Abstract
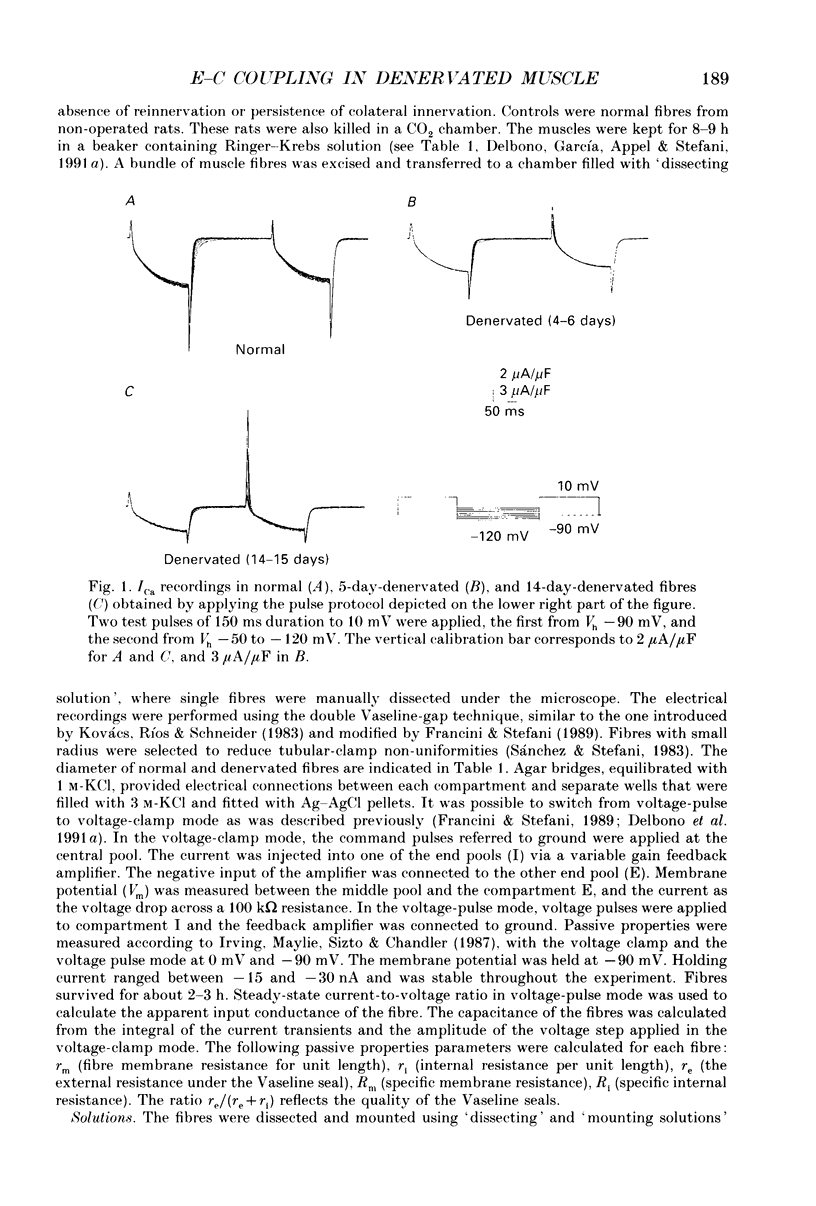
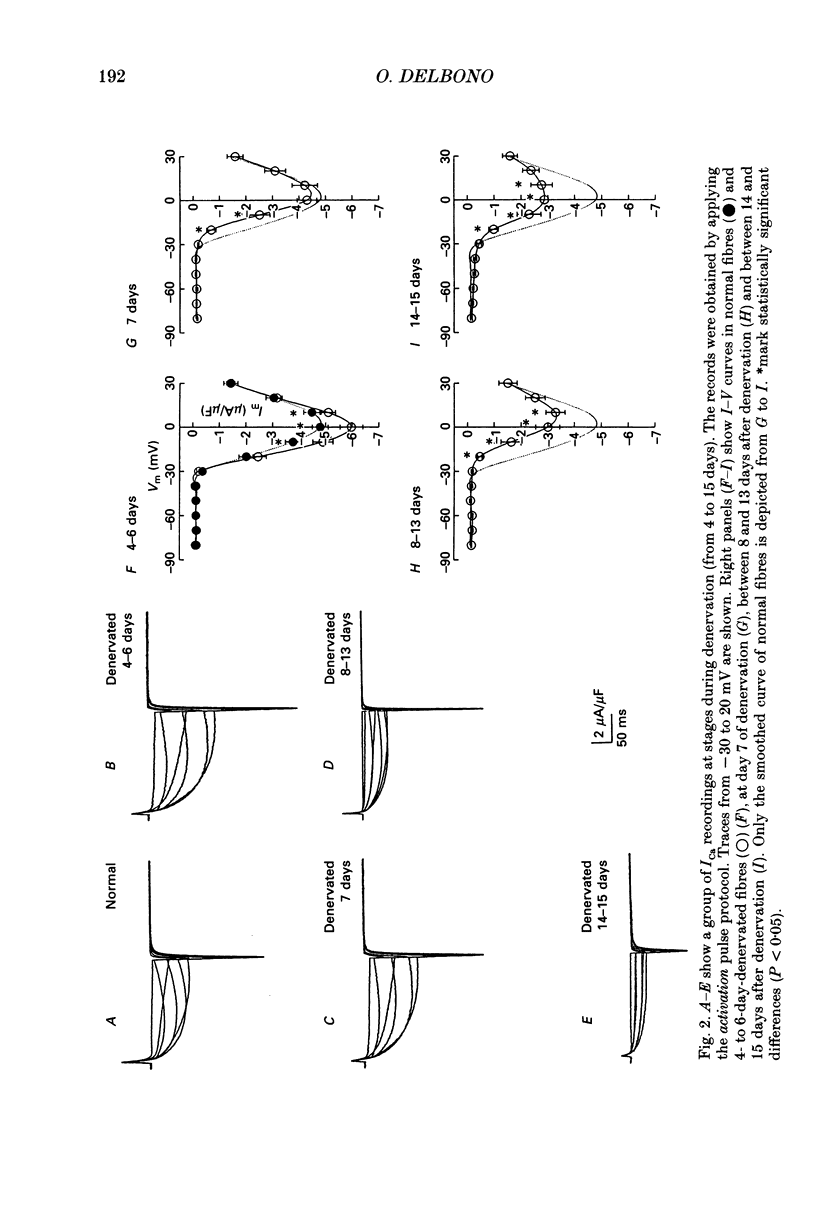
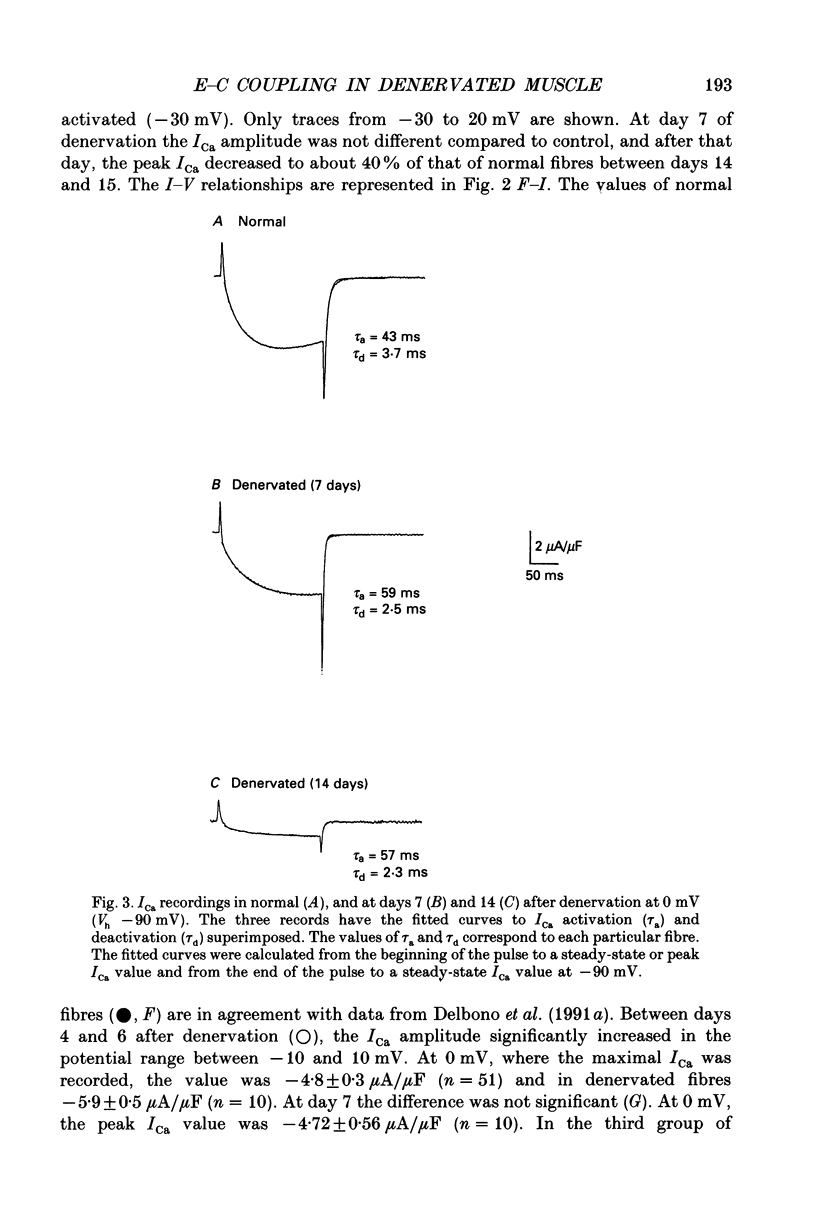
1. Calcium current (ICa) activation was studied in denervated extensor digitorum longus muscle fibres of the rat. Denervation was performed by surgically removing 6-8 mm of the sciatic nerve at the sciatic notch. Controls were normal fibres from non-operated rats. Electrical recordings were carried out using the double Vaseline-gap technique. 2. Current-voltage (I-V) curves showed that the ICa amplitude increased during the first 4-6 days after denervation and subsequently decreased during the second week. Between days 4 and 6 after denervation, the peak ICa amplitude (at 0 mV) was -5.9 +/- 0.5 microA/microF (mean +/- S.E.M.) as compared with -4.8 +/- 0.3 microA/microF in normal fibres. Between days 14 and 15 after denervation, the ICa amplitude was -2.9 +/- 0.4 microA/microF. 