Significance
Nitrifiers live via oxidation of ammonia as their cellular fuel. In doing so, they drive key transformations in the nitrogen cycle. Many products and by-products of their metabolisms are environmental and atmospheric pollutants of concern. Key among these by-products, is nitrous oxide (N2O), a greenhouse gas and ozone-depleting agent. Ammonia oxidizing archaea (AOA) specifically have been identified as major N2O producers. We have characterized a multicopper oxidase in a lineage unique to AOA that effects the selective oxidation of the nitrification intermediate hydroxylamine (NH2OH) to nitroxyl (HNO), which ultimately forms N2O and N2. This result affords one means by which AOA may produce N2O and identifies a physiologically relevant mechanism for the formation of the enigmatic nitrogen oxide HNO.
Keywords: nitrification, nitrous oxide, enzymology, bioinorganic chemistry
Abstract
Ammonia oxidizing archaea (AOA) are among the most abundant microorganisms on earth and are known to be a major source of nitrous oxide (N2O) emissions, although biochemical origins of this N2O remain unknown. Enzymological details of AOA nitrogen metabolism are broadly unavailable. We report the recombinant expression, purification, and characterization of a multicopper oxidase, Nmar_1354, from the AOA Nitrosopumilus maritimus. We show that Nmar_1354 selectively produces nitroxyl (HNO) by coupling the oxidation of the obligate nitrification intermediate hydroxylamine (NH2OH) to dioxygen (O2) reduction. This HNO undergoes several downstream reactions, although the major fates are production of N2 via reaction with NH2OH and dimerization with itself to yield N2O. These results afford one plausible enzymatic origin for N2O release by AOA. Moreover, these results reveal a physiologically relevant enzymatic reaction for producing HNO, an enigmatic nitrogen oxide speculated to be operative in cellular signaling and in energy transduction.
Ammonia oxidizing archaea (AOA) are among the most abundant microorganisms on Earth (1–3). Since the first AOA were reported in 2005 (4), they have been found in almost every ecosystem where they carry out nitrification—aerobic oxidation of ammonia (NH3) to nitrite (NO2–)—as their primary metabolism (5). It is now recognized that these organisms dominate nitrification in many ecosystems. Remarkably, AOA have been identified as key emitters of the potent greenhouse gas nitrous oxide (N2O), particularly in the oceans (6–9). Moreover, AOA participate in carbon cycling and produce numerous secondary metabolites of importance to other organisms (10–12).
Despite their ubiquity and the global impact of the AOA metabolism, the fundamental reactions underlying AOA metabolism remain largely uncharacterized due to the difficulty of growing AOA in pure culture (5, 13). In 2010, the first AOA genome—from the marine archaeon Nitrosopumilus maritimus—revealed many differences from that of ammonia-oxidizing bacteria (AOB) despite their similarities to the better-characterized metabolism of AOB nitrification. These discrepancies have since been supported by a more global analysis of multiple AOA genomes (14–16). Specifically, AOA lack genes encoding the multi c-heme enzyme hydroxylamine oxidoreductase (HAO) responsible for the generation of reducing equivalents for respiration from hydroxylamine (NH2OH), the first common obligate intermediate of AOA and AOB metabolism (17). In fact, AOA lack any canonical c-heme biosynthesis pathways, strongly suggesting distinct mechanisms operative in NH2OH oxidation (18). The presence of 24 genes in N. maritimus encoding proteins bearing type 1 (T1, blue) copper domains, including multicopper oxidases (MCOs) (19) led to an early suggestion that AOA may substitute Cu for the Fe-based NH2OH oxidation machinery of AOB (14, 20). Subsequent comparative proteomics analysis indicated that AOA MCOs are not conserved among the core clusters of orthologous groups (COGs) in these organisms (15). This suggests that the MCOs are not constituents of the primary metabolism. However, the genomic abundance of these MCOs prompted us to explore their reactivity.
Recent work has implicated NH2OH as a principal source of N-atoms in AOA-derived N2O (6). This N2O production, however, has previously been speculated to occur via abiotic reactions between pathway intermediates of ammonia oxidation, presumably facilitated by free redox-active transition metals (21). Moreover, the limited studies of NH2OH reactivity with Cu show that N2O is a major product (22). Consequently, we speculated that N2O emission by AOA could be attributed to the reaction of NH2OH with a Cu enzyme, namely, one or more of their MCOs. Previously, we had shown that AOB can generate N2O via the reaction of NH2OH with heme-bound nitric oxide (NO), the product of HAO and thus second obligate intermediate of bacterial NH3 oxidation (23). This reaction can be considered a waste pathway because no net electron flow to respiration is generated from NH2OH oxidation. Moreover, N2O exhibits no apparent bioenergetic role in nitrifiers, especially given that both AOB and AOA lack copies of the only known enzyme family to reduce N2O, N2O reductase (24–27). Consequently, N2O production by AOA could occur via reactions that lie off of the primary metabolism, meaning that the operative enzymes need not be constituents of COGs (28, 29).
The numerous MCOs encoded by AOA have been grouped by lineage (15). AOA produce a number of homologs to Cu nitrite reductases (NirK), which are MCOs bearing T1 Cu centers for electron transfer and type 2 (T2) Cu centers for catalysis (30). In addition, AOA encode numerous two-domain, homotrimeric MCOs (2dMCOs) bearing trinuclear Cu centers comprising T2 and binuclear type 3 (T3) sites (31, 32). Of note is a lineage of unusual 2dMCOs bearing an additional C-terminal pendant T1-containing cupredoxin domain. This lineage, MCO4, is unique to AOA, where it is found in the order Nitrososphaerales and the genera Nitrosopumilus and Nitrosarchaeum (15). Thus, we chose to study Nmar_1354, an MCO4 from the model marine AOA N. maritimus.
Purification and Characterization of Nmar_1354.
