Abstract
Passive antibody therapies, typically administered via parenteral routes, have played a crucial role in the initial response to the COVID-19 pandemic. However, the ongoing evolution of SARS-CoV-2 has revealed significant limitations of this approach, primarily due to mutational escape and the inadequate delivery of antibodies to the upper respiratory tract. To overcome these challenges, we propose a novel prophylactic strategy involving the intranasal delivery of an antibody in combination with an Fc-binding nanodisc. This nanodisc, engineered to specifically bind to the Fc regions of IgG antibodies, served two key functions: extending the antibody's half-life in the larynx and trachea, and enhancing its neutralization efficacy. Notably, Sotrovimab, an FDA-approved monoclonal antibody that has experienced a significant decline in neutralizing potency due to viral evolution, exhibited robust antiviral activity when complexed with the nanodisc against all tested Omicron variants. Furthermore, the Fc-binding nanodisc significantly boosted the antiviral efficacy of the soluble angiotensin-converting enzyme 2 (sACE2) Fc fusion protein, which possesses broad but modest antiviral activity. In ACE2 transgenic mice, the Fc-binding nanodisc protected better than sACE2-Fc alone with two more log reduction in lung viral titer. Therefore, the intranasal Fc-binding nanodisc offers a promising and powerful approach to counteract the diminished antiviral activity of neutralizing antibodies caused by mutational escape, effectively restoring antiviral efficacy against various evolving SARS-CoV-2 variants.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12951-025-03100-y.
Keywords: Antibody, SARS-CoV-2, Nanodisc, Antiviral, COVID19
Introduction
COVID-19 continues to oscillate between periods of decline and resurgence. In the ongoing fight against coronavirus disease 2019 (COVID-19), passive antibody therapy has been a crucial tool during the early stages of the pandemic when vaccines were not yet widely accessible. This therapeutic approach involves administering convalescent plasma and neutralizing antibodies to rapidly confer immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), playing a pivotal role in managing severe cases effectively. Initially, convalescent plasma from recovered patients was utilized, significantly reducing viral load and mortality rates when administered early in the disease's progression [1, 2]. Subsequently, neutralizing antibodies (nAbs), particularly those targeting the receptor-binding domain (RBD) of the viral spike protein, were developed. Combinations like casirivimab and imdevimab have demonstrated the ability to reduce COVID-19-related hospitalizations and mortality by up to 70% in high-risk individuals [3, 4]. These nAbs function by preventing the virus from binding to angiotensin-converting enzyme 2 (ACE2) receptors on human cells, a crucial step for viral entry and infection, thereby mitigating disease severity [5]. However, the continuous evolution of the virus has led to the emergence of various variants, including Omicron, which has shown resistance to all FDA-approved nAbs [6–11]. Neutralizing antibodies have been shown to even exert selection pressure, contributing to the accumulation of viral mutants [12]. To counteract the reduced efficacy of nAbs against Omicron variants, several strategies have been explored. These include combining antibodies such as imdevimab-casirivimab and bamlanivimab-etesevimab, redesigning non-neutralizing antibodies into broad-spectrum inhibitors [13], using computational techniques to restore the potency of approved antibodies, and developing bispecific antibodies that enhance neutralization by targeting multiple epitopes [14–16].