3. The time constant of ICa activation (tau a) was significantly increased by denervation. At 0 mV, tau a in normal fibres was 44.8 +/- 1.4 ms. Between 4 and 6 days after denervation tau a was 58.1 +/- 4.8 ms, and between 14 and 15 days after denervation, 55.8 +/- 3.8 ms. 4. The time constant of deactivation (tau d) decreased after denervation. At -10 mV, the tau d in normal fibres was 103.4 +/- 14 ms. The value decreased to 74.5 +/- 8.6 and 74.0 +/- 17 ms between days 4 and 6 and days 14 and 15 of denervation respectively. 5. Charge movement (Qon) was reduced progressively without major changes in the steepness (k) and position on the voltage axis of the Qon-Vm relationship. The fitted parameters under control were Qmax = 15.4 nC/microF, mid-point potential Vq1/2 = -25.2 mV and k = 11.9 mV. Between days 14 and 15 of denervation, the values for Qmax, Vq1/2 and k were 6.7 nC/microF, -36.8 mV and 11.3 mV respectively. 6. Calcium permeability (PCa) in normal and denervated fibres at stages during denervation was calculated according to the Hodgkin-Huxley model. At 0 mV PCa was 1.24 x 10(-5) cm/s in normal fibres, and 7.43 x 10(-6) cm/s after 2 weeks of denervation. 7. The m infinity-Vm relationship was shifted to more positive potentials after denervation without significant changes in the steepness factor k. The V1/2 value in normal fibres was -4.4 mV, and 5.8 mV after two weeks of denervation. 8. The ICa sensitivity to nifedipine was not modified in the different groups of denervated fibres studied. With 10 microM-nifedipine, the 1-(ICa in nifedipine/ICa control) relationships were 0.74 +/- 0.03 in normal fibres and 0.76 +/- 0.12, 14 days after denervation.