We constructed a recombinant expression platform for Nmar_1354. The Nmar_1354 gene was codon-optimized for Escherichia coli and cloned into a pET-22(b)+ vector. To preclude binding of adventitious Cu, we eschewed a His6 tag. Holo Nmar_1354 can be purified to homogeneity following column chromatography with a yield of 3 mg/L. The protein is unstable to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) conditions, with lanes containing purified protein showing multiple bands (SI Appendix, Fig. S1). Mass spectrometry (MS) revealed that these bands correspond to Nmar_1354. Blue-Native PAGE shows three bands (SI Appendix, Fig. S2). Upon excision and submission to SDS-PAGE, these bands show the same fragmentation as samples analyzed directly by SDS-PAGE. Size-exclusion chromatography reveals that purified Nmar_1354 elutes as one band at a molecular weight consistent with the crystallographically characterized protein complex (SI Appendix, Figs. S3 and S4). UV/vis absorption spectroscopy reveals characteristic bands at 600 nm and ca. 350 nm for T1 Cu (S 3pπ → Cu 3d) and binuclear T3 centers, respectively (SI Appendix, Fig. S5) (19). X-band electron paramagnetic resonance (EPR) spectroscopy reveals the presence of two distinct S = 1/2 spin systems, one corresponding to T1 Cu and the other to T2 Cu centers (SI Appendix, Fig. S6). Allowing the relative abundance of the spin systems to float as a fitting parameter shows that for every T1 Cu center, there are 0.5 ± 0.1 T2 Cu centers (SI Appendix, Table S1). The presence of these two spin systems is consistent with the standard 2dMCO arrangement of a T1 Cu center and a dioxygen-reducing (O2) trinuclear Cu site. However, the stoichiometry is consistent with additional T1 Cu centers, indicating that the extra cupredoxin domains are present and metalated.
Structure.
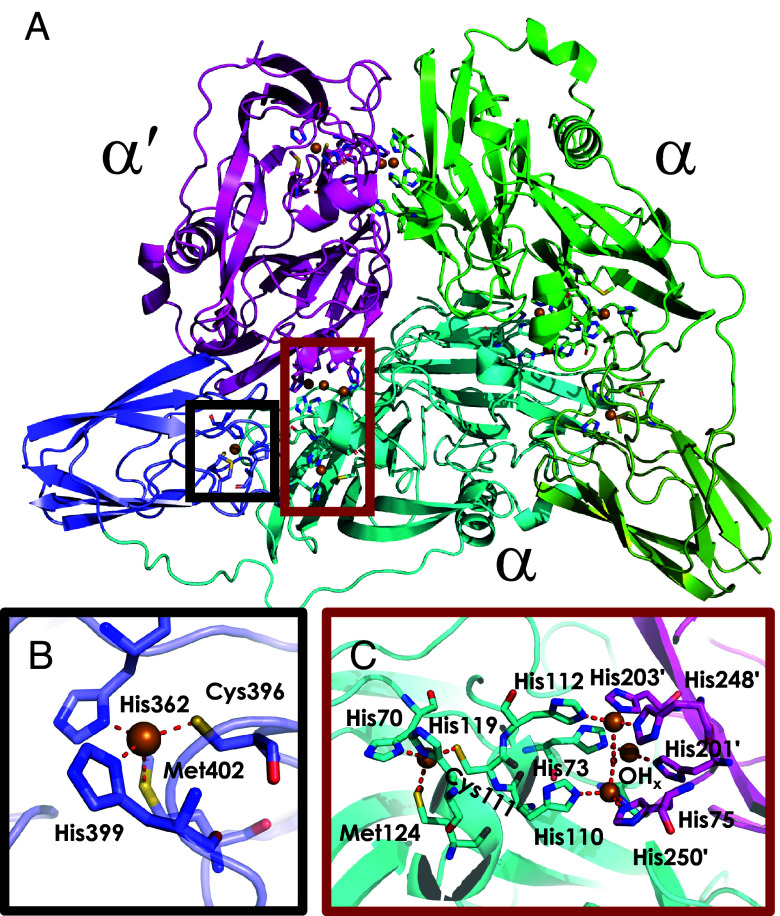
We identified suitable conditions for crystallization of Nmar_1354, yielding crystals that afforded high-quality X-ray diffraction data to 1.8 Å. The crystals used to obtain the structure were harvested, washed with buffer, and analyzed by SDS-PAGE, revealing the same fragmentation pattern as the freshly chromatographed-material. The protein crystallizes in the P1211 spacegroup with a large unit cell comprising four proteins in the asymmetric unit. Nmar_1354 presents as ααα′ heterotrimers, where the α units bear the additional, “dangling” cupredoxin domain connected to the canonical 2dMCO via an ordered but unstructured 15 amino acid linker (Fig. 1A). Both the dangling and 2dMCO T1 Cu sites comprise the standard His2Cys inner-sphere coordination sphere in addition to weak axial Met ligation (Fig. 1 B and C) (19). Within the canonical 2dMCO units, the T1 Cu sites are connected to the trinuclear copper sites via Cys–His diads, with T1Cu–T3Cu distances of 12.6 Å (Fig. 1C) (19, 31). The dangling cupredoxin T1Cu sites are situated 13.5 Å from the 2dMCO T1Cu sites.
Fig. 1.
1.84 Å resolution crystal structure of Nmar_1354. (A) Quaternary structure of one of the four identical Nmar_1354 complexes in the asymmetric unit revealing the presence of two two-domain multicopper oxidase (2dMCO) monomers bearing “dangler” cupredoxin units and one without this unit, comprising an ααα′ structure. (B) Type 1 (T1) copper binding site of one of the dangler cupredoxins. (C) T1 and type 2/type 3 trinuclear copper sites constituted at the interface of two of the 2dMCO units. PDBID: 9CCK.
Production of N2O via Nitroxyl.