The emergence of SARS-CoV-2 variants, such as Omicron, which have adapted to infect the upper respiratory tract more effectively—particularly the nasal passages and throat—has resulted in increased transmissibility [17–19]. Although these variants often cause milder symptoms, their enhanced ability to spread complicates efforts to control the virus. This shift in viral behavior has prompted a growing interest in alternative administration strategies for antibody therapies, with intranasal delivery emerging as a promising approach [20]. Intranasal administration allows antibodies to directly target the primary sites of viral entry in the upper respiratory tract. Pharmacokinetic studies in mice have demonstrated that inhaled antibodies can achieve significantly higher concentrations in the pulmonary epithelial lining fluid compared to serum, leading to more effective lung delivery [21–26]. Pharmacokinetic studies in mice have shown that inhaled nAbs can achieve concentrations in the pulmonary epithelial lining fluid approximately 50-fold higher than those in serum [27], compared to only 0.2% of a parenterally administered dose reaching these areas [28]. Additionally, it has been demonstrated that aerosol delivery of antibodies can significantly limit viral replication and reduce lung pathology in non-human primates [29], highlighting the potential of aerosolized applications in treating respiratory viral infections. However, despite this success, the studies also revealed a limited therapeutic impact due to the rapid clearance of delivered antibodies in the upper respiratory tract, potentially reducing their effectiveness against recently circulating Omicron variants.
Nanomaterials have emerged as promising antiviral agents due to their unique physicochemical properties and versatile mechanisms of action. Nanomaterials can exhibit potent antiviral activity by interacting directly with viral structures. For instance, nanoparticles have been shown to effectively bind to viral particles, thereby inhibiting or inactivating the virus [30, 31]. Tapered chiral nanoparticles have been shown induce agglutination of several strains of coronaviruses, including the highly transmissible Omicron variants [32]. We have also shown that the use of nanodiscs as antiviral agents to inhibit a wide range of viruses, including influenza A virus and SARS-CoV-2 [33–37]. Nanodiscs are discoidal lipid bilayers, with their two hydrophobic acyl tail edges concealed by two molecules of membrane scaffold proteins (MSPs) [38]. Among the nanodiscs developed as antivirals, the antibody-nanodisc complex (ANC) has been proposed to overcome the limitations of neutralizing antibodies (nAbs) against influenza viruses [37]. By incorporating Fc-binding peptides into nanodiscs (F-NDs), these F-NDs can form ANCs spontaneously with nAbs. The F-NDs are endocytosed into cells infected with the influenza virus through the affinity of nAbs for the virus. Within the endosome, membrane fusion occurs between the viral envelope and the nanodisc, rather than the envelope-endosome fusion, resulting in viral RNA release into the endosome instead of the cytosol. This mechanism significantly reduces the morbidity and mortality of mice caused by the influenza virus post intravenous administration of MEDI8852-ANC. Furthermore, it has been demonstrated that these ANCs remain stable in mice, as they were detected in complex form in lung tissue following administration.
In this study, we address two critical challenges using nanodiscs: restoring the antiviral efficacy of antibodies that have diminished effectiveness against recently circulating SARS-CoV-2 variants and prolonging the retention of antibodies in the upper respiratory tract to ensure sustained protection at the crucial initial sites of viral entry. To achieve these objectives, we further engineered the previous version of Fc-binding nanodiscs (F-NDs) to improve their stability. These F-NDs were designed to bind a variety of Fc fusion proteins, including single-chain variable fragment (scFv)-Fc, immunoglobulin G (IgG), and soluble ACE2-Fc (sACE2)-Fc, facilitating the formation of ANCs. sACE2-Fc is an immunoadhesin, created by conjugating the soluble region of ACE2, the primary receptor for SARS-CoV-2, with the Fc region of an antibody at its C-terminus. Unlike monoclonal antibodies, which typically neutralize SARS-CoV-2 by binding to specific epitopes on the Spike protein, sACE2-Fc acts as a decoy receptor. Both molecules contain Fc regions and represent potent antiviral agents, making them suitable candidates for forming ANC models with Fc-binding nanodiscs. By allowing sACE2-Fc and antibodies to directly bind viral antigens while the nanodiscs physically obstruct virus particles, ANCs enhance antiviral defense. The ANCs significantly enhanced the antiviral activity of sACE2-Fc and various nAbs. In a humanized mouse model, the sACE2-Fc ANC demonstrated superior antiviral efficacy compared to sACE2-Fc alone. Moreover, the FDA-approved S309 antibody (Sotrovimab), which had lost its neutralizing efficacy against Omicron variants while retaining binding capability, regained its neutralizing efficacy against all tested Omicron variants upon ANC formation. This demonstrates that F-NDs serve as effective antiviral adjuvants for various Fc fusion proteins, including nAbs and sACE2-Fc, not only broadening their antiviral spectrum but also enhancing their efficacy. Furthermore, the prolonged retention of IgGs in the larynx and trachea in the presence of F-NDs increases the protective effects of nAbs or sACE2-Fc against the highly transmissible, recently circulating Omicron variants.