Full text
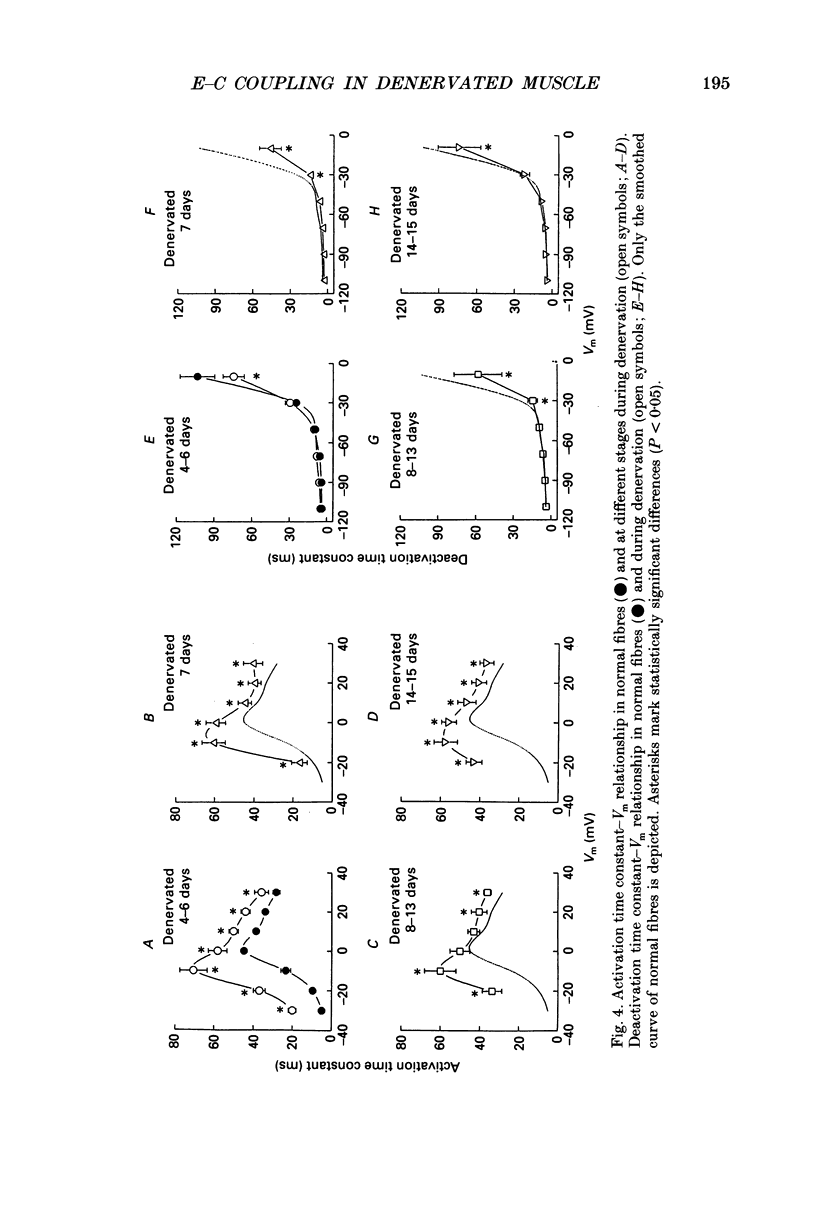
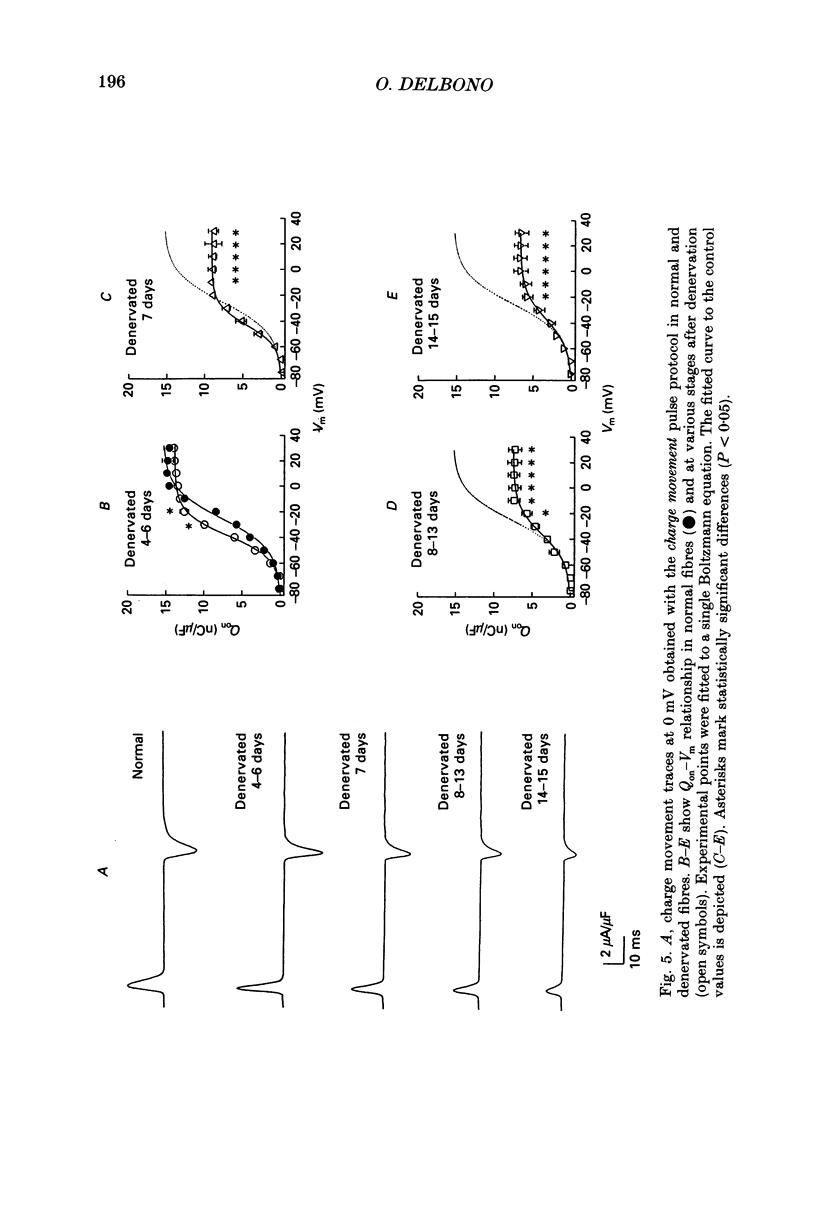
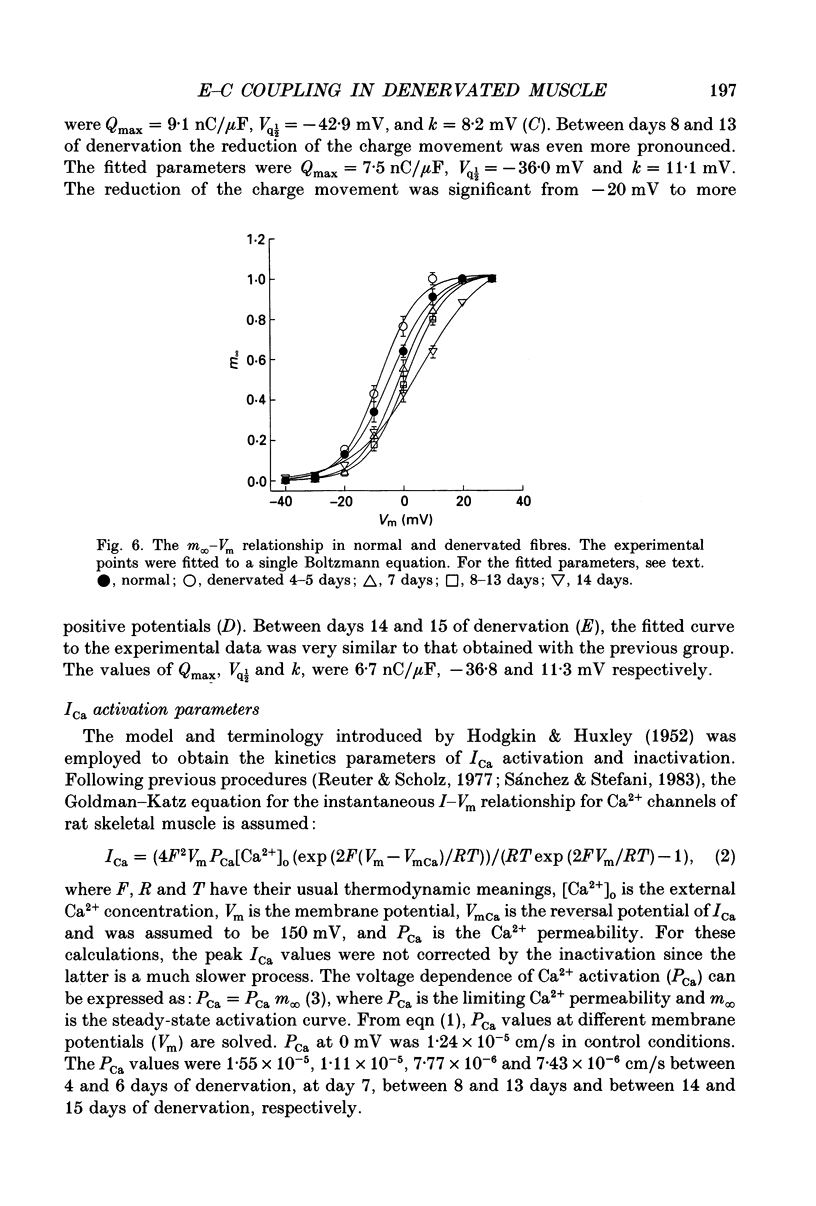
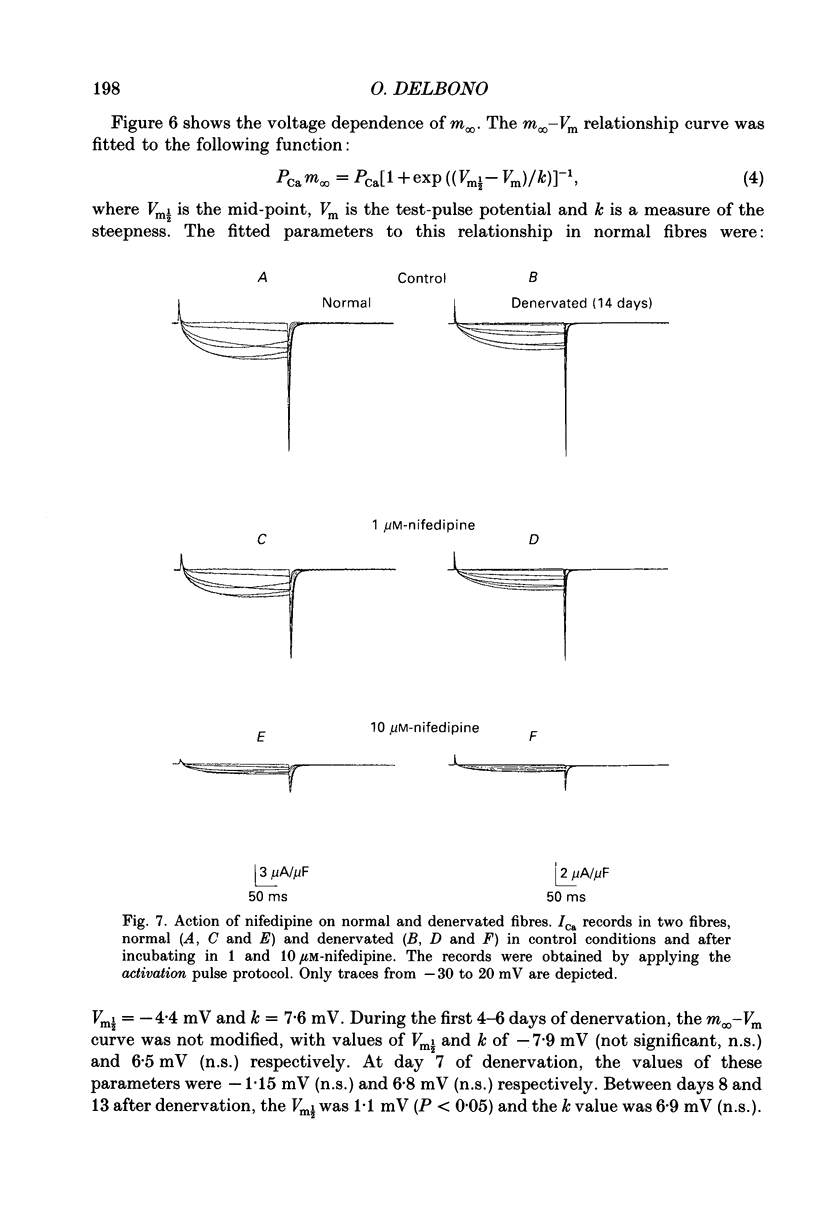
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brum G., Ríos E., Stéfani E. Effects of extracellular calcium on calcium movements of excitation-contraction coupling in frog skeletal muscle fibres. J Physiol. 1988 Apr;398:441–473. doi: 10.1113/jphysiol.1988.sp017052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-sensitive ion channels. Science. 1988 Oct 7;242(4875):50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Conte Camerino D., De Luca A., Mambrini M., Vrbovà G. Membrane ionic conductances in normal and denervated skeletal muscle of the rat during development. Pflugers Arch. 1989 Mar;413(5):568–570. doi: 10.1007/BF00594192. [DOI] [PubMed] [Google Scholar]
- Cota G., Stefani E. Effects of external calcium reduction on the kinetics of potassium contractures in frog twitch muscle fibres. J Physiol. 1981 Aug;317:303–316. doi: 10.1113/jphysiol.1981.sp013826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbono O., García J., Appel S. H., Stefani E. Calcium current and charge movement of mammalian muscle: action of amyotrophic lateral sclerosis immunoglobulins. J Physiol. 1991 Dec;444:723–742. doi: 10.1113/jphysiol.1991.sp018903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbono O., Kotsias B. A. Calcium action potentials in innervated and denervated rat muscle fibres. Pflugers Arch. 1991 Apr;418(3):284–291. doi: 10.1007/BF00370528. [DOI] [PubMed] [Google Scholar]
- Dulhunty A. F., Gage P. W. Effects of extracellular calcium concentration and dihydropyridines on contraction in mammalian skeletal muscle. J Physiol. 1988 May;399:63–80. doi: 10.1113/jphysiol.1988.sp017068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty A. F., Gage P. W. Excitation-contraction coupling and charge movement in denervated rat extensor digitorum longus and soleus muscles. J Physiol. 