Treatment of 3 µM Nmar_1354 with varied, excess concentrations of NH2OH under buffered (100 mM HEPES, pH 8.0) aerobic conditions promotes consumption of O2 as measured by a Clark electrode (Fig. 2 A and D and SI Appendix, Fig. S7). This reactivity shows saturation behavior, although it cannot be modeled with simple Michaelis–Menten kinetics (vide infra). Gas chromatrography-MS (GC-MS) analysis reveals N2O to be a significant but nonstoichiometric product of this reaction (Fig. 2B). No NH3 is produced, obviating the possibility of NH2OH disproportionation. To rule out the reaction of NH2OH with adventitious Cu, we carried out analogous experiments using CuSO4 in place of Nmar_1354. These experiments revealed rapid consumption of O2, production of N2O, and no production of NH3 (SI Appendix, Fig. S8). However, this reaction was completely inhibited by the addition of excess ethylenediaminetetraacetic acid (EDTA) in agreement with previous studies (22). The addition of excess EDTA to the aerobic reaction between Nmar_1354 and NH2OH showed no inhibitory effect indicating that the reactivity shown by Nmar_1354 is not due to adventitious metal binding or free metal ions in the buffer. Moreover, the absence of NH2OH-driven O2 consumption by purified E. coli copper efflux oxidase (CuEO) (33) indicates that this reactivity is not general to MCOs (SI Appendix, Fig. S9).
Fig. 2.

Reactivity of Nmar_1354 with NH2OH. (A) Kinetics assayed by O2 respirometry (Clark-style electrode) monitoring O2-dependent consumption of NH2OH according to Eq. 1. (B) N2O production assayed by gas chromatograph-mass spectrometry (GC-MS) of the headspace of sealed reaction vials containing varied concentrations of NH2OH (red) compared to theoretical yields from a global kinetics model (gray, see SI Appendix). (C) Fluorescence response yields for reactions containing 10 mM NH2OH, and 20 μM 2-oxo-2H-chromen-7-yl 2-(diphenylphosphaneyl)benzoate (P-CM) probe assayed after 2 h of reaction. Condition 1: P-CM alone. Condition 2: P-CM + Nmar_1354. Condition 3: P-CM + 10 mM NH2OH. Condition 4: P-CM + Nmar_1354 + 10 mM NH2OH. All reactions in (A–C) c were carried out at 25 °C in 100 mM HEPES buffer at pH 8.0 that was saturated with air. Reactions involving Nmar_1354 were carried out with 3 µM protein. (D) Real-time MS (m/z = 30, 46) (Left axis) of an aerobic reaction containing 6 μM Nmar_1354 and 20 mM 15NH2OH in 100 mM HEPES at pH 8.0. Reactions were initiated at time “0” by the addition of Nmar_1354 to the reaction mixture. Right axis: independent respirometry measurement under identical conditions to the real-time MS showing that the production of 15N15N and 15N15NO ceases upon complete consumption of O2.
An early study by Anderson (22) of aerobic Cu reactivity with NH2OH revealed N2O as a major product. Anderson hypothesized that nitroxyl (HNO) was the actual product of NH2OH oxidation by Cu, although no direct evidence has been afforded. HNO has been the subject of substantial attention due to its implication as a cell signaling agent with orthogonal activity to NO (34). This was recently elaborated upon in a report showing that HNO can be formed as an enzymatic product in eukaryotes, where it is produced from NO via the reaction of S-nitrosothiol with lipoate thiol moieties (35). Direct detection of HNO is challenging due to its high second-order rate constant (8 × 106 M–1 s–1 at pH 7.4) for the formation of N2O via dimerization (36). Moreover, HNO reacts with all of the other reaction constituents under consideration: With O2, it forms peroxynitrite (ONOO–) (3.0 × 103 – 1.8 × 104 M–1 s–1 at pH 7.4) and with NH2OH, it forms N2 (4 × 103 – 2.1 × 104 M–1 s–1 at pH 7.4) (34, 37–41). To furnish some indirect evidence for HNO formation, we spiked the HNO donor Angeli’s salt (Na2N2O3) into aerobic reactions of Nmar_1354 with 15NH2OH. GC-MS data revealed the formation of all possible isotopes of N2O: 14N14NO (Angeli’s salt derived H14NO dimerization), 14N15NO/15N14NO (15NH2OH/Nmar_1354 H15NO reacting with Angeli’s salt derived H14NO), and 15N15NO (dimerization of H15NO from 15NH2OH/Nmar_1354) (SI Appendix, Fig. S14). Furthermore, GC-MS studies with Nmar_1354 showed a significantly higher N2O yield at pH 6.0 than at pH 8.0, consistent with Anderson’s observations of diminished reactivity of HNO with NH2OH at more acidic pH (22). We hypothesize the enzymatic oxidation of NH2OH to HNO by Nmar_1354 take place according to Eq. 1.
| [1] |
HNO is also known to rapidly react with phosphines to form ylides and phosphine oxides, enabling the development of fluorometric assays for this fleeting species (41, 42). We employed 2-oxo-2H-chromen-7-yl 2-(diphenylphosphaneyl)benzoate (P-CM), a turn-on fluorometric sensor that exhibits high specificity for HNO over other possible constituents of our reactions including metal ions, nitric oxide, etc (43). We observed a 3.6-fold fluorescence turn on when we included this reagent in our assays (Fig. 2C). This response is comparable to previously reported results for detection of HNO in the presence of bovine serum to simulate the consumption of some HNO by reactivity with amino acid sidechains, e.g., thiolates (43).
Given the reactivity of HNO with itself and other reaction constituents, we assembled a global kinetic model to facilitate interpretation of the saturation kinetics data as well as the yields of N2O relative to input NH2OH (44). This model employed previously published rate constant data for all HNO reactions with the exception of reactions with protein sidechains, which given the relative abundance of Nmar_1354, we assumed to be negligible. We also assumed instantaneous transfer of O2 into buffer. (Further details are included in Materials and Methods and in SI Appendix.) Using this model, we obtained a good fit to the saturation kinetics curve, yielding a KM of 22 ± 5 mM for NH2OH and a kcat of 0.3 ± 0.1 s–1, giving a specificity constant kcat/KM of 13.6 ± 0.5 M–1 ∙s–1. The kinetic model also accurately reproduces the magnitude of and trend in N2O yield as a function of input NH2OH (Fig. 2A).