Materials and methods
Cells
Madin-Darby canine kidney (MDCK; Korean Cell Line Bank, Seoul, Republic of Korea) cells were maintained in minimum essential medium (MEM; Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA), 100 U/mL penicillin G, 100 µg/mL streptomycin, and 0.25 µg/mL amphotericin B (Hyclone). Human epithelial lung carcinoma (A549; Korean Cell Line Bank) cells were cultured in Roswell Park Memorial Institute 1640 medium (RPMI; Hyclone) with the same supplements as the MDCK medium. Both cell types were incubated at 37 ℃ in a 5% CO2 humidified environment and passaged upon reaching 80%–90% confluence. Human embryonic kidney (HEK) 293F cells (Invitrogen, Carlsbad, CA, USA) were grown in FreeStyle 293 expression medium (Invitrogen) in a shaking incubator at 125 rpm, 37 ℃, and 8% CO2, and subcultured at a density of 2–3 × 106 viable cells/mL. Vero E6 cells (ATCC, Manassas, VA, USA and HEK 293 T (Korean Cell Line Bank were cultured in Dulbecco's Modified Eagle Medium (DMEM; Hyclone) supplemented with 10% FBS and antibiotics under similar conditions.
Monoclonal antibody selection through phage display and panning
A diverse set of antibodies binding to the spike (S) protein of SARS-CoV-2 was obtained using a human scFv library constructed with the phagemid vector, pDR-D1, as previously described [39]. The scFv-displaying phages were rescued using VCSM13 helper phage (Stratagene, La Jolla, CA, USA) and subjected to three rounds of panning against recombinant S protein subunits, S1 or S2 (SinoBiological). To exclude nonspecific binders, subtraction panning was performed using BSA-coated immunotubes (Nunc, Naperville, IL, USA). The phages were then incubated in S1 or S2-coated immunotubes for 2 h at 25 ℃. Unbound phages were removed by washing with PBST, and the remaining S1 or S2-bound phages were eluted, amplified, and used for the next round of panning. After the final round of panning, fifty colonies were randomly selected from the output plate. Phages were rescued from the culture of each colony and applied onto S1 or S2-coated microtiter plate wells. Phage binding was detected using enzyme-linked immunosorbent assay with horseradish peroxidase (HRP)-conjugated anti-M13 antibody (GE Healthcare, Chicago, IL, USA). Clones showing specific binding to S1 or S2 were subjected to DNA sequencing, and the nucleotide sequences were analyzed using IMGT/V-QUEST (https://www.imgt.org/IMGT_vquest/input).
Preparation of scFv-Fc and analysis of binding kinetics
The genes encoding the selected scFv clones were cloned into pDR-OriP-Fc vector for transient expression of scFv-Fc as described previously [40]. The constructed expression plasmid was transfected using polyethlyenimine (PEI; Polysciences, Warrington, PA, USA) into HEK 293E (CRL-10852, ATCC) cells, and the transfected cells were cultured for seven days at 32 ℃ in 8% CO2 while being fed with 15% glucose (Thermo Fisher Scientific, Waltham, MA, USA) and 200 × Glutamax (Thermo Fisher Scientific) twice. The produced scFv-Fc in the supernatant was purified by affinity chromatography based on a Protein G-agarose column (Merck Millipore, Darmstadt, Germany). Binding activities of the purified scFv-Fc was measured by an indirect ELISA. Serial dilutions of scFv-Fc were applied to S1 or S2-coated wells, and bound scFv-Fc was detected by HRP-conjugated anti-human IgG Fc antibody produced in goat (Jackson ImmunoResearch, West Grove, PA, USA). Apparent dissociation constant (KD) for each binding interaction was determined by binding curve fitting as described previously [41].