1985 Jan;358:75–89. doi: 10.1113/jphysiol.1985.sp015541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty A. F., Gage P. W., Valois A. A. Indentations in the terminal cisternae of denervated rat EDL and soleus muscle fibers. J Ultrastruct Res. 1984 Jul;88(1):30–43. doi: 10.1016/s0022-5320(84)90179-5. [DOI] [PubMed] [Google Scholar]
- Duval A., Léoty C. Changes in the ionic currents sensitivity to inhibitors in twitch rat skeletal muscles following denervation. Pflugers Arch. 1985 Apr;403(4):407–414. doi: 10.1007/BF00589254. [DOI] [PubMed] [Google Scholar]
- Engel A. G., Stonnington H. H. Trophic functions of the neuron. II. Denervation and regulation of muscle. Morphological effects of denervation of muscle. A quantitative ultrastructural study. Ann N Y Acad Sci. 1974 Mar 22;228(0):68–88. doi: 10.1111/j.1749-6632.1974.tb20503.x. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D., Melzer W., Pohl B., Zöllner P. Fast gating kinetics of the slow Ca2+ current in cut skeletal muscle fibres of the frog. J Physiol. 1990 Jun;425:347–367. doi: 10.1113/jphysiol.1990.sp018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finol H. J., Lewis D. M., Owens R. The effects of denervation on contractile properties or rat skeletal muscle. J Physiol. 1981;319:81–92. doi: 10.1113/jphysiol.1981.sp013893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francini F., Stefani E. Decay of the slow calcium current in twitch muscle fibers of the frog is influenced by intracellular EGTA. J Gen Physiol. 1989 Nov;94(5):953–969. doi: 10.1085/jgp.94.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J., Avila-Sakar A. J., Stefani E. Repetitive stimulation increases the activation rate of skeletal muscle Ca2+ currents. Pflugers Arch. 1990 Apr;416(1-2):210–212. doi: 10.1007/BF00370245. [DOI] [PubMed] [Google Scholar]