The assembled kinetic model indicates that N2 should be a major product via the reaction of NH2OH with HNO. To test this, we carried out similar assays and monitored species by MS in real time using a modified differential electrochemical MS (DEMS) apparatus (45). Consistent with the predictions of this model, we observed concomitant production of 15N15N with 15N15NO when 15NH2OH was supplied as the N-atom source. Production of these products ceased upon complete consumption of O2, consistent with the role of O2 as the terminal electron acceptor in this reaction (Fig. 2D). The kinetic model also predicts the formation of peroxynitrite (ONOO–), which is known to decompose predominantly to nitrate (NO3–). In a reaction containing 0.5 mM NH2OH and ambient O2, 40 µM NO3– is produced, consistent with the model.
Implications.
Together, these results establish precedent for enzymatic production of HNO and precedent for NH2OH as a substrate of a copper enzyme. Overall, the expression of Nmar_1354 in N. maritimus (46) and homologs in other AOA (47), the production of NH2OH by AOA (17), and the aerobic nature of AOA all enable N2O production by this mechanism. NH2OH-driven O2 consumption by an MCO4 provides a plausible explanation for the promotion of O2 consumption by AOA when treated in vivo with NH2OH (48), Thus, these results establish a plausible enzymatic origin for N2O production by one of Earth’s principal producers of this greenhouse gas—AOA. The contribution of MCO4 orthologs is likely more relevant in soil than in marine environments. Case in point, Nitrosopelagicus brevis, a highly ubiquitous ocean AOA, lacks an MCO4 ortholog, whereas most soil AOA (with the exception of acidophilic Nitrosotalea) bear MCO4 orthologs (15). These results also offer a mechanism by which AOA may produce N2 via the reaction of enzymatically produced HNO with NH2OH. N2 has been otherwise implicated as a product of NO dismutation by a heretofore unknown enzyme/mechanism in these organisms (49).
The modest specificity constant for NH2OH oxidation by Nmar_1354 is consistent with the production of N2O being a waste pathway, presumably to prevent the accumulation of NH2OH to cytotoxic levels. That reducing equivalents from NH2OH are directly transferred to O2 without the involvement of any respiratory apparatus is also consistent with this reaction occurring off the primary metabolic pathway. More rapid consumption of NH2OH via MCO-driven oxidation to HNO would deplete a critical obligate intermediate from productive participation in AOA metabolism. Consequently, the functional equivalent in AOA to the bacterial HAO remains elusive.
It remains to be seen whether there is some functional role for HNO in the AOA metabolism. Moreover, the production of HNO via NH2OH as a reducing equivalent for an MCO may have biocatalytic utility. These remain active areas of inquiry in our laboratory.
Materials and Methods
General Considerations.
Milli-Q water (18.2 MΩ; Millipore) was used in the preparation of all buffers and solutions. UV-visible (UV-vis) absorption spectra were obtained using a Cary 60 UV-vis spectrometer (Agilent) with temperature control set to 25 °C. Continuous-wave EPR spectra were measured at X-band (9.3 GHz) using a Bruker Elexsys-II spectrometer maintained at 77 K by a flow cryostat (Oxford) cooled with liquid N2. Spectra were simulated using EasySpin (50). Fluorescence data were obtained using a BioTek Cytation 5 plate reader with a DAPI filter cube using an excitation wavelength of 377 nm and an emission wavelength of 447 nm (BioTek Instruments). Kinetics and activity assay data were measured using a Clark oxygen electrode (Oxygraph+ system, Hansatech). Data were fit using Igor Pro version 9.05 (WaveMetrics). The HNO-donor disodium diazen-1- ium-1,2,2-triolate (Angeli’s salt, Cayman Chemicals) was used to generate the N2O calibration curve for GC experiments as well as for mixed-isotope N2O measurements. 15N-labeled hydroxylamine (15NH2OH) was purchased from Sigma-Aldrich. All other chemicals were purchased from VWR International. All reactions were performed in technical triplicate using air-saturated buffer solutions.
Nmar_1354 Gene Synthesis.
The Nmar_1354 gene was codon optimized for expression in E. coli, synthesized, and cloned into the pET22(b)+ vector with the His6 tag removed by GenScript. The codon-optimized gene sequence is as follows:
GCTAGCTTAATTCACGGAAATCGTGCCCTTCATCCACGGATGCAGGGTGCAGATATAGTCGAACTCACCGGGGTTGTCGAACGAGTAGGACCAGGTGTTGCCTGCCTGGATAAAACCGGAATCGAAGCTGTTTTCGTTATCGGTAACCGTGTGCACAACGCTATCGTCATTGGTCCAGGTCACAGTTGTACCGCTATCAACTTGAATCGCTATTGGATCGTAGCTTTCGGTAATTTCCGGATTCCATGAATCTTTCACAATGGAAACTTTATCGGCGCTGACCTTCTCCGATGAAATTGCCGCGCTCTCACCCAGATTTTCGTAGCTGATCAGCTGCGGCTGCTCCAAGGTGCGGATCAGGTCTTCCTGCCATTCAACCATACTGTAGCTACCTTTGTTATTAGACGGACGCTGCACATCGGACAGGCTGCTCGCGTCCTCGAGGACCTCAATCATTGCCATGCTACCACGCTCTTCTTGGATGCCATGTACGTGGAACAGGTACATACCCGGCTGGGTCCAGGTAGCTTCGATGATCGCGGTGTCGCCGTTACCGATCAGGTGAGTCTGCGCATAATACGGTTCATTCCACAGAATGCCGCTCGGATAGACCTTCATGATGGTGCTGTGAACGTGAAACGGGCTTTGCAGGACACCGCCGATACCAACAACATAAAAACGGGTCAACTCGCCAGACACCACGCGAATCGGGTTGTCCATATACTGGCCAGCATAACCGTTGATCGGGTAGAATTCGGTGAAGTGCTCTAGAGCGTTGTTCGGGTCGAACTCGGACAAGGTAAAGAAGTATTCACGCGCCGGATCCATCGGACGAATCGCCGGATCGATAATCATCGTACCAAACATACCCATGCGCACGTGCTCGCTGGTCGGAAACGCATGACAATGGTACATAAACAGACCGGCCTCTTCTGCAACAAAATGATACGTGTACTCTTCGCCCGGCATGATCATCGGAAAAACGCCGTCATTAGCAGAATCATGGGTGCCGTGGAAATGAATGGTGTGCGCGTACGGGGTGTCGTTCACGAACTTTACAGTCACGTCGTCACCTTCGCTGAAACGCAGAGTTGGCGCCGGCACCGTGCCGTTATACGTCCACACTTTTGCTCTAACTCCCGGCGAAACCTCGATTTCCGCGTCTTGGGCAATCAAGGTGTAAGATAACGTTTGCGGATTAGGGTTTTTGAACTTGGGAAATGTATCTGAACTGTCCATATG
Protein Expression and Purification of Nmar_1354.