Preparation of sACE2-Fc
A total of 125 µg of plasmids encoding sACE2-Fc (Addgene plasmid #145163; Cambridge, MA, USA) were transiently transfected into 200 mL of HEK293F cells at a density of 1 × 106 cells/mL. The transfection was performed using a DNA to PEImax (Polysciences) ratio of 1:3. After a 72-h incubation, the culture medium was centrifuged at 6000 × g for 10 min to remove cells and debris. The supernatant was then purified using Protein G resin (GE Healthcare). Protein concentration was determined using a detergent-compatible protein assay (Bio-Rad, Hercules, CA, USA), with bovine serum albumin (BSA; Thermo Fisher Scientific) as the standard.
Expression and purification of MSP-related proteins
The MSP-related plasmid (MSP1E3D1, #20,066, Addgene; MSP2N2, # 29,520, Addgene) was transformed into Escherichia coli BL21 (DE3). A single colony was cultured in 10 mL of Terrific Broth (Sigma-Aldrich) containing 50 μg/mL kanamycin (BIO BASIC, Inc., Markham, ON, Canada) and incubated overnight at 37 ℃ with shaking at 200 rpm. This pre-culture was then used to inoculate 600 mL of Terrific Broth with 50 μg/mL kanamycin and cultured at 37 ℃ with shaking at 180 rpm until the OD600 reached 1.0. Protein expression was induced with 0.2 mM isopropyl β-D-thiogalactoside (GoldBio, Olivette, MO, USA), and the culture was incubated at 25 ℃ with shaking at 150 rpm for 5 h. Bacterial cells were collected by centrifugation at 6000 rpm for 5 min. The cell pellet was resuspended in a basic buffer (40 mM Tris, pH 8.0, 300 mM NaCl, 1% Triton X-100, 2 mM Tris(2-carboxyethyl)phosphine hydrochloride (TCEP), and 1 mM AEBSF (Enzo Life Sciences, Inc., Farmingdale, NY, USA) and lysed via sonication on ice using a 60% probe amplitude for 5 min (1 s ON, 1 s OFF). The lysate was centrifuged at 13,000 rpm for 30 min and filtered through a 0.45-μm-pore-size bottle-top filter (Sartorius). The supernatant was incubated with Work Beads NIMAC (Bio-Works, Boston, MA, USA) overnight at 4 ℃ under rotating conditions. Bound proteins were washed with two buffers: (1) basic buffer with 50 mM sodium cholate, and (2) basic buffer with 30 mM imidazole. Proteins were eluted using elution buffer (basic buffer with 500 mM imidazole, pH 8.0) and desalted with storage buffer (40 mM Tris, 100 mM NaCl, 10% glycerol, and 1 mM AEBSF, pH 7.4). Protein concentration was determined using a detergent-compatible protein assay (Bio-Rad) with bovine serum albumin (BSA; Thermo Fisher Scientific) as the standard. For animal studies, endotoxin was removed using a 0.5 mL Pierce High-Capacity Endotoxin Removal Spin Column (Thermo Fisher Scientific).