- Gonoi T., Hasegawa S. Post-natal disappearance of transient calcium channels in mouse skeletal muscle: effects of denervation and culture. J Physiol. 1988 Jul;401:617–637. doi: 10.1113/jphysiol.1988.sp017183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann E., Sandow A. Caffeine-induced contracture and potentiation of contraction in normal and denervated rat muscle. Life Sci. 1965 Jun;4(11):1149–1156. doi: 10.1016/0024-3205(65)90104-9. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Thesleff S. Studies on tetrodotoxin resistant action potentials in denervated skeletal muscle. Acta Physiol Scand. 1971 Nov;83(3):382–388. doi: 10.1111/j.1748-1716.1971.tb05091.x. [DOI] [PubMed] [Google Scholar]
- Henderson L. P., Lechleiter J. D., Brehm P. Single channel properties of newly synthesized acetylcholine receptors following denervation of mammalian skeletal muscle. J Gen Physiol. 1987 Jun;89(6):999–1014. doi: 10.1085/jgp.89.6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. The effect of diameter on the electrical constants of frog skeletal muscle fibres. J Physiol. 1972 Feb;221(1):105–120. doi: 10.1113/jphysiol.1972.sp009742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving M., Maylie J., Sizto N. L., Chandler W. K. Intrinsic optical and passive electrical properties of cut frog twitch fibers. J Gen Physiol. 1987 Jan;89(1):1–40. doi: 10.1085/jgp.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsias B. A., Muchnik S. Mechanical and electrical properties of denervated rat skeletal muscles. Exp Neurol. 1987 Sep;97(3):516–528. doi: 10.1016/0014-4886(87)90110-5. [DOI] [PubMed] [Google Scholar]
