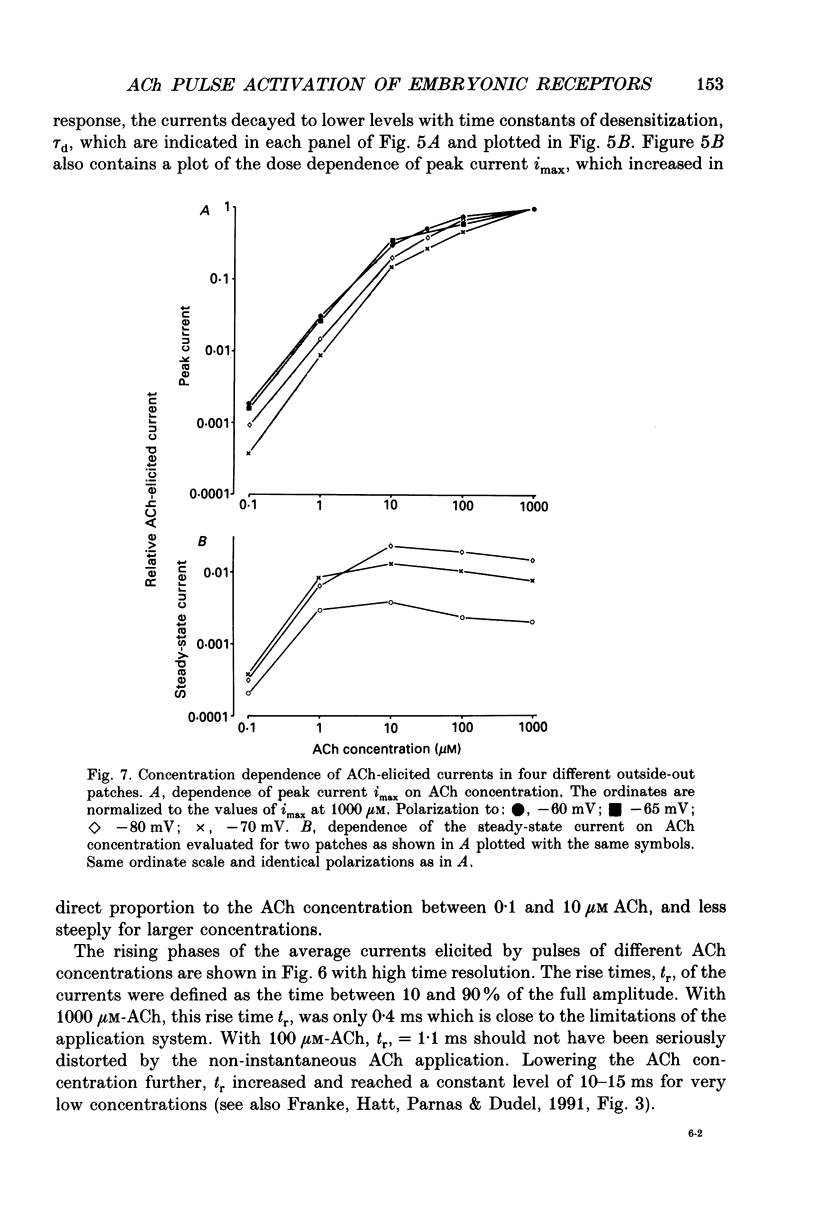
1. Pulses of acetylcholine (ACh) in concentrations between 0.1 and 1000 microM were applied repetitively to outside-out patches of enzymatically denervated (14 days) mouse muscle with the liquid filament switch. Solutions superfusing the patch could be changed rapidly (within 0.2 ms). 2. Single-channel activity was studied under steady-state conditions in the outside-out and in the cell-attached mode. The single-channel conductance was 26 pS in outside-out patches, characteristic for embryonic-like channels. Apparent mean open time was about 2.5 ms, a shorter component of closed times was 800 microseconds and burst length was about 5 ms. 3. Channel currents elicited by pulses of ACh were averaged. The time-to-peak current was concentration dependent and decreased from a level of about 10 ms below 10 microM to about 400 microseconds at 100 microM-ACh. 4. For a typical experiment, the average peak current, imax, increased from -0.4 pA with 0.1 microM to -82 pA with 1000 microM-ACh, close to the value at saturation. The half-maximal response was at 60 microM-ACh. The dose-response curves for imax had double-logarithmic slopes of 1.1-1.3, consistent with two binding sites at the embryonic nicotinic acetylcholine receptor (nAChR). 5. The current elicited by ACh pulses decreased rapidly after the peak. The time constant of desensitization increased from 20-50 ms with 1000 microM-ACh to up to more than a second with 1 microM-ACh. 6. The current in steady state (fully desensitized) increased up to 10 microM-ACh, but decreased slightly to values of imax/100 to imax/500 when higher concentrations were applied. 7. In addition to the well-known differences between adult and embryonic nAChR concerning the apparent mean open time and burst length, we found differences in the slope of the dose-response curve for imax, in the ratio of peak to steady-state response, and in the rise time of the response.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brehm P., Kidokoro Y., Moody-Corbett F. Acetylcholine receptor channel properties during development of Xenopus muscle cells in culture. J Physiol. 1984 Dec;357:203–217. doi: 10.1113/jphysiol.1984.sp015497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Kullberg R. Acetylcholine receptor channels on adult mouse skeletal muscle are functionally identical in synaptic and nonsynaptic membrane. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2550–2554. doi: 10.1073/pnas.84.8.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H. R., Sakmann B. Gating properties of acetycholine receptor in newly formed neuromuscular synapses. Nature. 1978 Jan 26;271(5643):366–368. doi: 10.1038/271366a0. [DOI] [PubMed] [Google Scholar]
