Abstract
1. Whole-cell voltage-clamp recordings were used to study the effects of (-)-baclofen and of gamma-aminobutyric acid (GABA) on neurones cultured from the ventral midbrain of embryonic rats. 2. Baclofen induced an outward current (IBac) at a holding potential of -60 mV. The maximal current was 80 pA, and half-maximal current was evoked by 5 microM-baclofen. The proportion of cells affected by baclofen was greater in 25-day-old cultures than in 14-day-old cultures. 3. IBac was blocked by barium (1 mM), and it reversed polarity at a potential that changed according to the Nernst equation when the extracellular potassium concentration was changed. The reversal potential was not different when recording electrodes contained caesium instead of potassium. 4. GABA (10-20 microM), in the presence of picrotoxin (50 microM) and bicuculline (50 microM), also evoked a small potassium current at -60 mV. There was no correlation between the amplitude of the potassium current caused by GABA and that caused by baclofen measured in the same neurones. 5. Spontaneous synaptic currents (up to hundreds of picoamps) were observed that were blocked by picrotoxin (20 microM; IPSCs) or by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 microM; EPSCs); the amplitude and frequency were strongly reduced by baclofen and by GABA. 6. Spontaneous synaptic currents of lower amplitudes (up to 60 pA) remained in the presence of tetrodotoxin. IPSCs (blocked by picrotoxin, reversal at -50 mV) and EPSCs (blocked by CNQX, reversal at 0 mV) were reduced in frequency by baclofen. GABA, in the presence of bicuculline and picrotoxin, had a similar effect on the EPSCs. This action of baclofen persisted in barium (1 mM), and was observed as readily in cells cultured for 14 days as those cultured for 25 days. 7. Some spontaneous synaptic currents remained in the presence of tetrodotoxin and cadmium (100 microM). Their frequency was reduced by baclofen. The effectiveness of baclofen was greater on cells that had been longer in culture. 8. It is concluded that activation of GABAB receptors has two main effects on neurones cultured from rat ventral midbrain. These are potassium conductance increase, and inhibition of the spontaneous release of GABA and excitatory amino acids; both effects can be observed in tetrodotoxin and cadmium.
Full text
PDF



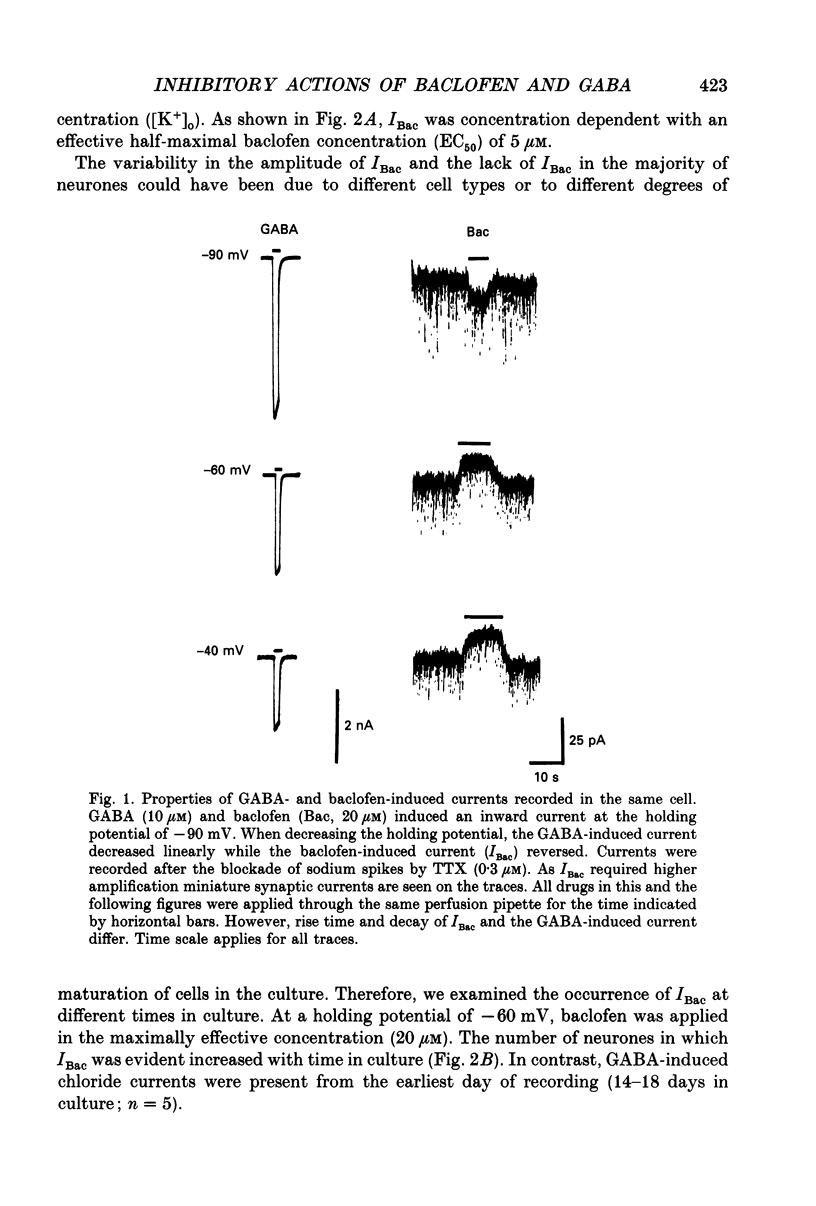



















Selected References
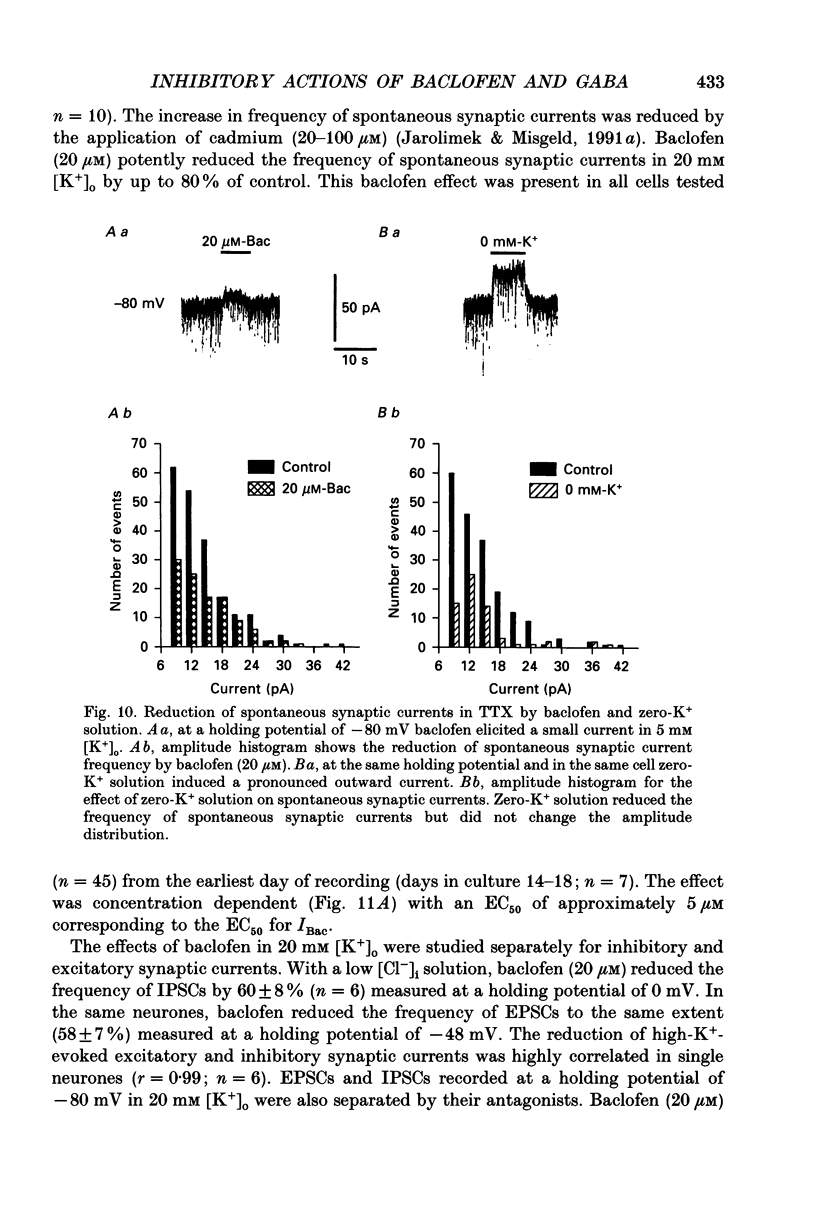
