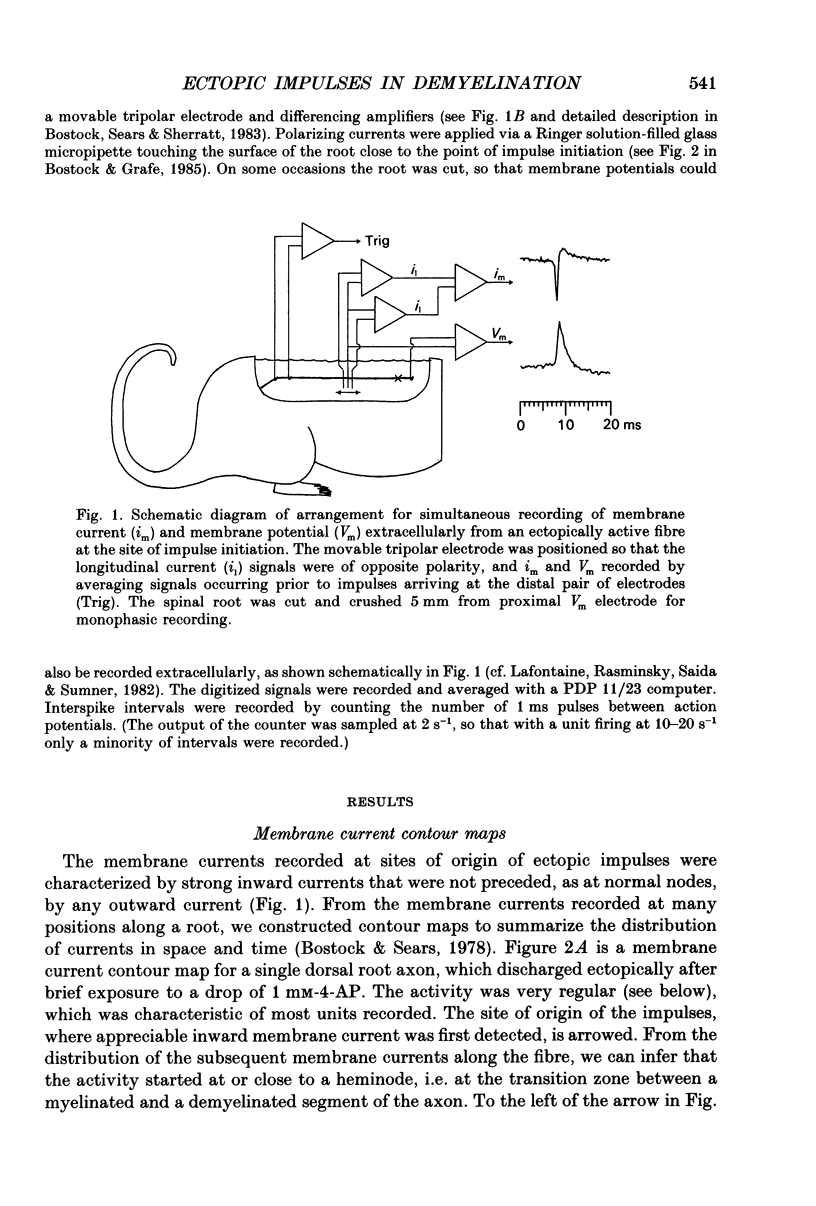
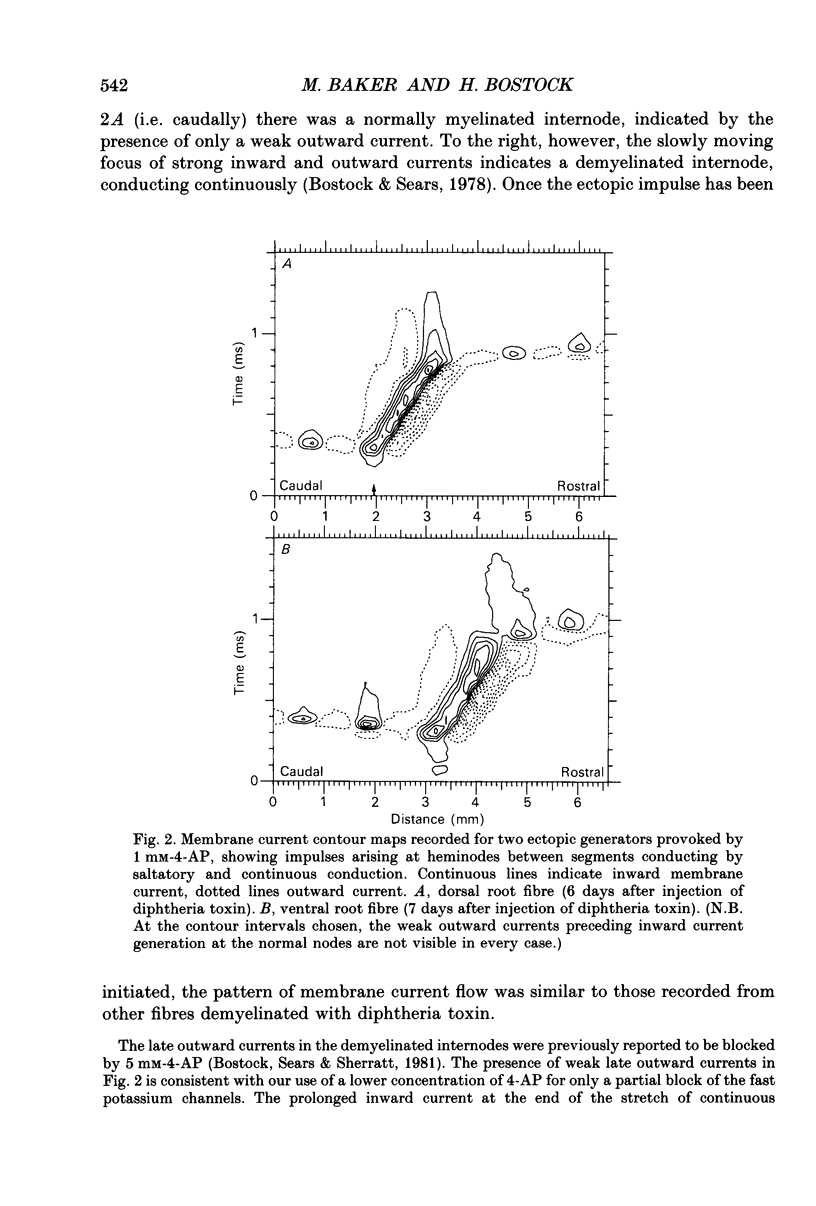
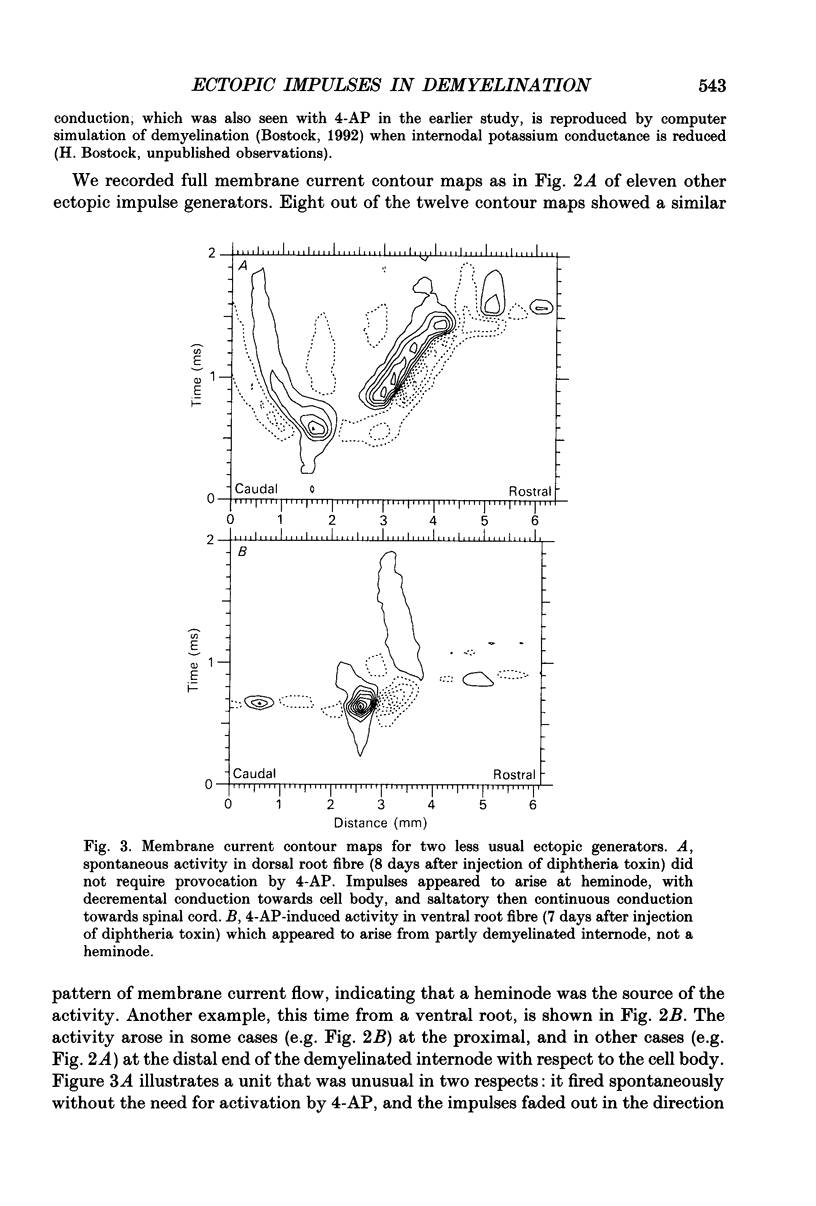
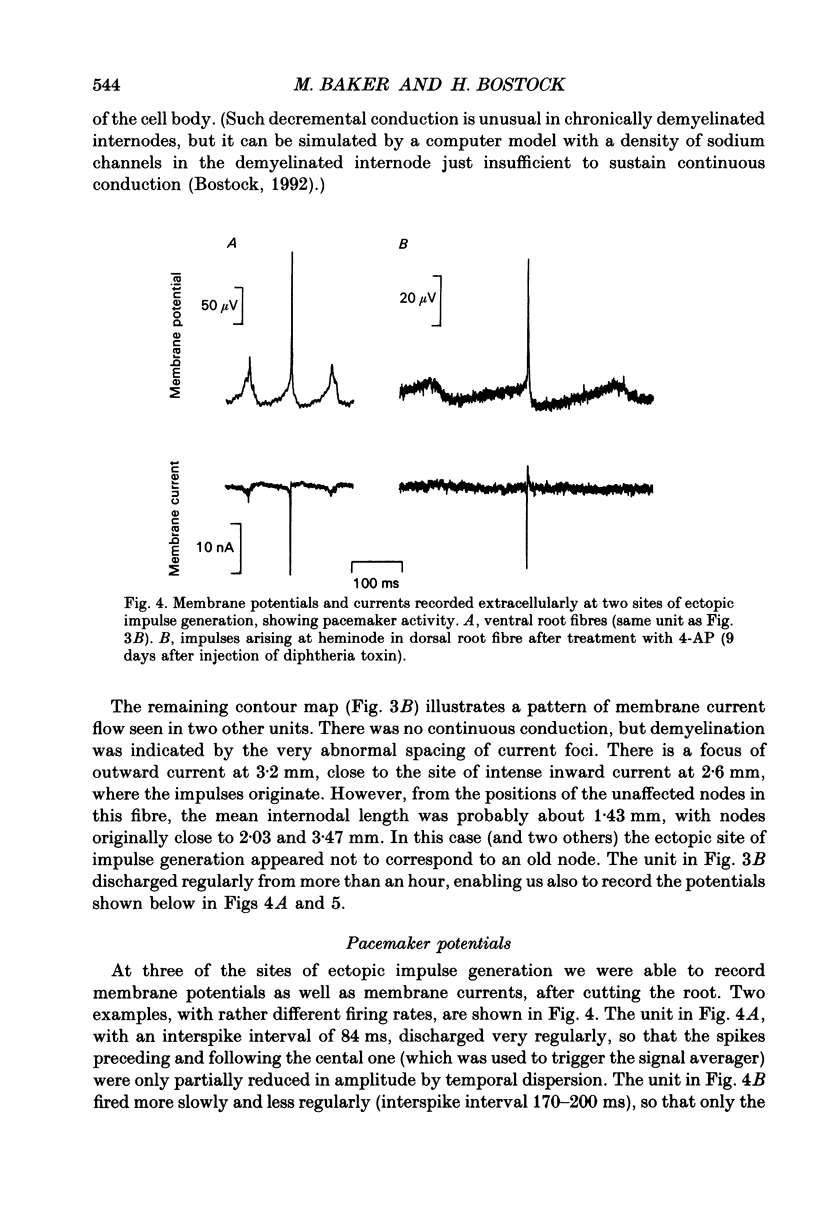
Abstract
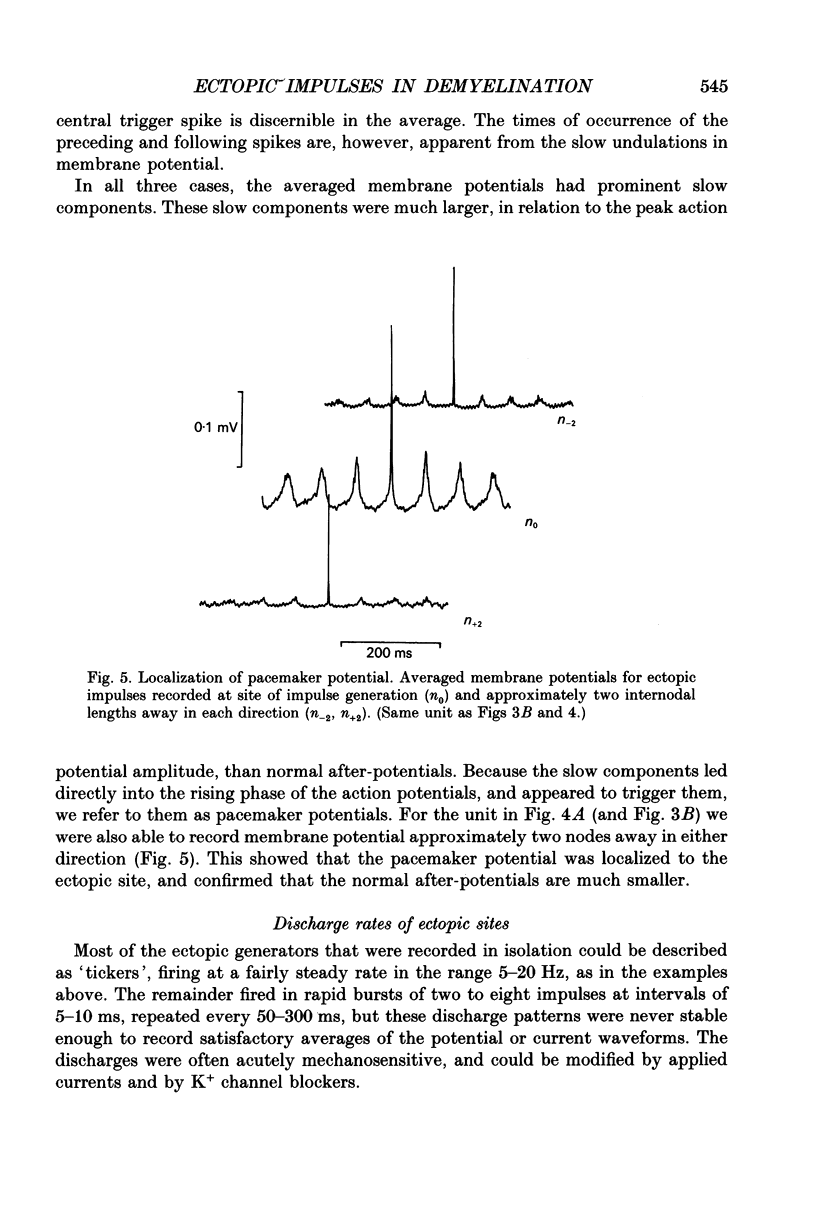
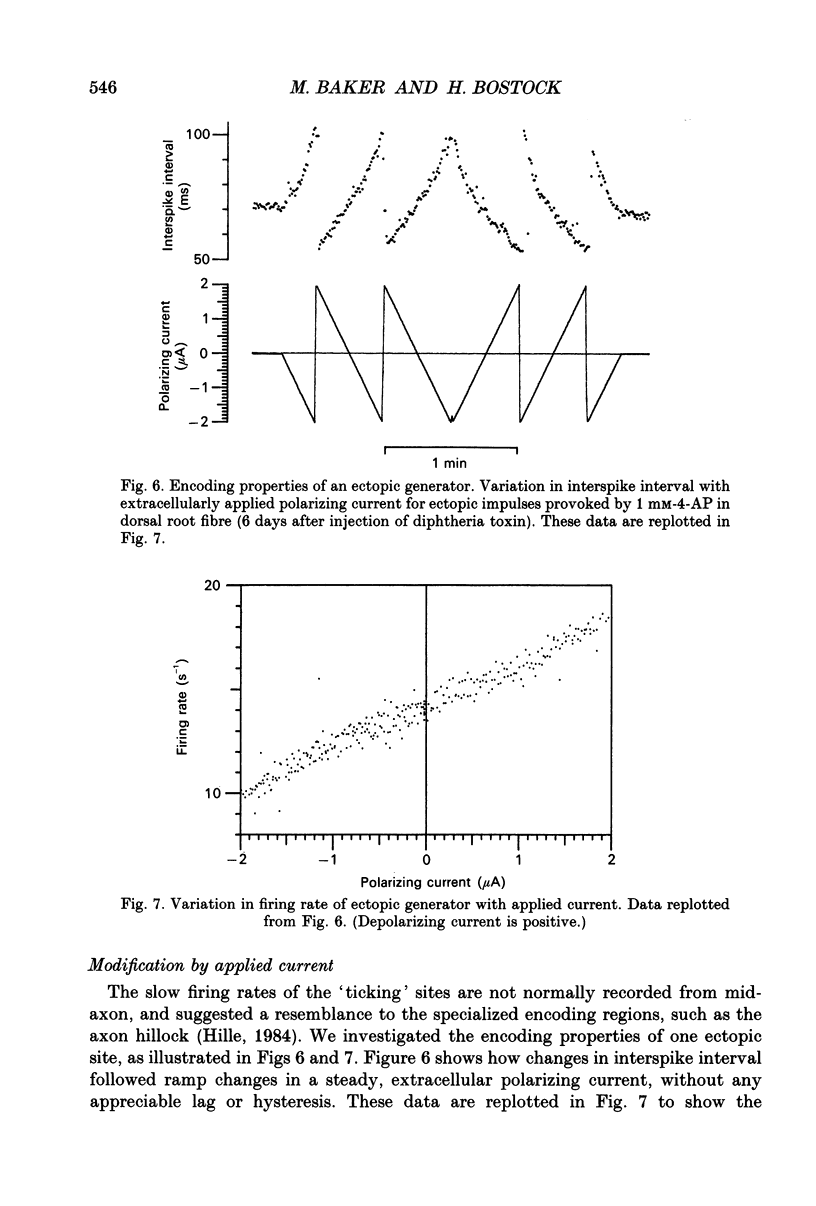
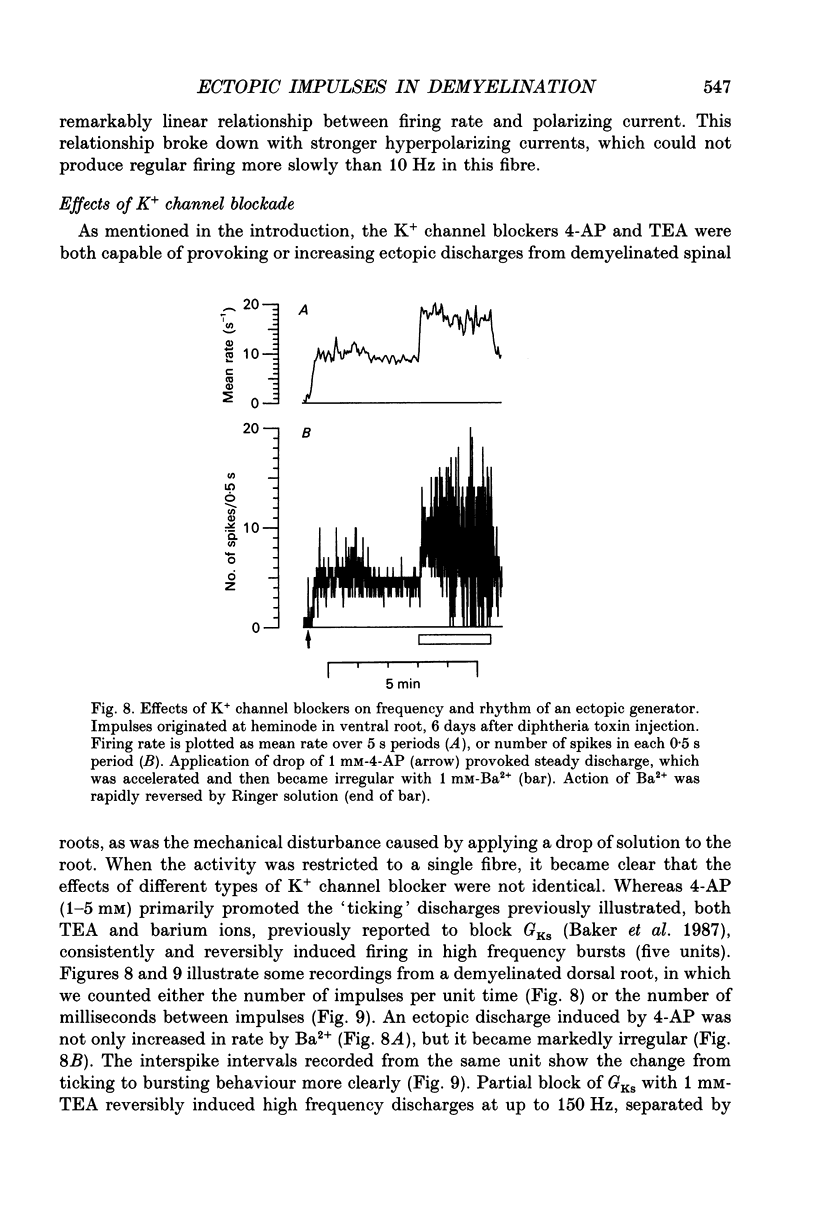
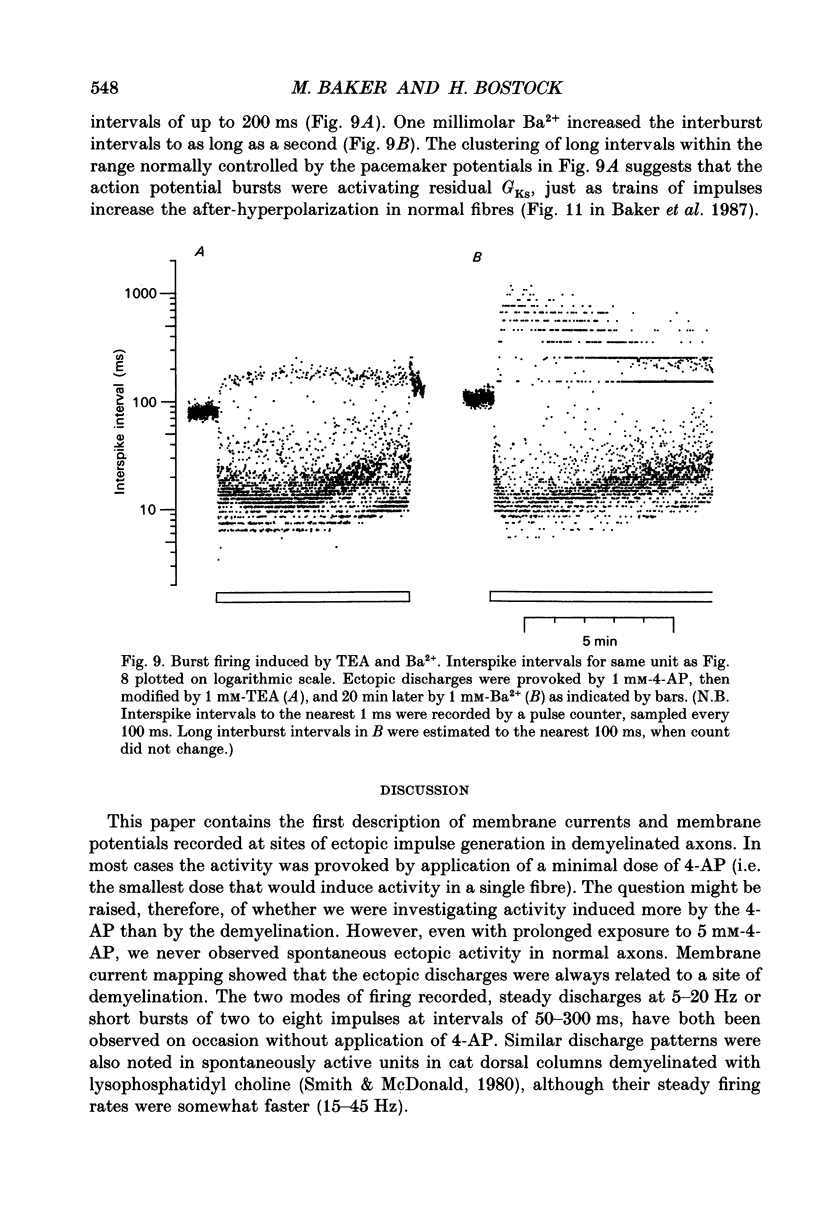
1. We have provoked ectopic discharges from demyelinated rat spinal roots by applying 1 mM-4-aminopyridine (4-AP), and recorded membrane currents and action potentials extracellularly by spike-triggered averaging. The demyelination was caused by intrathecal injection of diphtheria toxin, 6-9 days previously. 2. Mapping the distribution of membrane currents in the vicinity of an ectopic site showed that in most cases (eight out of twelve recorded) the impulses arose from one end of a continuously conducting internode, and conducted in both directions. In the remaining cases the impulses also arose from a site of demyelination. 