Abstract
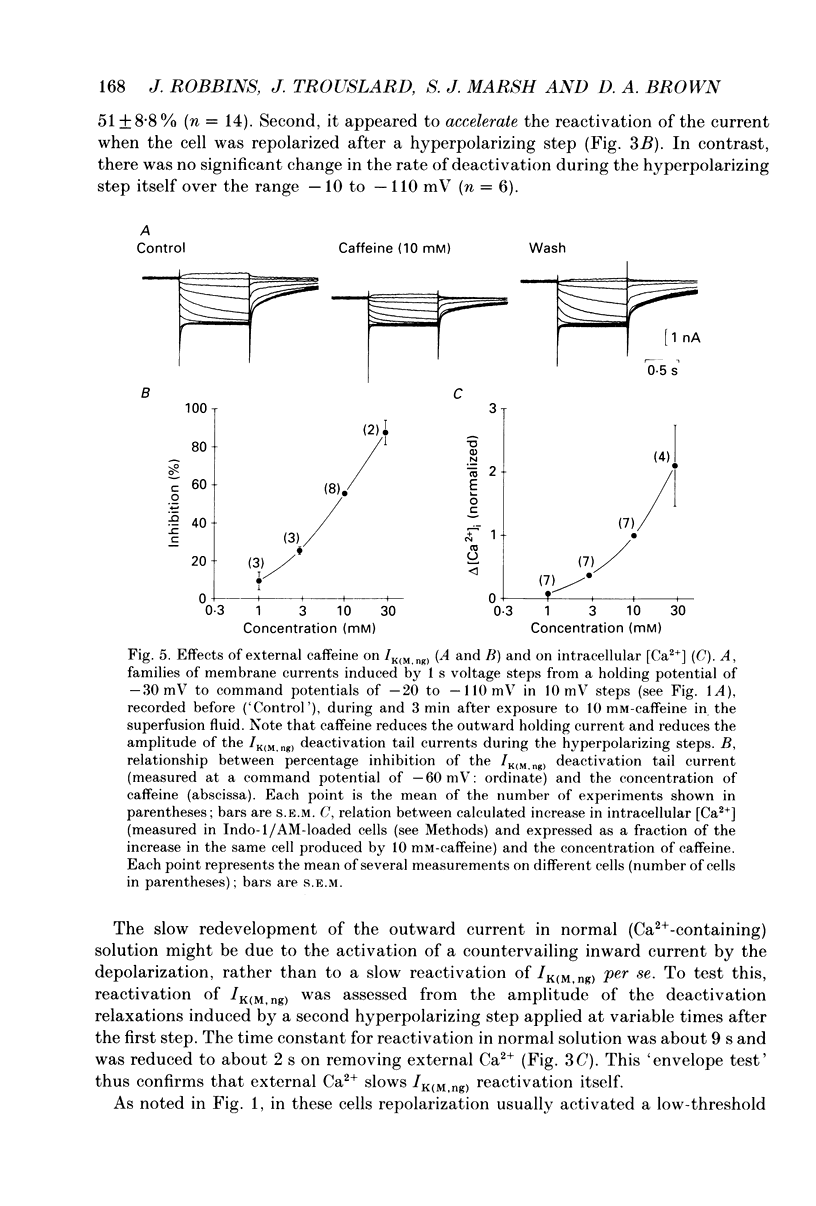
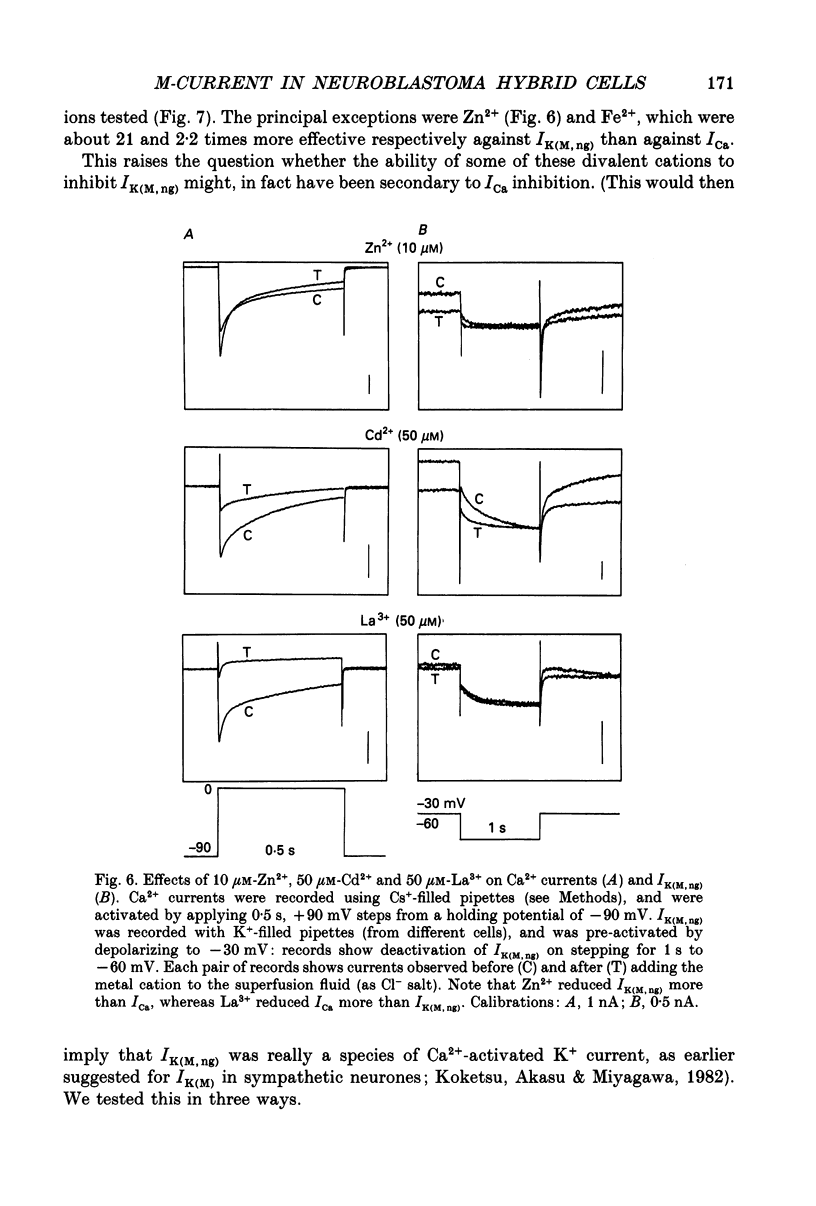
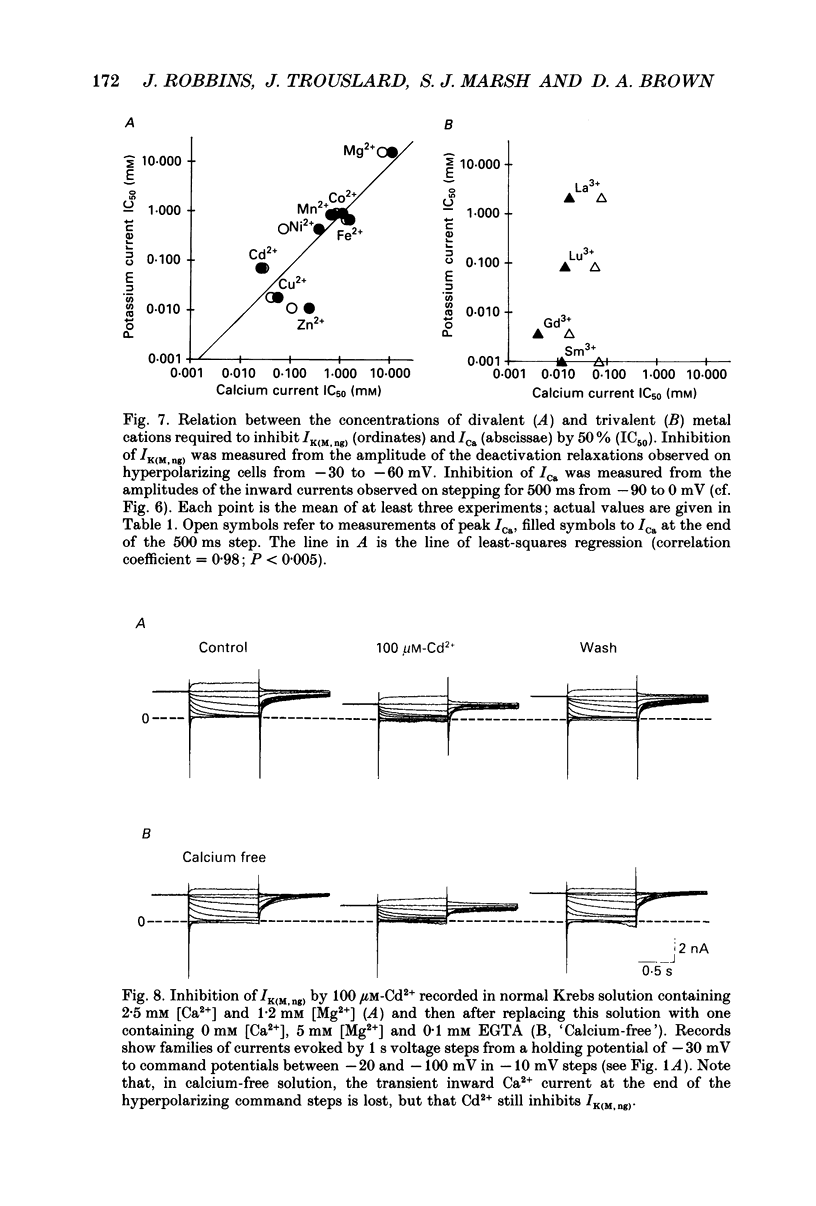
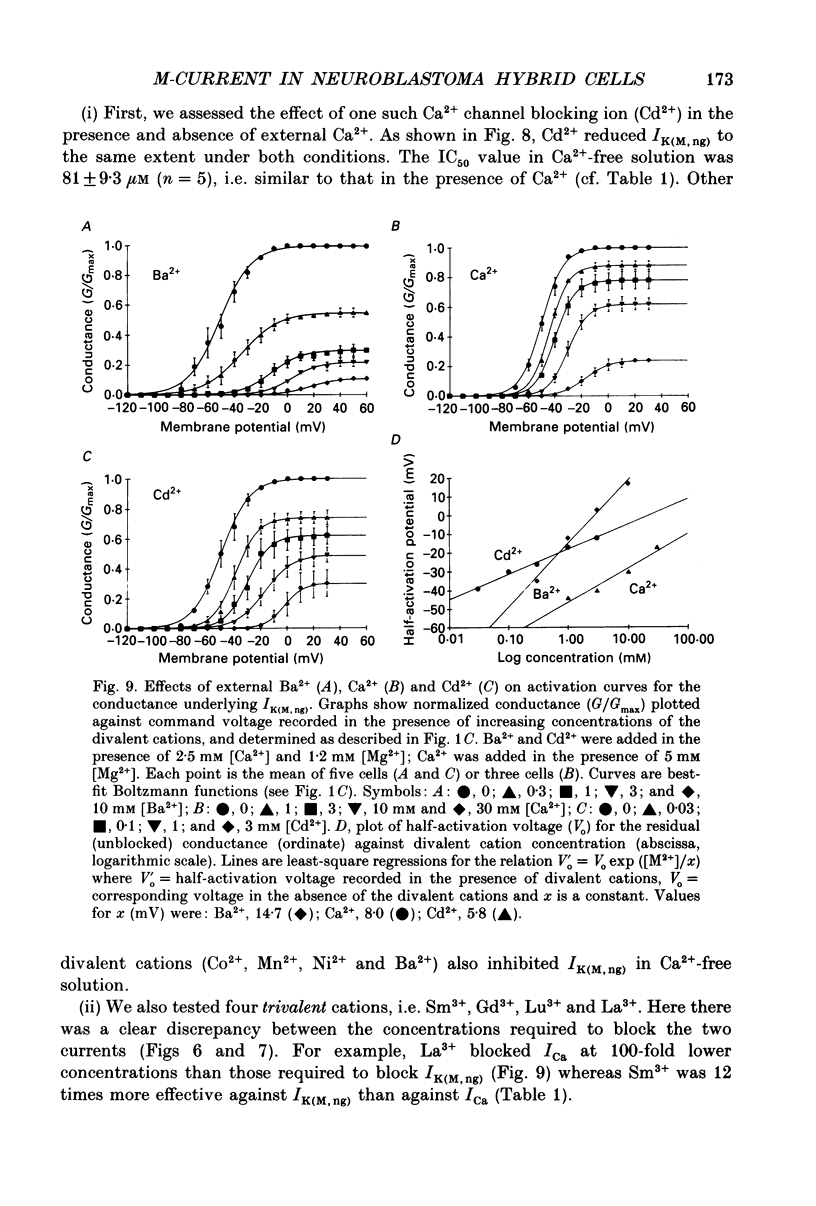
1. The M-like current IK(M,ng) in differentiated NG108-15 mouse neuroblastoma x rat glioma hybrid cells has been studied using tight-seal, whole-cell patch-clamp recording. 2. When calculated from steady-state current-voltage curves, the conductance underlying IK(M,ng) showed a Boltzmann dependence on voltage with half-activation voltage Vo = -44 mV (in 3 mM [K+]) and slope factor (a) = 8.1 mV/e-fold increase in conductance. In 12 mM [K+] Vo = -38 mV and a = 6.9 mV. The deactivation reciprocal time constant accelerated with hyperpolarization with slope factor 17 mV/e-fold voltage change. 3. The reversal potential for deactivation tail currents varied with external [K+] as if PNa/PK were 0.005. 4. Steady-state current was increased on removing external Ca2+. In the presence of external Ca2+, reactivation of IK(M, ng) after a hyperpolarizing step was delayed. This delay was preceded by an inward Ca2+ current, and coincided with an increase in intracellular [Ca2+] as measured with Indo-1 fluorescence. Elevation of intracellular [Ca2+] with caffeine also reduced IK(M, ng). 5. IK(M, ng) was inhibited by external divalent cations in decreasing order of potency (mM IC50 in parentheses): Zn2+ (0.011) greater than Cu2+ (0.018) greater than Cd2+ (0.070) greater than Ni2+ (0.44) greater than Ba2+ (0.47) greater than Fe2+ (0.69) greater than Mn2+ (0.86) greater