Protein expression and purity were assayed by SDS-PAGE, shown below, as well as UV-vis absorption spectroscopy. BL21(DE3) E. coli cells were transformed with the plasmid and single colonies were picked and grown in terrific broth (TB) medium at 30 °C to an OD600 of ~1.0. The growth was then cooled to 20 °C and was brought to 0.75 mM in CuSO4 and allowed to equilibrate for 1 h. The medium was then brought to 0.4 mM in isopropyl β-D-1-thiogalactopyranoside (IPTG) and the cells were allowed to induce for 18 h before harvesting. Cells were lysed via the osmotic shock procedure using a lysis buffer of 50 mM tris(hydroxymethyl)aminomethane (Tris) (pH 8.0) and 0.01% TritonX. The soluble fraction of the lysate was brought to 1 mM in CuSO4 and allowed to sit at 4 °C overnight. The lysate was directly applied to a Q-sepharose FPLC column (Cytiva Life Sciences) equilibrated in 50 mM tris (pH 8.0). A linear NaCl gradient was run (0 to 500 mM NaCl) and Nmar_1354 elutes from ~33 to 40 mS/cm as evidenced by blue fractions and by SDS-PAGE. These blue fractions were pooled and concentrated to a volume of 2 mL and applied to a Superdex 200 pg size exclusion column (Cytiva) equilibrated in 50 mM tris (pH 8.0) and 150 mM NaCl. Blue fractions were pooled from this column and the protein was buffer exchanged into 50 mM 2-[Bis-(2-hydroxy-ethyl)-amino]-2-hydroxymethyl-propane-1,3-diol (Bis-Tris) (pH 6.0) and spun down to remove precipitated protein. The blue supernatant was applied to a Q-sepharose column in the same buffer and eluted over a linear NaCl gradient of 0 to 500 mM NaCl, where the blue fractions were pooled and concentrated to a volume of 2 mL. This nearly pure Nmar_1354 was then applied to a pg 75 superdex column, which yielded pure Nmar_1354. Nmar_1354 is not stable to SDS-PAGE conditions, as the SDS-PAGE of pure protein shows multiple bands. These bands were verified to be Nmar_1354 by MS. Additionally, the SDS-PAGE of crystals which were picked and dissolved in SDS buffer show the same profile. Pure fractions of Nmar_1354 were pooled and buffer exchanged into 50 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES) (pH 8.0) for storage. Before use in any experiments, pure Nmar_1354 was treated with a 10-fold excess of ethylenediaminetetraacetic acid (EDTA) to remove any adventitious metals from solution or weakly bound to the protein. The protein was then desalted using a PD-10 column (Cytiva) equilibrated in 50 mM HEPES (pH 8.0).
Gene Synthesis of CuEO.
CuEO from E. coli was synthesized and cloned into the pET22(b)+ vector with the poly His tag removed by GenScript.
Protein Expression and Purification of CuEO.
Protein expression and purity were assayed by SDS-PAGE as well as UV-vis absorption spectroscopy. BL21(DE3) E. coli cells were transformed with the plasmid and single colonies were picked and grown in TB medium at 30 °C to an OD600 of ~1.0. The growth was then cooled to 20 °C and was brought to 0.75 mM in CuSO4 and allowed to equilibrate for 1 h. The medium was then brought to 0.4 mM in IPTG and the cells were allowed to induce for 18 h before harvesting. Cells were lysed via the osmotic shock procedure using a lysis buffer of 50 mM Tris (pH 8.0), 0.01% TritonX. The soluble fraction of the lysate was brought to 1 mM in CuSO4 and allowed to sit at 4 °C overnight. The lysate was directly applied to a Q-sepharose FPLC column (Cytiva Life Sciences) equilibrated in 50 mM Tris (pH 8.0). A linear NaCl gradient was run (0 to 500 mM NaCl) and Nmar_1354 elutes from ca. 6 to 12 mS/cm as evidenced by blue fractions and by SDS-PAGE. These blue fractions were pooled and concentrated to a volume of 2 mL and applied to a Superdex 200 size exclusion column equilibrated in 50 mM Tris (pH 8.0) and 150 mM NaCl. Blue fractions eluted in a single peak and showed only a single band by SDS-PAGE at ca. 50 kDa.
Protein Crystallization.
Pure Nmar_1354 was buffer exchanged into 50 mM potassium phosphate dibasic (pH 7.0) and brought to a concentration of 12.5 mg/mL, as determined by the A280, assuming 1 absorbance unit (AU) = 1 mg/mL. The buffer used for precipitation was 100 mM BIS-TRIS propane (pH 7.0) and 1.3 to 1.8 M ammonium sulfate. Crystals were grown using a 1:1 v/v ratio of the protein and precipitant solutions via the sitting drop method. Cornflower blue crystals grew as plates after ca. 8 d.
Crystallographic Data Collection.