- Brett R. S., Dilger J. P., Adams P. R., Lancaster B. A method for the rapid exchange of solutions bathing excised membrane patches. Biophys J. 1986 Nov;50(5):987–992. doi: 10.1016/S0006-3495(86)83539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes J. P., Hall Z. W. Acetylcholine receptors in normal and denervated rat diaphragm muscle. II. Comparison of junctional and extrajunctional receptors. Biochemistry. 1975 May 20;14(10):2100–2106. doi: 10.1021/bi00681a009. [DOI] [PubMed] [Google Scholar]
- Cachelin A. B., Colquhoun D. Desensitization of the acetylcholine receptor of frog end-plates measured in a Vaseline-gap voltage clamp. J Physiol. 1989 Aug;415:159–188. doi: 10.1113/jphysiol.1989.sp017717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Ogden D. C. Activation of ion channels in the frog end-plate by high concentrations of acetylcholine. J Physiol. 1988 Jan;395:131–159. doi: 10.1113/jphysiol.1988.sp016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger J. P., Brett R. S. Direct measurement of the concentration- and time-dependent open probability of the nicotinic acetylcholine receptor channel. Biophys J. 1990 Apr;57(4):723–731. doi: 10.1016/S0006-3495(90)82593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J., Franke C., Hatt H. Rapid activation, desensitization, and resensitization of synaptic channels of crayfish muscle after glutamate pulses. Biophys J. 1990 Mar;57(3):533–545. doi: 10.1016/S0006-3495(90)82569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J., Franke C. Single glutamate-gated synaptic channels at the crayfish neuromuscular junction. II. Dependence of channel open time on glutamate concentration. Pflugers Arch. 1987 Mar;408(3):307–314. doi: 10.1007/BF02181474. [DOI] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Schuetze S. M. A post-natal decrease in acetylcholine channel open time at rat end-plates. J Physiol. 1980 Jun;303:125–137. doi: 10.1113/jphysiol.1980.sp013275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C., Hatt H., Dudel J. Liquid filament switch for ultra-fast exchanges of solutions at excised patches of synaptic membrane of crayfish muscle. Neurosci Lett. 1987 Jun 15;77(2):199–204. doi: 10.1016/0304-3940(87)90586-6. [DOI] [PubMed] [Google Scholar]
- Franke C., Hatt H., Dudel J. Steep concentration dependence and fast desensitization of nicotinic channel currents elicited by acetylcholine pulses, studied in adult vertebrate muscle. Pflugers Arch. 1991 Jan;417(5):509–516. doi: 10.1007/BF00370947. [DOI] [PubMed] [Google Scholar]
- Franke C., Hatt H., Parnas H., Dudel J. Kinetic constants of the acetylcholine (ACh) receptor reaction deduced from the rise in open probability after steps in ACh concentration. Biophys J. 1991 Nov;60(5):1008–1016. doi: 10.1016/S0006-3495(91)82138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Franco A., Jr, Gardner P. D., Lansman J. B., Forsayeth J. R., Hall Z. W. Properties of embryonic and adult muscle acetylcholine receptors transiently expressed in COS cells. Neuron. 1990 Aug;5(2):147–157. doi: 10.1016/0896-6273(90)90305-y. [DOI] [PubMed] [Google Scholar]