than Co2+ (0.92) greater than Ca2+ (5.6) greater than Mg2+ (16) greater than Sr2+ (33). This was not secondary to inhibition of ICa since: (i) inhibition persisted in Ca(2+)-free solution; (ii) La3+ did not inhibit IK(M, ng) at concentrations which inhibited ICa; and (iii) organic Ca2+ channel blockers were ineffective. Inhibition comprised both depression of the maximum conductance and a positive shift of the activation curve. Addition of Ca2+ (10 microM free [Ca2+]) or Ba2+ (1 mM total [Ba2+]) to the pipette solution did not significantly change IK(M, ng). 6. IK(M, ng) was reduced by 9-amino-1,2,3,4-tetrahydroacridine (IC50 8 microM) and quinine (30 microM) but was insensitive to tetraethylammonium (IC50 greater than 30 mM), 4-aminopyridine (greater than 10 mM), apamin (greater than 3 microM) or dendrotoxin (greater than 100 nM). 7. IK(M, ng) was inhibited by bradykinin (1-10 microM) or angiotensin II (1-10 microM), but not by the following other receptor agonists: acetylcholine (10 mM), muscarine (10 microM), noradrenaline (100 microM), adrenaline (100 microM), dopamine (100 microM), histamine (100 microM), 5-hydroxytryptamine (10 microM), Met-enkephalin (1 microM), glycine (100 microM), gamma-aminobutyric acid (100 microM) or baclofen (500 microM).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


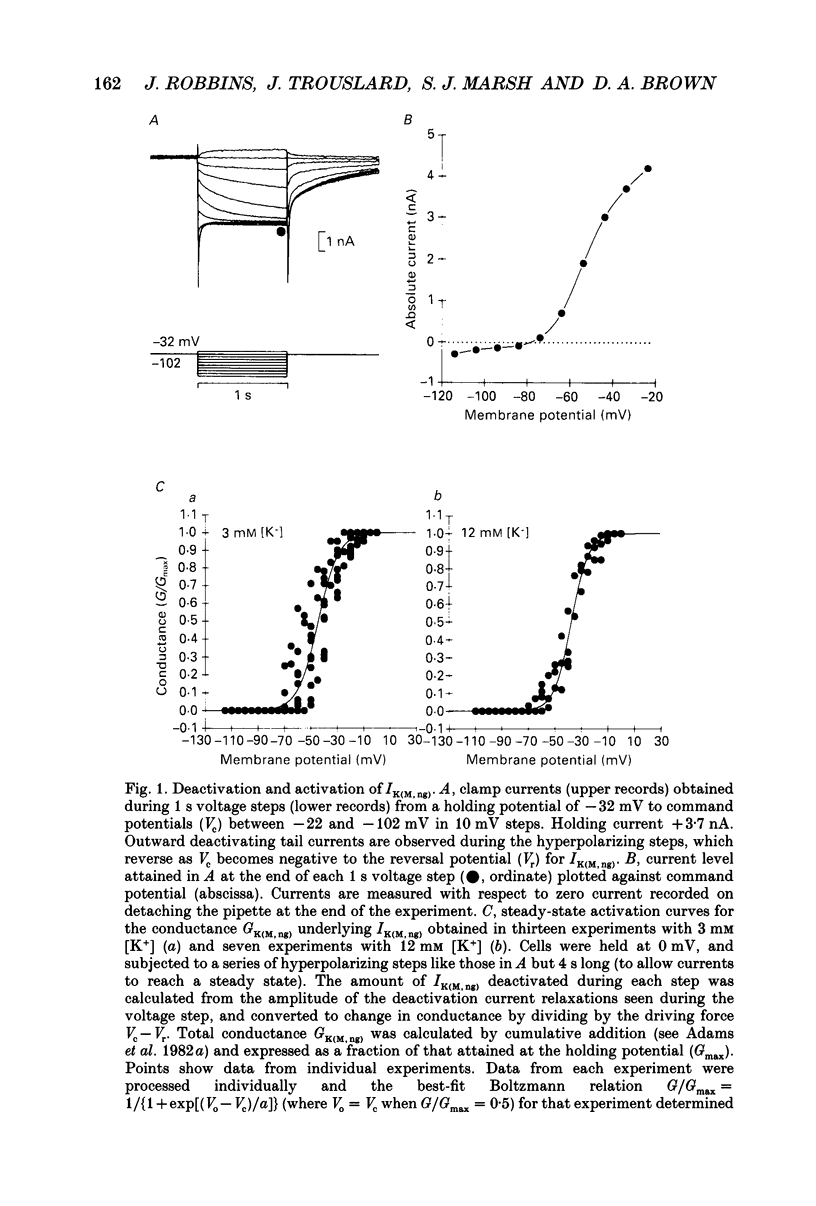

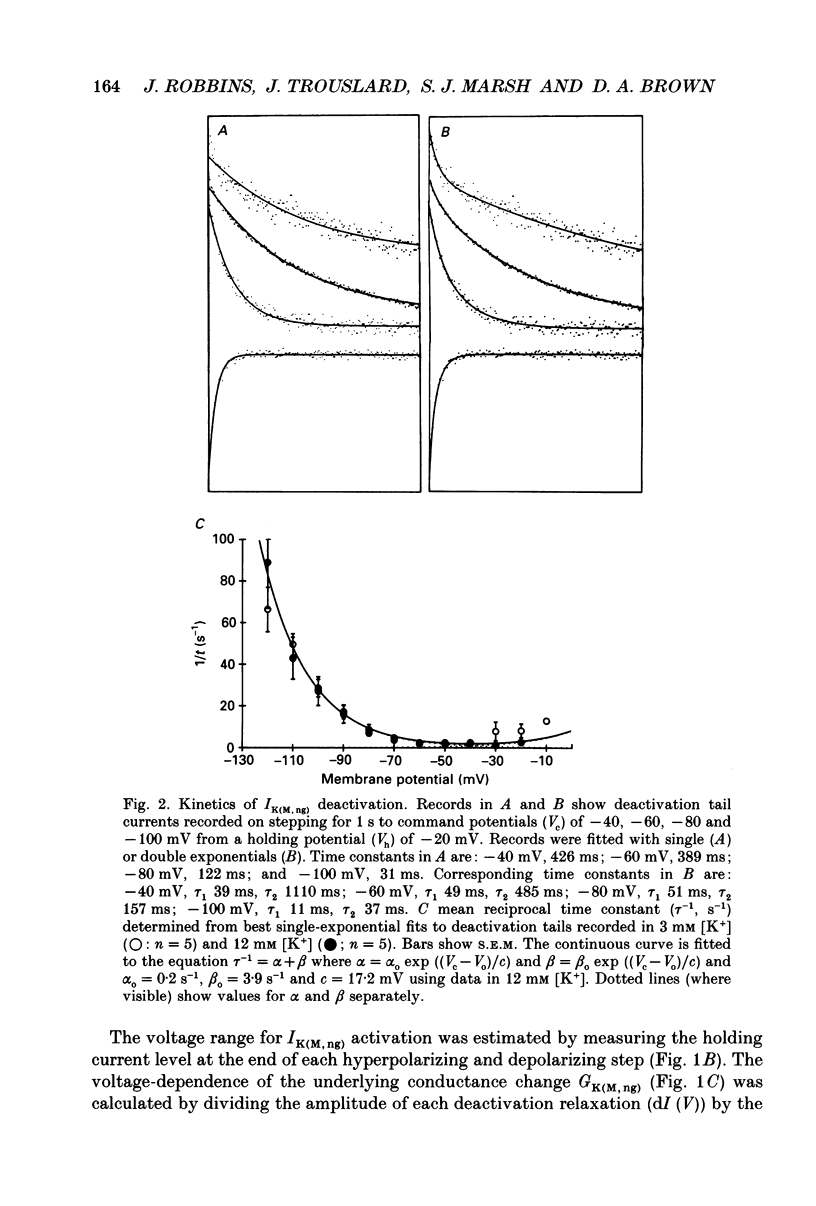

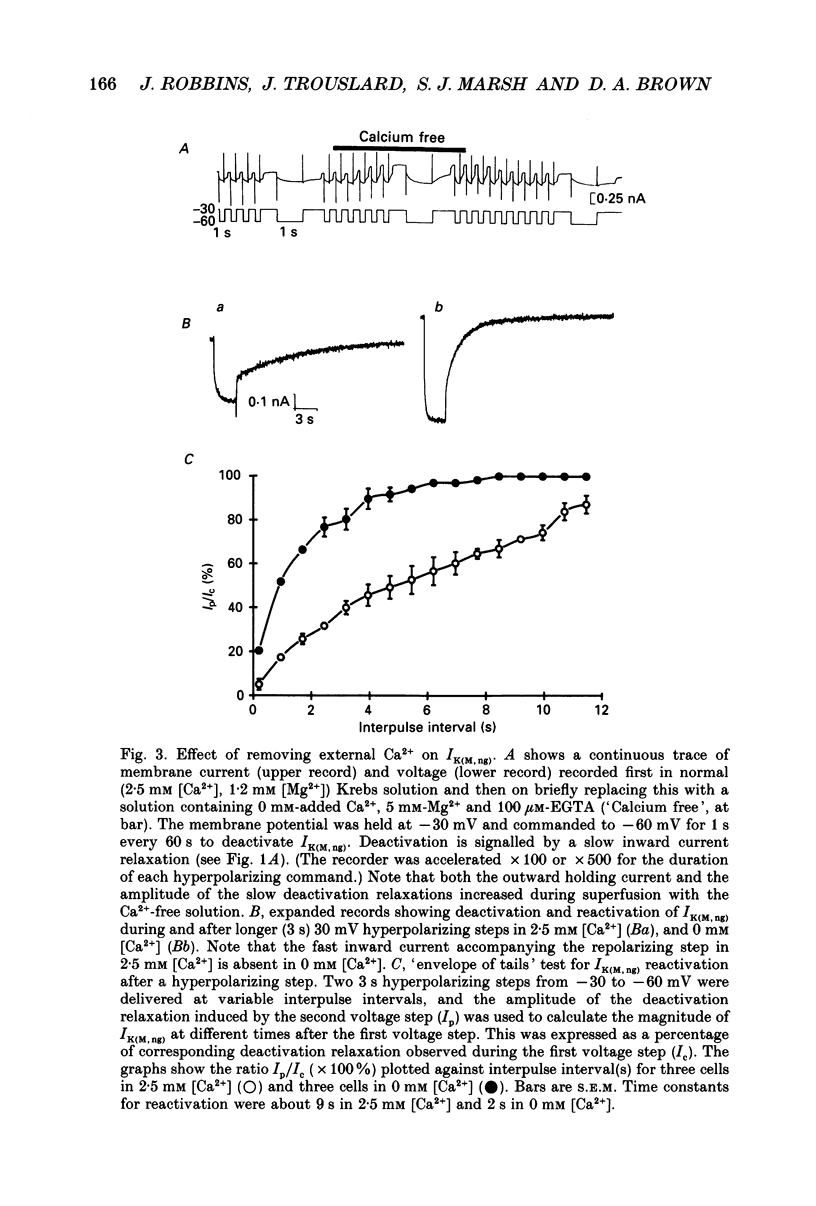
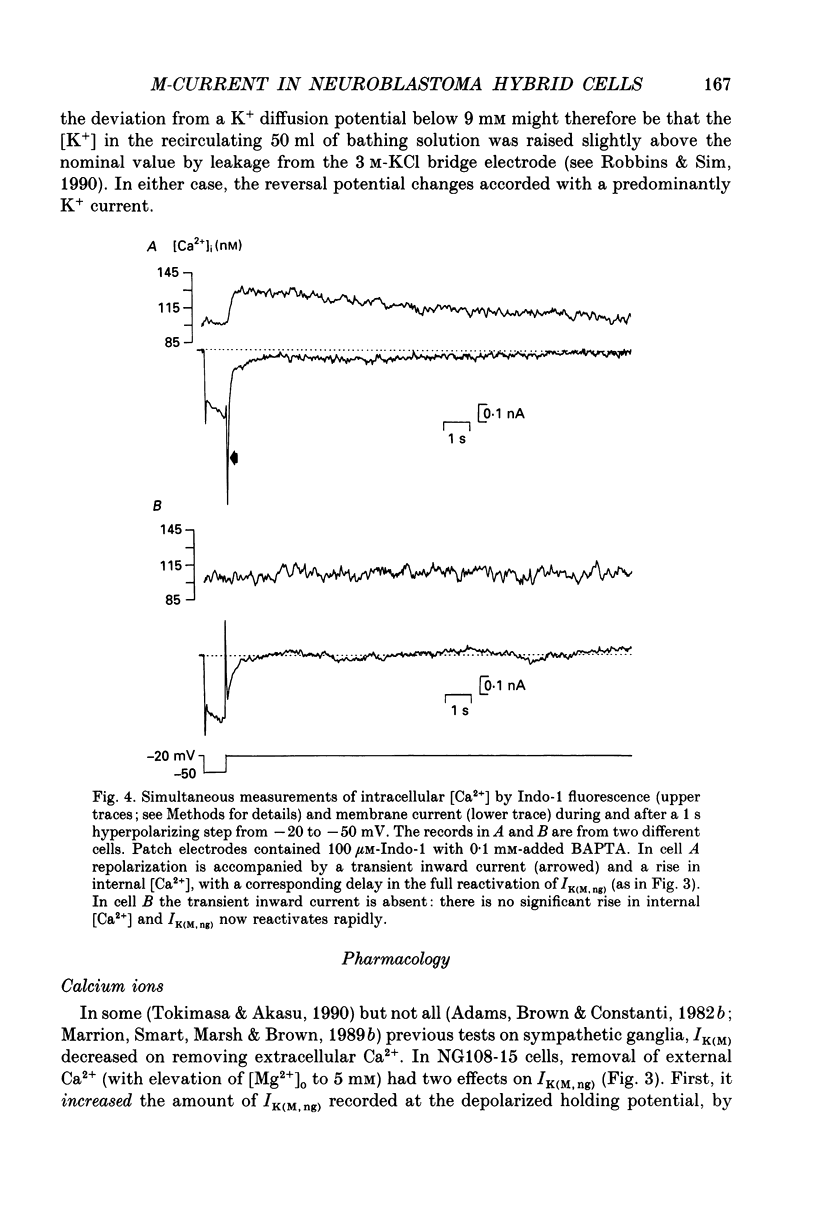



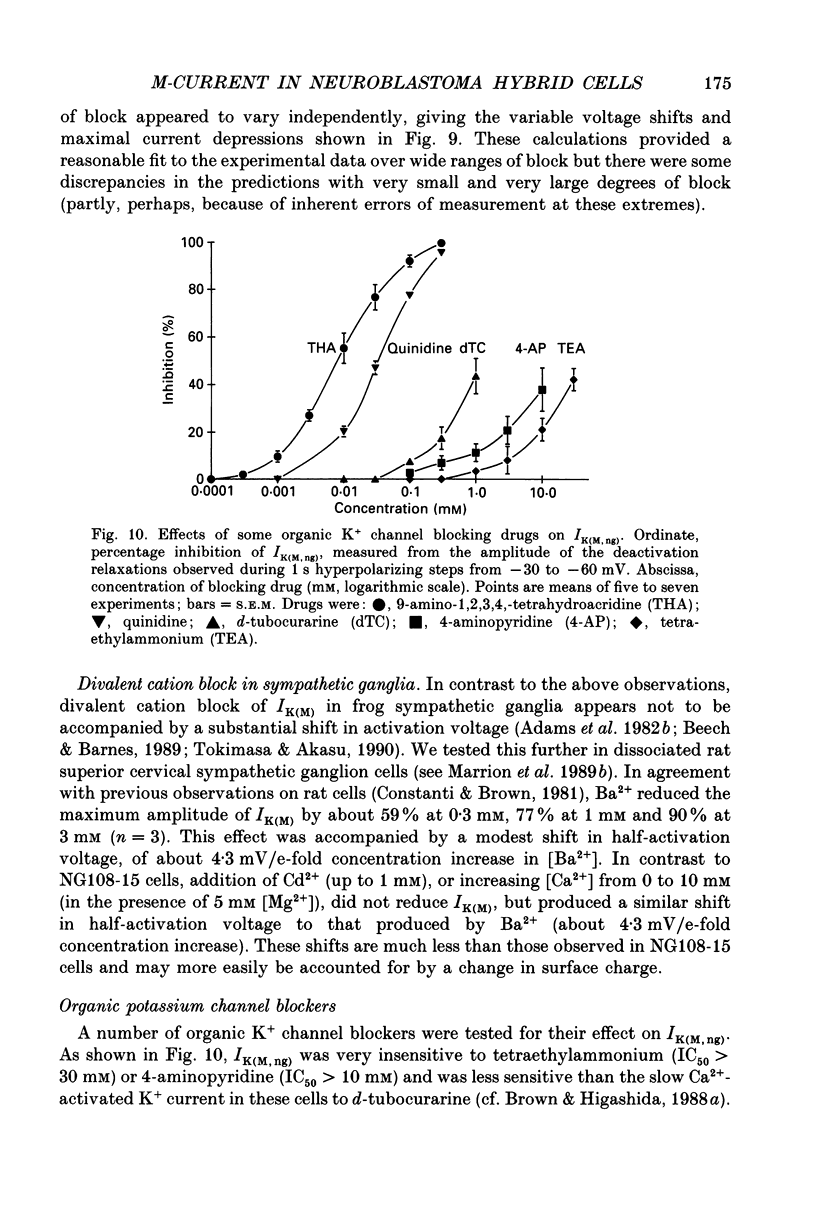