X-ray diffraction measurements were carried out at beamline 24-ID-C of the Advanced Photon Source (APS) Northeastern Collaborative Access Team (NE-CAT). Crystals were irradiated at 100 K using X-rays with a wavelength (λ) of 0.979 Å. X-ray diffraction data were indexed, integrated, scaled, and merged using the programs XDS3 (51) and CCP4 7.0 (52). An initial model was generated in Phenix 1.2 (53) using the molecular replacement method and an AlphaFold2 (54) model of a truncated version of the protein consisting of only the first 300 amino acids. The remaining amino acids containing the unstructured linker and C-terminal cupredoxin domain were manually built in to fit the experimental electron density. Refinements and completion of model building were conducted using Phenix and Coot 0.9 (55), respectively. PyMol2.6 (56) was used to create figures.
Enzyme Assay Conditions.
Activity of Nmar_1354 toward NH2OH was assayed by Oxygraph+ system, where consumption of O2 was used as a handle for catalytic activity. All experiments were conducted in 100 mM HEPES (pH 8.0) and all reagents were dissolved in the same buffer. Fresh NH2OH stocks were prepared the same day as the measurements were taken to ensure reproducibility. For generating kinetics data, the Oxygraph+ system was equilibrated at 25 °C using a water jacket and with 100 rpm stirring using a magnetic stir bar. Then, 1 mL of 100 mM HEPES (pH 8.0) and 3 µM protein was allowed to equilibrate for ca. 5 min to establish a baseline and then the reaction was initiated by addition of a stock NH2OH solution via Hamilton syringe.
NH3 production was assayed using Berthelot’s method (57) on reaction filtrate (to remove protein) of a solution of 3 µM Nmar_1354 and 10 mM NH2OH in 100 mM HEPES (pH 8.0). This assay revealed no significant formation of NH3 vs. a control containing no Nmar_1354.
To assay for nitrite (NO2–) production, reaction filtrate (as mentioned above) was subjected to Griess reagent (Thermo Scientific) (58). This assay revealed no significant formation of NO2– vs. a control lacking Nmar_1354.
Nitrate (NO3–) production was assayed by subjecting reaction filtrate to excess NADH and nitrate reductase and subsequent filtration and reaction with Griess reagent as mentioned above and quantified via a calibration curve obtained using a standard NO2– stock. While this quantity cannot be checked against the quantity of N2 produced due to our inability to experimentally quantify N2 production, it does fall between predicted values generated by our theoretical model at low (10 µM O2, 10 µM HOONO) and high (250 µM O2, 160 µM HOONO) constant concentrations of O2 in solution.
MS.
Headspace GC-MS studies for N2O detection and quantitation were performed in 2 mL clear GC vials using 0.5 mL total reaction volumes. Reactions were set up, capped, and allowed to react overnight before data collection of the headspace. All reactions were initiated by the addition of NH2OH and quickly capped to prevent loss of volatile products. Data were analyzed using Xcalibur4 (Thermo Scientific) and integrated to determine total amounts of N2O using ProteoWizard (59) and Metaboseek (60) software. For pH-dependence studies, the same protocol was used with 50 mM 1,4-Piperazinediethanesulfonic acid (PIPES) (pH 6.0) buffer instead of HEPES.
Real-time MS measurements for N2 detection were performed using a modified DEMS setup to track in situ/operando evolution of 15N-labeled or 14N-labeled N2 and N2O. Reactions were set up in a liquid feeder cell open to ambient air where 3 mL total reaction mixtures were used. MS data collection was started with 100 mM HEPES (pH 8.0) buffer and allowed to collect for ~5 min to establish a baseline followed by the addition of NH2OH to 20 mM and this mixture was allowed to collect for an additional ~5 min to establish a baseline with 15N present. The reaction was initiated by addition of Nmar_1354 to 6 µM.
In a conventional DEMS cell, a porous Teflon membrane (a thickness of 75 mm, a porosity of 50%, and a pore size of 20 nm, Gore-Tex), mechanically supported on a stainless-steel frit, was used as the interface between the aqueous solution and vacuum. The gases, generated from the reaction, can evaporate through Teflon membrane into the vacuum, and subsequently be detected with a quadrupole mass spectrometer (HiQuad 300, Pfeiffer Vacuum).
Fluorescence Assays.
Synthesis and purification of “turn on” probe 2-oxo-2H-chromen-7-yl 2-(diphenylphosphaneyl) benzoate (P-CM) was performed according to previously reported procedures (43). P-CM was added to reactions via a DMSO stock solution. Then, 1 mL total reaction volumes in Eppendorf tubes were used in all reactions and all were done in technical triplicate. Further, 100 mM HEPES (pH 8.0) was brought to 25 µM in P-CM and 3 µM in Nmar_1354. The reactions were initiated by the addition of NH2OH to 10 mM. These solutions were capped, inverted 10 times, and allowed to react for 2 h. The solutions were then placed into a centrifuge and spun at 13,000 rpm for 30 min to remove any precipitated P-CM or protein. Then, 200 µL of supernatant was added to a black-walled 96-well plate for fluorescence measurement.
Kintecus Modeling.
Global kinetics modeling of Nmar_1354 was performed using Kintecus software (44). Rate constants used for the reactions were obtained from the literature in the case of HNO reactivity with NH2OH and reactivity with other HNO molecules as described in the main text. Other rate constants were determined experimentally. Of note, reactivity between HNO and O2 was omitted and O2 concentration was kept constant due to the complexity of modeling O2 diffusion into solution, consumption by HNO, and consumption by Nmar_1354. Additionally, the kinetics of O2 binding to Nmar_1354 was calculated to be 4,100 M−1 s−1 through Michaelis–Menten kinetics analysis of the rate of O2 consumption as a function of O2 concentration in our oxygen electrode studies. Here, we assumed excess NH2OH, so that the Km would not be influenced by the rate of binding of NH2OH, which we found to be a fair assumption by comparing Km values from oxygen electrode experiments using varying [NH2OH] values.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank Tyler Schwertfeger for assistance with GC-MS data acquisition and analysis. We are grateful to Christa Schleper and Logan Hodgskiss for discussions of AOA proteomics and metabolism. We also thank Melissa Bollmeyer, Alex Laughlin, Colby Gekko, Rachael Coleman, and Samantha MacMillan for feedback on this manuscript. This work is based upon research conducted at the Northeastern Collaborative Access Team (NECAT) beamlines, which are funded by the National Institute of General Medical Sciences (NIGMS) from the NIH. This research used resources of the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory. EPR data were acquired at the National Biomedical Resource for Advanced ESR Spectroscopy (ACERT), which is supported by NIGMS. This work was supported by NIH grants R35GM124908 (K.M.L.), P30 GM124165 (NECAT), and 1R24GM146107 (ACERT), Department of Energy Office of Science grant DE-AC02-06CH11357 (NECAT), and the NSF Center for Synthetic Organic Electrochemistry, grant number CHE-1740656 (H.D.A.).