Nanodisc preparation
To construct either empty nanodiscs or ganglioside-incorporated nanodiscs, the molar ratio of phospholipids to gangliosides was adjusted accordingly. For empty nanodiscs, a molar ratio of 100:0 phospholipids to gangliosides were used, consisting entirely of either 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), or 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) purchased from Avanti Polar Lipids (Alabaster, AL, USA). For ganglioside-embedded nanodiscs, a ratio of 70:30 phospholipids to gangliosides were employed, consisting of 70 mol% DMPC and 30 mol% ganglioside GM1 (Avanti Polar Lipids). The lipid mixtures were dissolved in chloroform, evaporated under nitrogen, and vacuum dried overnight. The dried lipid film was rehydrated with nanodisc assembly buffer (20 mM Tris/HCl, 100 mM NaCl, 0.5 mM EDTA, 50 mM sodium cholate, pH 7.4), sonicated at 55 ℃ for 15 min, and mixed with membrane scaffold proteins. SM-2 bio-beads (Bio-Rad) were added to the mixture to remove the detergent, and it was incubated at room temperature for 5 h. The nanodiscs were purified by size exclusion chromatography (SEC) using a Superose 6 Increase 10/300 GL column (GE Healthcare). Appropriate fractions were collected and concentrated using an Amicon Ultra centrifugal filter (10 kDa cutoff) (EMD Millipore, Billerica, MA, USA).
Dynamic light scattering (DLS)
The hydrodynamic diameter and size distribution of the nanodiscs were measured using a Litesizer 500 instrument (Anton Paar, Graz, Austria). Measurements were performed at 25 ℃ using the volume measurement mode.
Enzyme-linked immunosorbent assay (ELISA)
Plates were coated with 50 µL/well of protein, diluted in carbonate-bicarbonate buffer (pH 9.6) to a final concentration of 5 µg/mL, and incubated overnight at 4 ℃. The plates were blocked with 100 µL/well of blocking buffer (4% skim milk in PBS) for 1 h at 4 ℃. After washing four times with 180 µL/well of PBS containing 0.05% Tween-20 (PBST), 50 µL/well of the tested samples, serially diluted in blocking buffer, were added and incubated for 1 h. The plates were washed four times, and 50 µL/well of goat anti-human Fc IgG (Sigma-Aldrich) with HRP-conjugated antibody (1:10,000 dilution in blocking buffer) was added and incubated for 1 h. The plates were washed again, and 50 µL/well of 1-step Ultra TMB-ELISA solution (Thermo Fisher Scientific, 34,028) was added. The reaction was stopped by adding 50 µL of 2 M sulfuric acid, and the absorbance was measured at 450 nm using a microplate reader (BioTek, Winooski, VT, USA).
Preparation of HIV-based lentiviral pseudovirus
HEK293T cells were co-transfected with 10 μg of a plasmid encoding NL4.3-mCherry-Luciferase (Addgene plasmid #44965) and 10 μg of a plasmid encoding the SARS-CoV-2 spike protein using a 1:3 ratio (w/w) of DNA to PEImax (Polyscience, Inc.). For the preparation of various SARS-CoVs pseudotyped virus, the following spike genes were utilized: SARS-CoV (VG40150-G-N, Sino Biological, Beijing, China), SARS-CoV-2 (2019-nCoV; VG40589-UT, Sino Biological), SARS-CoV-2 Delta (Addgene plasmid #172320), SARS-CoV-2 Omicron BA.1 (VG40835-UT, Sino Biological), SARS-CoV-2 Omicron BA.2 (plv-spike-v12, InvivoGen), SARS-CoV-2 Omicron BA.4 (VG40877-UT, Sino Biological), SARS-CoV-2 Omicron BA.2.75 (Addgene plasmid #190012), SARS-CoV-2 Omicron BF.7 (VG40917-UT, Sino Biological), SARS-CoV-2 Omicron BQ.1.1 (VG40916-UT, Sino Biological), and SARS-CoV-2 Omicron XBB (VG40915-UT, Sino Biological). The transfection procedure was performed in Opti-MEM medium (Thermo Fisher Scientific), and the cells were incubated for 48–72 h. Post-incubation, the culture medium was subjected to centrifugation at 800 × g for 10 min, and the supernatant was subsequently filtered through a 0.45 μm filter. The filtered medium containing the pseudovirus was then stored at -80 ℃.