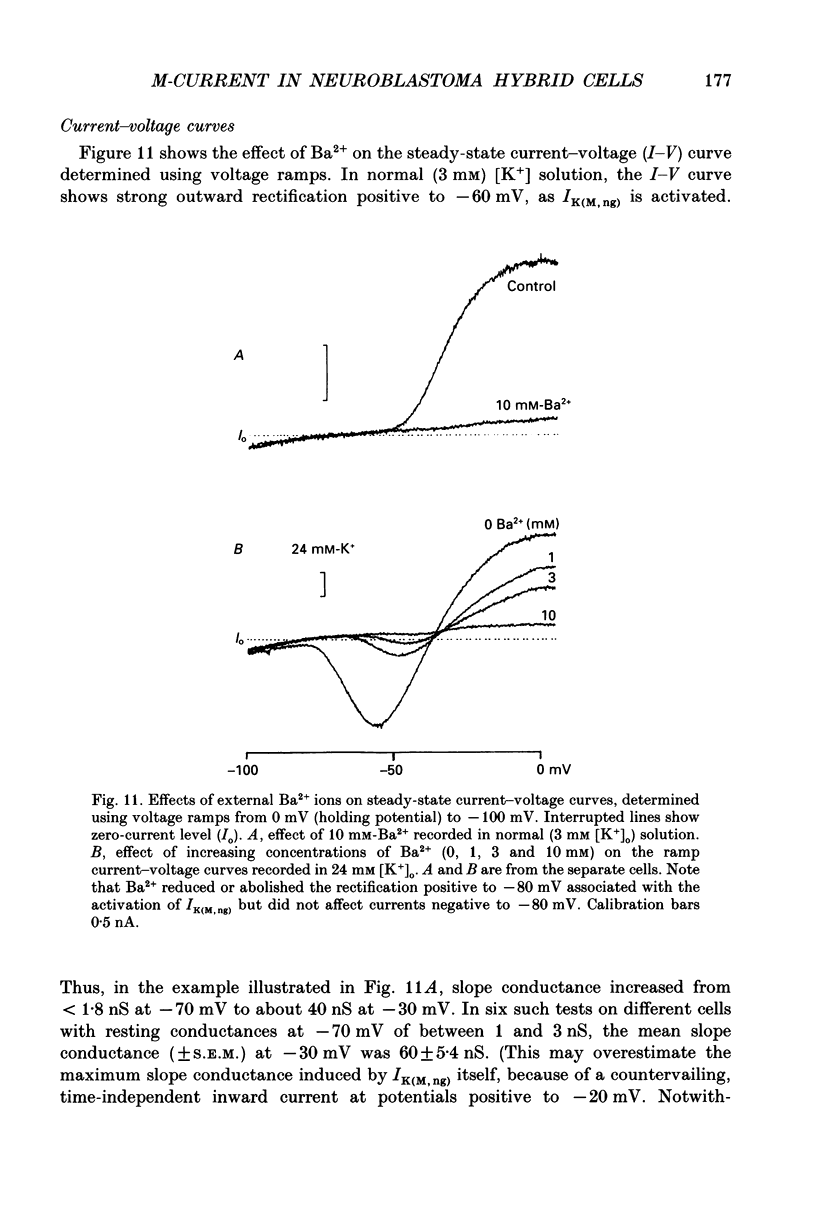

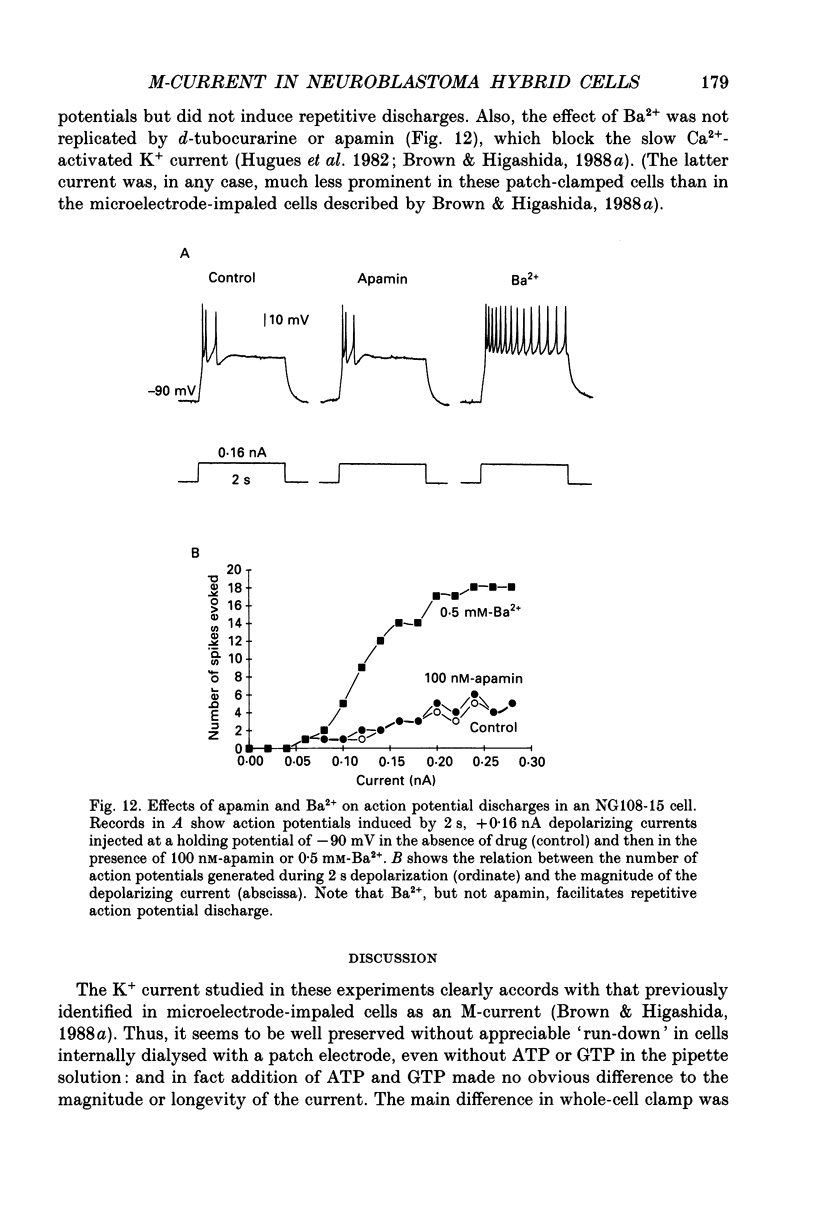





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Sadoshima J. Caffeine affects four different ionic currents in the bull-frog sympathetic neurone. J Physiol. 1989 May;412:221–244. doi: 10.1113/jphysiol.1989.sp017612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Barnes S. Characterization of a voltage-gated K+ channel that accelerates the rod response to dim light. Neuron. 1989 Nov;3(5):573–581. doi: 10.1016/0896-6273(89)90267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bernheim L., Mathie A., Hille B. Intracellular Ca2+ buffers disrupt muscarinic suppression of Ca2+ current and M current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):652–656. doi: 10.1073/pnas.88.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Membrane current responses of NG108-15 mouse neuroblastoma x rat glioma hybrid cells to bradykinin. J Physiol. 1988 Mar;397:167–184. doi: 10.1113/jphysiol.1988.sp016994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Higashida H. Voltage- and calcium-activated potassium currents in mouse neuroblastoma x rat glioma hybrid cells. J Physiol. 1988 Mar;397:149–165. doi: 10.1113/jphysiol.1988.sp016993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A. M currents. Ion Channels. 1988;1:55–94. doi: 10.1007/978-1-4615-7302-9_2. [DOI] [PubMed] [Google Scholar]
- Constanti A., Brown D. A. M-Currents in voltage-clamped mammalian sympathetic neurones. Neurosci Lett. 1981 Jul 17;24(3):289–294. doi: 10.1016/0304-3940(81)90173-7. [DOI] [PubMed] [Google Scholar]