- Kotsias B. A., Venosa R. A. Role of sodium and potassium permeabilities in the depolarization of denervated rat muscle fibres. J Physiol. 1987 Nov;392:301–313. doi: 10.1113/jphysiol.1987.sp016781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs L., Rios E., Schneider M. F. Measurement and modification of free calcium transients in frog skeletal muscle fibres by a metallochromic indicator dye. J Physiol. 1983 Oct;343:161–196. doi: 10.1113/jphysiol.1983.sp014887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D. Components of charge movement in rabbit skeletal muscle: the effect of tetracaine and nifedipine. J Physiol. 1986 Jul;376:85–100. doi: 10.1113/jphysiol.1986.sp016143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Walsh T. Calcium currents, charge movement and dihydropyridine binding in fast- and slow-twitch muscles of rat and rabbit. J Physiol. 1987 Dec;393:595–617. doi: 10.1113/jphysiol.1987.sp016843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorković H. Force and membrane potential in acetylcholine and potassium contractures of denervated mouse muscles. Pflugers Arch. 1985 May;404(1):50–55. doi: 10.1007/BF00581490. [DOI] [PubMed] [Google Scholar]
- Mejía-Alvarez R., Fill M., Stefani E. Voltage-dependent inactivation of T-tubular skeletal calcium channels in planar lipid bilayers. J Gen Physiol. 1991 Feb;97(2):393–412. doi: 10.1085/jgp.97.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obejero Paz C. A., Delbono O., Muchnik S. Effects of actinomycin D on contractile properties of denervated rat skeletal muscle. Exp Neurol. 1986 Dec;94(3):509–518. doi: 10.1016/0014-4886(86)90234-7. [DOI] [PubMed] [Google Scholar]
- Pappone P. A. Voltage-clamp experiments in normal and denervated mammalian skeletal muscle fibres. J Physiol. 1980 Sep;306:377–410. doi: 10.1113/jphysiol.1980.sp013403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios E., Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature. 1987 Feb 19;325(6106):717–720. doi: 10.1038/325717a0. [DOI] [PubMed] [Google Scholar]