Pseudovirus inhibition assay
The pseudovirus inhibition assay was conducted to evaluate the antiviral activity of various inhibitors against the SARS-CoV-2 pseudovirus. HEK293T cells were co-transfected with plasmids encoding human ACE2 (HG10108-CH; Sino Biological) and human TMPRSS2 (HG13070-CH; Sino Biological). The transfected cells were then seeded into white 96-well plates at a density of 2 × 104 cells per well and incubated at 37 ℃ with 5% CO2 for 24 hours. Subsequently, serial dilutions of the inhibitors were prepared and pre-incubated with pseudovirus (2.0 × 105 relative luminescence unit/mL) at 37 ℃ for 1 hour. The inhibitor-virus mixtures were then added to the prepared HEK293T cells and incubated for 48 hours. Following the incubation period, the supernatant was discarded, and the cells were lysed. Luminescence was measured using a Luciferase Assay Kit (Promega, Madison, Wisconsin, USA). To determine relative infectivity, the positive control consisted of cells not infected with the pseudovirus, while the negative control consisted of cells infected with the pseudovirus. The difference between the experimental value and the positive control was compared to the difference between the negative control and the positive control, with the resulting percentage indicating the relative infectivity. The half-maximal inhibitory concentration (IC50) values were calculated using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA).
Cell viability test
Cytotoxicity of the inhibitors was evaluated using the CellTiter-Glo® Luminescent Cell Viability Assay (Promega). Vero E6 cells were seeded in 96-well plates at a density of 2 × 104 cells/well and incubated overnight at 37 ℃ in a 5% CO2 incubator. The cells were treated with serial dilutions of the inhibitors and incubated for 48 h. CellTiter-Glo® reagent was added to each well, and luminescence was measured using a microplate reader (BioTek). Cell viability was calculated as a percentage of the luminescence in treated wells compared to untreated control wells.
Electron microscopy
A negative-staining procedure was used for electron microscopy analysis. Ten microliters of the nanodisc sample were placed on carbon-coated gold grids (TED PELLA, Inc., Redding, CA, USA) for 1 min, and the excess sample was removed by blotting with filter paper. The grids were then washed with 10 µL of ultrapure water and stained with 5 µL of 1% (w/v) uranyl acetate (UA) for 20 s. Excess liquid was removed with filter paper at each step. The prepared grids were air-dried before TEM analysis. All specimens were analyzed using a JEM 3010 microscope (JEOL Ltd., Tokyo, Japan) equipped with energy-filtering capabilities. The microscope operated at an accelerating voltage of 300 kV.
Cytopathic effect (CPE) inhibition assay
Vero E6 cells were cultured in a humidified incubator at 37 ℃ with 5% CO2 and passaged upon reaching 80–90% confluence. The cells were infected with 100 × tissue culture infective dose 50% (TCID50) of the Beta-CoV/Korea/KCDC03/2020 strain. Serial dilutions of each inhibitor were mixed with the virus and added to the cells. After 1 h of incubation at 37 ℃, the supernatant was replaced with fresh medium containing the respective inhibitor, and the cells were incubated for 96 h post-infection for Vero E6 cells at 37 ℃. Cell viability was measured using the CellTiter-Glo® Luminescent Cell Viability Assay (Promega), and the results were analyzed to determine antiviral efficacy.