3. The 4-AP-induced activity resembled the activity occurring spontaneously in some preparations, and was often highly regular (5-20 Hz). Recordings of membrane potential revealed a pacemaker potential, which was localized to the site of impulse initiation. One ectopic site was tested with applied currents and found to have a linear current-frequency relation for steady currents. 4. The time course of the pacemaker potential resembled that of the small after-hyperpolarization seen in normal fibres, due to a slow K+ conductance (GKs). Tetraethylammonium and barium ions, which block GKs, made spontaneously active fibres fire much more rapidly, or to fire bursts of action potentials. 5. Possible mechanisms for these ectopic discharges are discussed. GKs appears to contribute to the pacing of the activity, but not its generation. The increased excitability of the active fibres could not be attributed directly to the loss of myelin, nor to extracellular K+ accumulation. We suggest that they may have been depolarized by stretch-activated or ligand-gated channels in the demyelinated axon membrane.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker M., Bostock H., Grafe P., Martius P. Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J Physiol. 1987 Feb;383:45–67. doi: 10.1113/jphysiol.1987.sp016395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H., Baker M. Evidence for two types of potassium channel in human motor axons in vivo. Brain Res. 1988 Oct 18;462(2):354–358. doi: 10.1016/0006-8993(88)90564-1. [DOI] [PubMed] [Google Scholar]
- Bostock H., Baker M., Reid G. Changes in excitability of human motor axons underlying post-ischaemic fasciculations: evidence for two stable states. J Physiol. 