- Gu Y., Hall Z. W. Immunological evidence for a change in subunits of the acetylcholine receptor in developing and denervated rat muscle. Neuron. 1988 Apr;1(2):117–125. doi: 10.1016/0896-6273(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Sakmann B. Multiple conductance states of single acetylcholine receptor channels in embryonic muscle cells. Nature. 1981 Dec 3;294(5840):462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- Jaramillo F., Schuetze S. M. Kinetic differences between embryonic- and adult-type acetylcholine receptors in rat myotubes. J Physiol. 1988 Feb;396:267–296. doi: 10.1113/jphysiol.1988.sp016962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo F., Vicini S., Schuetze S. M. Embryonic acetylcholine receptors guarantee spontaneous contractions in rat developing muscle. Nature. 1988 Sep 1;335(6185):66–68. doi: 10.1038/335066a0. [DOI] [PubMed] [Google Scholar]
- Költgen D., Brinkmeier H., Jockusch H. Myotonia and neuromuscular transmission in the mouse. Muscle Nerve. 1991 Aug;14(8):775–780. doi: 10.1002/mus.880140813. [DOI] [PubMed] [Google Scholar]
- Land B. R., Salpeter E. E., Salpeter M. M. Kinetic parameters for acetylcholine interaction in intact neuromuscular junction. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7200–7204. doi: 10.1073/pnas.78.11.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. J., Nakajima S., Nakajima Y., Carlson C. G. Early development of two types of nicotinic acetylcholine receptors. J Neurosci. 1988 Nov;8(11):4038–4048. doi: 10.1523/JNEUROSCI.08-11-04038.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Maconochie D. J., Knight D. E. A method for making solution changes in the sub-millisecond range at the tip of a patch pipette. Pflugers Arch. 1989 Sep;414(5):589–596. doi: 10.1007/BF00580996. [DOI] [PubMed] [Google Scholar]
- Mishina M., Takai T., Imoto K., Noda M., Takahashi T., Numa S., Methfessel C., Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986 May 22;321(6068):406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Parnas H., Flashner M., Spira M. E. Sequential model to describe the nicotinic synaptic current. Biophys J. 1989 May;55(5):875–884. doi: 10.1016/S0006-3495(89)82886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Schuetze S. M., Role L. W. Developmental regulation of nicotinic acetylcholine receptors. Annu Rev Neurosci. 1987;10:403–457. doi: 10.1146/annurev.ne.10.030187.002155. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by high concentrations of agonist. J Physiol. 1987 Apr;385:325–359. doi: 10.1113/jphysiol.1987.sp016496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by low concentrations of agonist. J Physiol. 1986 Apr;373:129–162. doi: 10.1113/jphysiol.1986.sp016039. [DOI] [PMC free article] [PubMed] [Google Scholar]