Author contributions
K.M.L. designed research; R.W.V., H.W., and K.M.L. performed research; H.W. and H.D.A. contributed new reagents/analytic tools; R.W.V. and K.M.L. analyzed data; and R.W.V., H.W., H.D.A., and K.M.L. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Preprint servers: A version of thismanuscript has been submitted to ChemRxiv and may be accessed at https://doi.org/10.26434/chemrxiv-2024-s3hx8. The work is available under a CC BY NC ND 4.0 license.
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
Protein structure data have been deposited in PDB (9CCK) (61). All other data are included in the manuscript and/or SI Appendix.
Supporting Information
References
- 1.Santoro A. E., Richter R. A., Dupont C. L., Planktonic Marine Archaea. Ann. Rev. Mar. Sci. 11, 131–158 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Martens-Habbena W., Berube P. M., Urakawa H., de la Torre J. R., Stahl D. A., Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461, 976–979 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Offre P., Spang A., Schleper C., Archaea in biogeochemical cycles. Annu. Rev. Microbiol. 67, 437–457 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Könneke M., et al. , Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437, 543–546 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Lancaster K. M., Caranto J. D., Majer S. H., Smith M. A., Alternative bioenergy: Updates to and challenges in nitrification metalloenzymology. Joule 2, 421–441 (2018). [Google Scholar]
- 6.Wan X. S., et al. , Pathways of N2O production by marine ammonia-oxidizing archaea determined from dual-isotope labeling. Proc. Natl. Acad. Sci. U.S.A. 120, e2220697120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santoro A. E., Buchwald C., McIlvin M. R., Casciotti K. L., Isotopic signature of N2O produced by marine ammonia-oxidizing archaea. Science 333, 1282–1285 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Buitenhuis E. T., Suntharalingam P., Le Quéré C., Constraints on global oceanic emissions of N2O from observations and models. Biogeosciences 15, 2161–2175 (2018). [Google Scholar]
- 9.Freing A., Wallace D. W. R., Bange H. W., Global oceanic production of nitrous oxide. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 1245–1255 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Könneke M., et al. , Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation. Proc. Natl. Acad. Sci. U.S.A. 111, 8239–8244 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayer B., et al. , Ammonia-oxidizing archaea release a suite of organic compounds potentially fueling prokaryotic heterotrophy in the ocean. Environ. Microbiol. 21, 4062–4075 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawley A. K., et al. , Diverse Marinimicrobia bacteria may mediate coupled biogeochemical cycles along eco-thermodynamic gradients. Nat. Commun. 8, 1507 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright C. L., Lehtovirta-Morley L. E., Nitrification and beyond: Metabolic versatility of ammonia oxidising archaea. ISME J. 17, 1358–1368 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker C. B., et al. , Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc. Natl. Acad. Sci. U.S.A. 107, 8818–8823 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerou M., et al. , Proteomics and comparative genomics of Nitrososphaera viennensis reveal the core genome and adaptations of archaeal ammonia oxidizers. Proc. Natl. Acad. Sci. U.S.A. 113, E7937–E7946 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerou M., et al. , Genomes of Thaumarchaeota from deep sea sediments reveal specific adaptations of three independently evolved lineages. ISME J. 15, 2792–2808 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vajrala N., et al. , Hydroxylamine as an intermediate in ammonia oxidation by globally abundant marine archaea. Proc. Natl. Acad. Sci. U.S.A. 110, 1006–1011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thöny-Meyer L., Cytochrome c maturation: A complex pathway for a simple task? Biochem. Soc. Trans. 30, A50 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Solomon E. I., et al. , Copper active sites in biology. Chem. Rev. 114, 3659–3853 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein L. Y., Insights into the physiology of ammonia-oxidizing microorganisms. Curr. Opin. Chem. Biol. 49, 9–15 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Stein L. Y., et al. , Comment on”A critical review on nitrous oxide production by ammonia-oxidizing archaea” by Lan Wu, Xueming Chen, Wei Wei, Yiwen Liu, Dongbo Wang, and Bing-Jie Ni. Environ. Sci. Technol. 55, 797–798 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Anderson J., The copper-catalysed oxidation of hydroxylamine. Analyst 89, 357–362 (1964). [Google Scholar]
- 23.Caranto J. D., Vilbert A. C., Lancaster K. M., Nitrosomonas europaea cytochrome P460 is a direct link between nitrification and nitrous oxide emission. Proc. Natl. Acad. Sci. U.S.A. 113, 14704–14709 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tourna M., et al. , Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc. Natl. Acad. Sci. U.S.A. 108, 8420–8425 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chain P., et al. , Complete genome sequence of the ammonia-oxidizing bacterium and obligate chemolithoautotroph Nitrosomonas europaea. J. Bacteriol. 185, 2759–2773 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norton J. M., et al. , Complete genome sequence of Nitrosospira multiformis, an ammonia-oxidizing bacterium from the soil environment. Appl. Environ. Microbiol. 74, 3559–3572 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spang A., et al. , The genome of the ammonia-oxidizing Candidatus Nitrososphaera gargensis: Insights into metabolic versatility and environmental adaptations. Environ. Microbiol. 14, 3122–3145 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Heil J., Vereecken H., Brüggemann N., A review of chemical reactions of nitrification intermediates and their role in nitrogen cycling and nitrogen trace gas formation in soil. Eur. J. Soil Sci. 67, 23–39 (2016). [Google Scholar]
- 29.Zhu-Barker X., Cavazos A. R., Ostrom N. E., Horwath W. R., Glass J. B., The importance of abiotic reactions for nitrous oxide production. Biogeochemistry 126, 251–267 (2015). [Google Scholar]