1991 Sep;441:537–557. doi: 10.1113/jphysiol.1991.sp018766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H., Grafe P. Activity-dependent excitability changes in normal and demyelinated rat spinal root axons. J Physiol. 1985 Aug;365:239–257. doi: 10.1113/jphysiol.1985.sp015769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H., Sears T. A., Sherratt R. M. The effects of 4-aminopyridine and tetraethylammonium ions on normal and demyelinated mammalian nerve fibres. J Physiol. 1981;313:301–315. doi: 10.1113/jphysiol.1981.sp013666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H., Sears T. A., Sherratt R. M. The spatial distribution of excitability and membrane current in normal and demyelinated mammalian nerve fibres. J Physiol. 1983 Aug;341:41–58. doi: 10.1113/jphysiol.1983.sp014791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H., Sears T. A. The internodal axon membrane: electrical excitability and continuous conduction in segmental demyelination. J Physiol. 1978 Jul;280:273–301. doi: 10.1113/jphysiol.1978.sp012384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe C. M., Kocsis J. D., Waxman S. G. Differences between mammalian ventral and dorsal spinal roots in response to blockade of potassium channels during maturation. Proc R Soc Lond B Biol Sci. 1985 May 22;224(1236):355–366. doi: 10.1098/rspb.1985.0037. [DOI] [PubMed] [Google Scholar]
- Bray G. M., Cullen M. J., Aguayo A. J., Rasminsky M. Node-like areas of intramembraneous particles in the unensheathed axons of dystrophic mice. Neurosci Lett. 1979 Jul;13(2):203–208. doi: 10.1016/0304-3940(79)90042-9. [DOI] [PubMed] [Google Scholar]
- Burchiel K. J. Abnormal impulse generation in focally demyelinated trigeminal roots. J Neurosurg. 1980 Nov;53(5):674–683. doi: 10.3171/jns.1980.53.5.0674. [DOI] [PubMed] [Google Scholar]