- Salvatori S., Damiani E., Zorzato F., Volpe P., Pierobon S., Quaglino D., Jr, Salviati G., Margreth A. Denervation-induced proliferative changes of triads in rabbit skeletal muscle. Muscle Nerve. 1988 Dec;11(12):1246–1259. doi: 10.1002/mus.880111209. [DOI] [PubMed] [Google Scholar]
- Schmid-Antomarchi H., Renaud J. F., Romey G., Hugues M., Schmid A., Lazdunski M. The all-or-none role of innervation in expression of apamin receptor and of apamin-sensitive Ca2+-activated K+ channel in mammalian skeletal muscle. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2188–2191. doi: 10.1073/pnas.82.7.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A., Kazazoglou T., Renaud J. F., Lazdunski M. Comparative changes of levels of nitrendipine Ca2+ channels, of tetrodotoxin-sensitive Na+ channels and of ouabain-sensitive (Na+ + K+)-ATPase following denervation of rat and chick skeletal muscle. FEBS Lett. 1984 Jun 25;172(1):114–118. doi: 10.1016/0014-5793(84)80885-6. [DOI] [PubMed] [Google Scholar]
- Smith P. B., Appel S. H. Development of denervation alterations in surface membranes of mammalian skeletal muscle. Exp Neurol. 1977 Jul;56(1):102–114. doi: 10.1016/0014-4886(77)90142-x. [DOI] [PubMed] [Google Scholar]
- Sánchez J. A., Stefani E. Kinetic properties of calcium channels of twitch muscle fibres of the frog. J Physiol. 1983 Apr;337:1–17. doi: 10.1113/jphysiol.1983.sp014607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesleff S., Ward M. R. Studies on the mechanism of fibrillation potentials in denervated muscle. J Physiol. 1975 Jan;244(2):313–323. doi: 10.1113/jphysiol.1975.sp010800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. E., Horn R. Functional differences between two classes of sodium channels in developing rat skeletal muscle. Science. 1986 Jul 18;233(4761):361–364. doi: 10.1126/science.2425432. [DOI] [PubMed] [Google Scholar]