- Docherty R. J. Gadolinium selectively blocks a component of calcium current in rodent neuroblastoma x glioma hybrid (NG108-15) cells. J Physiol. 1988 Apr;398:33–47. doi: 10.1113/jphysiol.1988.sp017027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. Theory and operation of a single microelectrode voltage clamp. J Neurosci Methods. 1984 Jun;11(2):101–127. doi: 10.1016/0165-0270(84)90029-3. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Higashida H., Kubo T., Maeda A., Akiba I., Bujo H., Mishina M., Numa S. Selective coupling with K+ currents of muscarinic acetylcholine receptor subtypes in NG108-15 cells. Nature. 1988 Sep 22;335(6188):355–358. doi: 10.1038/335355a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hamprecht B. Structural, electrophysiological, biochemical, and pharmacological properties of neuroblastoma-glioma cell hybrids in cell culture. Int Rev Cytol. 1977;49:99–170. doi: 10.1016/s0074-7696(08)61948-8. [DOI] [PubMed] [Google Scholar]
- Hugues M., Romey G., Duval D., Vincent J. P., Lazdunski M. Apamin as a selective blocker of the calcium-dependent potassium channel in neuroblastoma cells: voltage-clamp and biochemical characterization of the toxin receptor. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1308–1312. doi: 10.1073/pnas.79.4.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höpp H. P., Reuter G., Reiser G., Hamprecht B. Angiotensin evokes in polyploid rat glioma cells hyperpolarization-depolarization responses and cross-desensitization with bradykinin. Brain Res. 1987 May 26;412(1):175–178. doi: 10.1016/0006-8993(87)91456-9. [DOI] [PubMed] [Google Scholar]