- Burchiel K. J. Ectopic impulse generation in demyelinated axons: effects of PaCO2, pH, and disodium edetate. Ann Neurol. 1981 Apr;9(4):378–383. doi: 10.1002/ana.410090411. [DOI] [PubMed] [Google Scholar]
- Calvin W. H., Devor M., Howe J. F. Can neuralgias arise from minor demyelination? Spontaneous firing, mechanosensitivity, and afterdischarge from conducting axons. Exp Neurol. 1982 Mar;75(3):755–763. doi: 10.1016/0014-4886(82)90040-1. [DOI] [PubMed] [Google Scholar]
- Calvin W. H., Loeser J. D., Howe J. F. A neurophysiological theory for the pain mechanism of tic douloureux. Pain. 1977 Apr;3(2):147–154. doi: 10.1016/0304-3959(77)90078-1. [DOI] [PubMed] [Google Scholar]
- Chiu S. Y., Schwarz W. Sodium and potassium currents in acutely demyelinated internodes of rabbit sciatic nerves. J Physiol. 1987 Oct;391:631–649. doi: 10.1113/jphysiol.1987.sp016760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S. Properties of potassium and sodium channels in frog internode. J Physiol. 1986 Dec;381:119–134. doi: 10.1113/jphysiol.1986.sp016317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. F., Calvin W. H., Loeser J. D. Impulses reflected from dorsal root ganglia and from focal nerve injuries. Brain Res. 1976 Oct 29;116(1):139–144. doi: 10.1016/0006-8993(76)90255-9. [DOI] [PubMed] [Google Scholar]
- Lafontaine S., Rasminsky M., Saida T., Sumner A. J. Conduction block in rat myelinated fibres following acute exposure to anti-galactocerebroside serum. J Physiol. 1982 Feb;323:287–306. doi: 10.1113/jphysiol.1982.sp014073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. E. Mechanosensitive ion channels. J Membr Biol. 1990 Feb;113(2):93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- Nordin M., Nyström B., Wallin U., Hagbarth K. E. Ectopic sensory discharges and paresthesiae in patients with disorders of peripheral nerves, dorsal roots and dorsal columns. Pain. 1984 Nov;20(3):231–245. doi: 10.1016/0304-3959(84)90013-7. [DOI] [PubMed] [Google Scholar]
- Rasminsky M. Hyperexcitability of pathologically myelinated axons and positive symptoms in multiple sclerosis. Adv Neurol. 1981;31:289–297. [PubMed] [Google Scholar]
- Röper J., Schwarz J. R. Heterogeneous distribution of fast and slow potassium channels in myelinated rat nerve fibres. J Physiol. 1989 Sep;416:93–110. doi: 10.1113/jphysiol.1989.sp017751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. J., McDonald W. I. Spontaneous and mechanically evoked activity due to central demyelinating lesion. Nature. 1980 Jul 10;286(5769):154–155. doi: 10.1038/286154a0. [DOI] [PubMed] [Google Scholar]