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade R., Malenka R. C., Nicoll R. A. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986 Dec 5;234(4781):1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Bekkers J. M., Stevens C. F. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989 Sep 21;341(6239):230–233. doi: 10.1038/341230a0. [DOI] [PubMed] [Google Scholar]
- Beyer C., Pilgrim C., Reisert I., Misgeld U. Cells from embryonic rat striatum cocultured with mesencephalic glia express dopaminergic phenotypes. Neurosci Lett. 1991 Jul 8;128(1):1–3. doi: 10.1016/0304-3940(91)90746-g. [DOI] [PubMed] [Google Scholar]
- Bijak M., Jarolimek W., Misgeld U. Effects of antagonists on quisqualate and nicotinic receptor-mediated currents of midbrain neurones in culture. Br J Pharmacol. 1991 Mar;102(3):699–705. doi: 10.1111/j.1476-5381.1991.tb12236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter T. J., Carlen P. L., Davies M. F., Kujtan P. W. gamma-Aminobutyric acid hyperpolarizes rat hippocampal pyramidal cells through a calcium-dependent potassium conductance. J Physiol. 1986 Apr;373:181–194. doi: 10.1113/jphysiol.1986.sp016041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. GABAB receptors and their significance in mammalian pharmacology. Trends Pharmacol Sci. 1989 Oct;10(10):401–407. doi: 10.1016/0165-6147(89)90188-0. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Kinetics and selectivity of a low-voltage-activated calcium current in chick and rat sensory neurones. J Physiol. 1987 May;386:547–570. doi: 10.1113/jphysiol.1987.sp016551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q. X., Stelzer A., Kay A. R., Wong R. K. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol. 1990 Jan;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Johnston G. A. Amino acid transmitters in the mammalian central nervous system. Ergeb Physiol. 1974;69(0):97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- Dale N., Kandel E. R. Facilitatory and inhibitory transmitters modulate spontaneous transmitter release at cultured Aplysia sensorimotor synapses. J Physiol. 1990 Feb;421:203–222. doi: 10.1113/jphysiol.1990.sp017941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz R. A., Lux H. D. gamma-Aminobutyric acid-induced depression of calcium currents of chick sensory neurons. Neurosci Lett. 1985 May 14;56(2):205–210. doi: 10.1016/0304-3940(85)90130-2. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Inhibition of calcium currents in cultured rat dorsal root ganglion neurones by (-)-baclofen. Br J Pharmacol. 