- Jones S. W. A muscarine-resistant M-current in C cells of bullfrog sympathetic ganglia. Neurosci Lett. 1987 Mar 9;74(3):309–314. doi: 10.1016/0304-3940(87)90315-6. [DOI] [PubMed] [Google Scholar]
- Jones S. W. On the resting potential of isolated frog sympathetic neurons. Neuron. 1989 Aug;3(2):153–161. doi: 10.1016/0896-6273(89)90028-7. [DOI] [PubMed] [Google Scholar]
- Kirkwood A., Simmons M. A., Mather R. J., Lisman J. Muscarinic suppression of the M-current is mediated by a rise in internal Ca2+ concentration. Neuron. 1991 Jun;6(6):1009–1014. doi: 10.1016/0896-6273(91)90240-z. [DOI] [PubMed] [Google Scholar]
- Koketsu K., Akasu T., Miyagawa M. Identification of gK systems activated by [Ca2+]. Brain Res. 1982 Jul 15;243(2):369–372. doi: 10.1016/0006-8993(82)90263-3. [DOI] [PubMed] [Google Scholar]
- Kuba K. Release of calcium ions linked to the activation of potassium conductance in a caffeine-treated sympathetic neurone. J Physiol. 1980 Jan;298:251–269. doi: 10.1113/jphysiol.1980.sp013079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion N. V., Smart T. G., Marsh S. J., Brown D. A. Muscarinic suppression of the M-current in the rat sympathetic ganglion is mediated by receptors of the M1-subtype. Br J Pharmacol. 1989 Oct;98(2):557–573. doi: 10.1111/j.1476-5381.1989.tb12630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion N. V., Zucker R. S., Marsh S. J., Adams P. R. Modulation of M-current by intracellular Ca2+. Neuron. 1991 Apr;6(4):533–545. doi: 10.1016/0896-6273(91)90056-6. [DOI] [PubMed] [Google Scholar]