- 30.Horrell S., Kekilli D., Strange R. W., Hough M. A., Recent structural insights into the function of copper nitrite reductases. Metallomics 9, 1470–1482 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Lawton T. J., Sayavedra-Soto L. A., Arp D. J., Rosenzweig A. C., Crystal structure of a two-domain multicopper oxidase. J. Biol. Chem. 284, 10174–10180 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhalnina K. V., et al. , Genome sequence of Candidatus Nitrososphaera evergladensis from group I.1b enriched from everglades soil reveals novel genomic features of the ammonia-oxidizing archaea. PLoS One 9, e101648 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts S. A., et al. , Crystal structure and electron transfer kinetics of CueO, a multicopper oxidase required for copper homeostasis in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 99, 2766–2771 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miranda K. M., et al. , A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc. Natl. Acad. Sci. U.S.A. 100, 9196–9201 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmieri E. M., et al. , Pyruvate dehydrogenase operates as an intramolecular nitroxyl generator during macrophage metabolic reprogramming. Nat. Commun. 14, 5114 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michalski R., et al. , The chemistry of HNO: Mechanisms and reaction kinetics. Front. Chem. 10, 930657 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shafirovich V., Lymar S. V., Nitroxyl and its anion in aqueous solutions: Spin states, protic equilibria, and reactivities toward oxygen and nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 99, 7340–7345 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liochev S. I., Fridovich I., The mode of decomposition of Angeli’s salt (Na2N2O3) and the effects thereon of oxygen, nitrite, superoxide dismutase, and glutathione. Free Radic. Biol. Med. 34, 1399–1404 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Smulik R., et al. , Nitroxyl (HNO) reacts with molecular oxygen and forms peroxynitrite at physiological pH. J. Biol. Chem. 289, 35570–35581 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson M. I., et al. , Kinetic feasibility of nitroxyl reduction by physiological reductants and biological implications. Free. Radic. Biol. Med. 47, 1130–1139 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smulik-Izydorczyk R., et al. , A kinetic study on the reactivity of azanone (HNO) toward its selected scavengers: Insight into its chemistry and detection. Nitric Oxide 69, 61–68 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Reisz J. A., Zink C. N., King S. B., Rapid and selective nitroxyl (HNO) trapping by phosphines: Kinetics and new aqueous ligations for HNO detection and quantitation. J. Am. Chem. Soc. 133, 11675–11685 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao G.-J., et al. , A highly sensitive and reductant-resistant fluorescent probe for nitroxyl in aqueous solution and serum. Chem. Commun. 50, 5790–5792 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Ianni J. C., “A comparison of the Bader-Deuflhard and the Cash-Karp Runge-Kutta integrators for the GRI-MECH 3.0 model based on the chemical kinetics code Kintecus” in Computational Fluid and Solid Mechanics 2003, Bathe K. J., Ed. (Elsevier Science Ltd, 2003), pp. 1368–1372. [Google Scholar]
- 45.Wang H., Dekel D. R., Abruña H. D., Unraveling the mechanism of ammonia electrooxidation by coupled differential electrochemical mass spectrometry and surface-enhanced infrared absorption spectroscopic studies. J. Am. Chem. Soc. 146, 15926–15940 (2024). [DOI] [PubMed] [Google Scholar]
- 46.Kraft B., et al. , Oxygen production by an ammonia-oxidizing archaeon. bioRxiv [Preprint] (2021). 10.1101/2021.04.01.436977 (Accessed 11 August 2024). [DOI]
- 47.Reyes C., et al. , Genome wide transcriptomic analysis of the soil ammonia oxidizing archaeon Nitrososphaera viennensis upon exposure to copper limitation. ISME J. 14, 2659–2674 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schatteman A., Wright C. L., Crombie A. T., Murrell J. C., Lehtovirta-Morley L. E., Hydrazines as substrates and inhibitors of the archaeal ammonia oxidation pathway. Appl. Environ. Microbiol. 88, e0247021 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kraft B., et al. , Oxygen and nitrogen production by an ammonia-oxidizing archaeon. Science 375, 97–100 (2022). [DOI] [PubMed] [Google Scholar]
- 50.Stoll S., Schweiger A., EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 178, 42–55 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Liebschner D., et al. , Macromolecular structure determination using X-rays, neutrons, and electrons: Recent developments in Phenix. Acta Cryst. D 75, 861–877 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kabsch W., XDS. Acta Cryst. D 66, 125–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winn M. D., et al. , Overview of the CCP4 suite and current developments. Acta Cryst. D 67, 235–242 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jumper J., et al. , Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emsley P., Lohkamp B., Scott W. G., Cowtan K., Features and development of Coot. Acta Cryst. D 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.The PyMOL Molecular Graphics System (Version 2.6, Schrödinger, LLC, New York, NY; ). [Google Scholar]
- 57.Sims G. K., Ellsworth T. R., Mulvaney R. L., Microscale determination of inorganic nitrogen in water and soil extracts. Commun. Soil Sci. Plant Anal. 26, 303–316 (2008). [Google Scholar]
- 58.Moorcroft M. J., Davis J., Compton R. G., Detection and determination of nitrate and nitrite: A review. Talanta 54, 785–803 (2001). [DOI] [PubMed] [Google Scholar]
- 59.Chambers M. C., et al. , A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 30, 918–920 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helf M. J., Foxx B. W., Artyukhin A. B., Zhang Y. K., Schroeder F. C., Comparative metabolomics with Metaboseek reveals functions of a conserved fat metabolism pathway in C. elegans. Nat. Commun. 13, 782 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voland R. W., Lancaster K. M., Multi-copper oxidase with a C-terminal cupredoxin domain from Nitrosopumilus maritimus. Protein Data Bank. https://www.rcsb.org/structure/9CCK. Deposited 21 June 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Protein structure data have been deposited in PDB (9CCK) (61). All other data are included in the manuscript and/or SI Appendix.