1986 May;88(1):213–220. doi: 10.1111/j.1476-5381.1986.tb09489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981 Aug;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. Two types of gamma-aminobutyric acid receptor on embryonic sensory neurones. Br J Pharmacol. 1981 Nov;74(3):579–585. doi: 10.1111/j.1476-5381.1981.tb10467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Pre- and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron. 1988 Sep;1(7):585–591. doi: 10.1016/0896-6273(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Finch D. M., Fisher R. S., Jackson M. B. Miniature excitatory synaptic currents in cultured hippocampal neurons. Brain Res. 1990 Jun 4;518(1-2):257–268. doi: 10.1016/0006-8993(90)90978-k. [DOI] [PubMed] [Google Scholar]
- Finch D. M., Jackson M. B. Presynaptic enhancement of synaptic transmission in hippocampal cell cultures by phorbol esters. Brain Res. 1990 Jun 4;518(1-2):269–273. doi: 10.1016/0006-8993(90)90979-l. [DOI] [PubMed] [Google Scholar]
- Fox S., Krnjević K., Morris M. E., Puil E., Werman R. Action of baclofen on mammalian synaptic transmission. Neuroscience. 1978;3(6):495–515. doi: 10.1016/0306-4522(78)90016-7. [DOI] [PubMed] [Google Scholar]
- Grassi F., Lux H. D. Voltage-dependent GABA-induced modulation of calcium currents in chick sensory neurons. Neurosci Lett. 1989 Oct 23;105(1-2):113–119. doi: 10.1016/0304-3940(89)90021-9. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison N. L. On the presynaptic action of baclofen at inhibitory synapses between cultured rat hippocampal neurones. J Physiol. 1990 Mar;422:433–446. doi: 10.1113/jphysiol.1990.sp017993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Sutor B., Zieglgänsberger W. Baclofen reduces post-synaptic potentials of rat cortical neurones by an action other than its hyperpolarizing action. J Physiol. 1987 Mar;384:539–569. doi: 10.1113/jphysiol.1987.sp016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Matsuo T., Ogata N. Baclofen activates voltage-dependent and 4-aminopyridine sensitive K+ conductance in guinea-pig hippocampal pyramidal cells maintained in vitro. Br J Pharmacol. 1985 Apr;84(4):833–841. doi: 10.1111/j.1476-5381.1985.tb17377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Matsuo T., Ogata N. Characterization of pre- and postsynaptic actions of (-)-baclofen in the guinea-pig hippocampus in vitro. Br J Pharmacol. 1985 Apr;84(4):843–851. doi: 10.1111/j.1476-5381.1985.tb17378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Matsuo T., Ogata N. Possible involvement of K+-conductance in the action of gamma-aminobutyric acid in the guinea-pig hippocampus. Br J Pharmacol. 