- Marsh S. J., Hubbard A., Brown D. A. Some Actions of 9-Amino-1,2,3,4-Tetrahydroacridine (THA) on Cholinergic Transmission and Membrane Currents in Rat Sympathetic Ganglia. Eur J Neurosci. 1990;2(12):1127–1134. doi: 10.1111/j.1460-9568.1990.tb00024.x. [DOI] [PubMed] [Google Scholar]
- McFadzean I., Mullaney I., Brown D. A., Milligan G. Antibodies to the GTP binding protein, Go, antagonize noradrenaline-induced calcium current inhibition in NG108-15 hybrid cells. Neuron. 1989 Aug;3(2):177–182. doi: 10.1016/0896-6273(89)90030-5. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel A. D., Stefanich E., Whiting R. L. PC12 phaeochromocytoma cells contain an atypical muscarinic receptor binding site. Br J Pharmacol. 1989 Jul;97(3):914–920. doi: 10.1111/j.1476-5381.1989.tb12032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Marty A., Fukuda K., Kubo T., Numa S. Intracellular calcium release mediated by two muscarinic receptor subtypes. FEBS Lett. 1988 Nov 21;240(1-2):88–94. doi: 10.1016/0014-5793(88)80345-4. [DOI] [PubMed] [Google Scholar]
- Ohmori H., Yoshii M. Surface potential reflected in both gating and permeation mechanisms of sodium and calcium channels of the tunicate egg cell membrane. J Physiol. 1977 May;267(2):429–463. doi: 10.1113/jphysiol.1977.sp011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Smith D. H., Ramachandran J., Capon D. J. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987 Dec 20;6(13):3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffinger P. J., Leibowitz M. D., Subers E. M., Nathanson N. M., Almers W., Hille B. Agonists that suppress M-current elicit phosphoinositide turnover and Ca2+ transients, but these events do not explain M-current suppression. Neuron. 1988 Aug;1(6):477–484. doi: 10.1016/0896-6273(88)90178-x. [DOI] [PubMed] [Google Scholar]
- Popov E. G., Gavrilov Y. Iu, Pozin EYa, Gabbasov Z. A. Multiwavelength method for measuring concentration of free cytosolic calcium using the fluorescent probe indo-1. Arch Biochem Biophys. 1988 Feb 15;261(1):91–96. doi: 10.1016/0003-9861(88)90107-5. [DOI] [PubMed] [Google Scholar]
- Robbins J., Caulfield M. P., Higashida H., Brown D. A. Genotypic m3-Muscarinic Receptors Preferentially Inhibit M-currents in DNA-transfected NG108-15 Neuroblastoma x Glioma Hybrid Cells. Eur J Neurosci. 1991;3(8):820–824. doi: 10.1111/j.1460-9568.1991.tb01678.x. [DOI] [PubMed] [Google Scholar]
- Robbins J., Sim J. A. A transient outward current in NG108-15 neuroblastoma x glioma hybrid cells. Pflugers Arch. 1990 Apr;416(1-2):130–137. doi: 10.1007/BF00370234. [DOI] [PubMed] [Google Scholar]
- Sala F., Hernández-Cruz A. Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J. 1990 Feb;57(2):313–324. doi: 10.1016/S0006-3495(90)82533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selyanko A. A., Smith P. A., Zidichouski J. A. Effects of muscarine and adrenaline on neurones from Rana pipiens sympathetic ganglia. J Physiol. 1990 Jun;425:471–500. doi: 10.1113/jphysiol.1990.sp018114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimasa T., Akasu T. Extracellular calcium ions are required for muscarine-sensitive potassium current in bullfrog sympathetic neurons. J Auton Nerv Syst. 1990 Feb;29(2):163–174. doi: 10.1016/0165-1838(90)90182-i. [DOI] [PubMed] [Google Scholar]
- Tokimasa T. Intracellular Ca2+-ions inactivate K+-current in bullfrog sympathetic neurons. Brain Res. 1985 Jul 1;337(2):386–391. doi: 10.1016/0006-8993(85)90081-2. [DOI] [PubMed] [Google Scholar]
- Tosaka T., Tasaka J., Miyazaki T., Libet B. Hyperpolarization following activation of K+ channels by excitatory postsynaptic potentials. Nature. 1983 Sep 8;305(5930):148–150. doi: 10.1038/305148a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel A., Marrion N. V., Lopez H., Adams P. R. Bradykinin inhibits a potassium M-like current in rat pheochromocytoma PC12 cells. FEBS Lett. 1989 Sep 11;255(1):42–46. doi: 10.1016/0014-5793(89)81057-9. [DOI] [PubMed] [Google Scholar]
- Wahl M., Lucherini M. J., Gruenstein E. Intracellular Ca2+ measurement with Indo-1 in substrate-attached cells: advantages and special considerations. Cell Calcium. 1990 Aug;11(7):487–500. doi: 10.1016/0143-4160(90)90081-5. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakel J. L., Jackson M. B. 5-HT3 receptors mediate rapid responses in cultured hippocampus and a clonal cell line. Neuron. 1988 Sep;1(7):615–621. doi: 10.1016/0896-6273(88)90111-0. [DOI] [PubMed] [Google Scholar]