1985 Oct;86(2):515–524. doi: 10.1111/j.1476-5381.1985.tb08923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarolimek W., Misgeld U. Reduction of GABAA receptor-mediated inhibition by the non-NMDA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione in cultured neurons of rat brain. Neurosci Lett. 1991 Jan 2;121(1-2):227–230. doi: 10.1016/0304-3940(91)90691-l. [DOI] [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. On the potassium conductance increase activated by GABAB and dopamine D2 receptors in rat substantia nigra neurones. J Physiol. 1988 Jul;401:437–453. doi: 10.1113/jphysiol.1988.sp017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann H. J., Prince D. A. Postnatal maturation of the GABAergic system in rat neocortex. J Neurophysiol. 1991 Feb;65(2):247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Klee M. R., Zeise M. L. Differences in baclofen-sensitivity between CA3 neurons and granule cells of the guinea pig hippocampus in vitro. Neurosci Lett. 1984 Jun 29;47(3):307–311. doi: 10.1016/0304-3940(84)90531-7. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Müller W., Brunner H. Effects of (-)baclofen on inhibitory neurons in the guinea pig hippocampal slice. Pflugers Arch. 1989 Jun;414(2):139–144. doi: 10.1007/BF00580955. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Swandulla D. Quisqualate receptor-mediated rhythmic bursting of rat hypothalamic neurons in dissociated cell culture. Neurosci Lett. 1989 Apr 10;98(3):291–296. doi: 10.1016/0304-3940(89)90416-3. [DOI] [PubMed] [Google Scholar]
- Müller W., Misgeld U. Carbachol reduces IK,baclofen, but not IK,GABA in guinea pig hippocampal slices. Neurosci Lett. 1989 Jul 31;102(2-3):229–234. doi: 10.1016/0304-3940(89)90083-9. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. A bicuculline-resistant inhibitory post-synaptic potential in rat hippocampal pyramidal cells in vitro. J Physiol. 1984 Mar;348:239–254. doi: 10.1113/jphysiol.1984.sp015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. 1984 Mar 29-Apr 4Nature. 308(5958):450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Ogata N., Abe H. Neuropharmacology in the brain slice: effects of substance P on neurons in the guinea-pig hypothalamus. Comp Biochem Physiol C. 1982;72(2):171–178. doi: 10.1016/0306-4492(82)90081-8. [DOI] [PubMed] [Google Scholar]
- Ogata N., Inoue M., Matsuo T. Contrasting properties of K+ conductances induced by baclofen and gamma-aminobutyric acid in slices of the guinea pig hippocampus. Synapse. 1987;1(1):62–69. doi: 10.1002/syn.890010109. [DOI] [PubMed] [Google Scholar]
- Ogata N. Pharmacology and physiology of GABAB receptors. Gen Pharmacol. 1990;21(4):395–402. doi: 10.1016/0306-3623(90)90687-h. [DOI] [PubMed] [Google Scholar]
- Ogata N. gamma-Aminobutyric acid (GABA) causes consistent depolarization of neurons in the guinea pig supraoptic nucleus due to an absence of GABAB recognition sites. Brain Res. 1987 Feb 17;403(2):225–233. doi: 10.1016/0006-8993(87)90059-x. [DOI] [PubMed] [Google Scholar]
- Osmanović S. S., Shefner S. A. Baclofen increases the potassium conductance of rat locus coeruleus neurons recorded in brain slices. Brain Res. 1988 Jan 12;438(1-2):124–136. doi: 10.1016/0006-8993(88)91331-5. [DOI] [PubMed] [Google Scholar]
- Pierau F. K., Zimmermann P. Action of a GABA-derivative on postsynaptic potentials and membrane properties of cats' spinal motoneurones. Brain Res. 1973 May 17;54:376–380. doi: 10.1016/0006-8993(73)90064-4. [DOI] [PubMed] [Google Scholar]
- Premkumar L. S., Chung S. H., Gage P. W. GABA-induced potassium channels in cultured neurons. Proc Biol Sci. 1990 Aug 22;241(1301):153–158. doi: 10.1098/rspb.1990.0079. [DOI] [PubMed] [Google Scholar]
- Saint D. A., Thomas T., Gage P. W. GABAB agonists modulate a transient potassium current in cultured mammalian hippocampal neurons. Neurosci Lett. 1990 Oct 2;118(1):9–13. doi: 10.1016/0304-3940(90)90236-3. [DOI] [PubMed] [Google Scholar]
- Seabrook G. R., Howson W., Lacey M. G. Electrophysiological characterization of potent agonists and antagonists at pre- and postsynaptic GABAB receptors on neurones in rat brain slices. Br J Pharmacol. 1990 Dec;101(4):949–957. doi: 10.1111/j.1476-5381.1990.tb14186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivilotti L., Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36(1):35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Misgeld U. Development and properties of synaptic mechanisms in a network of rat hypothalamic neurons grown in culture. J Neurophysiol. 1990 Sep;64(3):715–726. doi: 10.1152/jn.1990.64.3.715. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Dichter M., Morad M. Quisqualate activates a rapidly inactivating high conductance ionic channel in hippocampal neurons. Science. 1989 Mar 17;243(4897):1474–1477. doi: 10.1126/science.2467378. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci. 1987 Oct;7(10):3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich S., Wollheim C. B. GTP-dependent inhibition of insulin secretion by epinephrine in permeabilized RINm5F cells. Lack of correlation between insulin secretion and cyclic AMP levels. J Biol Chem. 1988 Jun 25;263(18):8615–8620. [PubMed] [Google Scholar]