Pharmacokinetics
Eight-week-old female BALB/c mice (Koatech, Inc., Pyeongtaek, Republic of Korea) were used in the pharmacokinetic study. Each mouse was intranasally administered with IgG and ANC, each containing an equivalent IgG concentration of 5 mg/kg. Tissues were harvested at various time points (2, 6, 12, 24, and 48 h) after intranasal administration from each group (n = 4 mice per group). The harvested organs were homogenized with 1X lysis buffer (Promega) using a bullet blender (Next Advance, Averill Park, NY, USA). To quantify the IgG concentration, homogenates were processed using a human IgG Fc-specific ELISA. A 96-well plate (SPL Life Sciences, Pocheon, Republic of Korea) was coated overnight at 4 ℃ with primary Goat Anti-Human IgG Fc (Sigma-Aldrich) dissolved in 0.05 M carbonate-bicarbonate buffer (pH 9.6) at 1 µg/mL, 50 µL per well. After blocking with 4% skim milk in PBST for 1 h, the plate was washed three times with PBST. Diluted organ homogenates (in PBS containing 1% skim milk) were added to each well (50 µL/well) and incubated for 1 h. Following three washes with PBST, the plate was incubated for 1 h with a 1:10,000 dilution of HRP-conjugated Goat Anti-Human IgG Fc (Abcam, Cambridge, UK) in PBS containing 1% skim milk. After the final wash, 50 µL/well of 1-step Ultra TMB-ELISA solution (Thermo Fisher Scientific) was added. The reaction was stopped by adding 50 µL of 2 M sulfuric acid, and the absorbance was measured at 450 nm using a microplate reader (BioTek, Winooski, VT, USA). Concentrations were determined based on absorbance readings and comparison with a standard curve. All experimental procedures for both pharmacokinetics and toxicology complied with the policies of the Institutional Animal Care and Use Committee of Sungkyunkwan University (SKKUIACUC2024-03–22-1).
Animal studies
Eight-week-old female C57BL/6 Cg-Tg (K-18 hACE2) 2Prlmn/J mice were obtained from Jackson Laboratory (Bar Harbor, Maine, USA). Mice were intranasally challenged with three or five times the mouse lethal dose (MLD50) of the Beta-CoV/Korea/KCDC03/2020 strain. To evaluate prophylactic efficacy, inhibitors were administered intranasally 24 h prior to virus inoculation. Mice were monitored daily for body weight and survival for up to 14 days. Mice losing more than 25% of their initial body weight were humanely euthanized. The remaining mice were euthanized at the end of the experiment. All SARS-CoV-2-related procedures were conducted in biosafety level three laboratories. The care and maintenance of the animals followed the recommendations and guidelines of the Ministry of Food and Drug Safety, Republic of Korea. This study was approved by the institutional animal care and use committee of Chungbuk National University (CBNUA-2207–23-02), and all experimental protocols adhered strictly to committee guidelines.
Viral tissue titration
Lung tissues were homogenized using bead disruption and centrifuged at 6000 rpm for 5 min. The resulting supernatants were serially diluted ten-fold and used to infect Vero E6 cells in 96-well plates, which were then incubated in a 5% CO2 incubator at 37 ℃ for 4 days. The infected cells were visualized by staining with 0.25% crystal violet. Viral titers were calculated using the Reed and Muench method and expressed as log10 TCID50 per gram of tissue.
Histopathological analysis
Mouse lung tissues were subjected to slide scanning following histopathological staining. The tissues were fixed with 10% neutral buffered formalin (Sigma-Aldrich), cut into 5 μm sections, and stained with hematoxylin and eosin (H&E). For immunohistochemistry (IHC) staining, the tissue sections were deparaffinized at 75 ℃ for 8 min and underwent cell conditioning at 95 ℃ for 44 min using Conditioner #1 and Standard CC1. Blocking was achieved using inhibitor CM at 37 ℃ for 4 min. Subsequently, a rabbit anti-SARS-CoV-2 nucleocapsid polyclonal antibody (Sinobiological) was used for primary antibody (1:2500) and incubated for 60 min. Following the primary antibody incubation, one drop of OmniMap anti-Rabbit HRP secondary antibody was applied for 16 min. For visualization, the samples were incubated with one drop each of DAB CM and H2O2 CM for 8 min, followed by incubation with one drop of Copper CM for 5 min. Counterstaining was performed by incubating the slides with hematoxylin for 8 min, followed by incubation with bluing reagent for 8 min as a post-counterstaining step. The slides were then cleaned in detergent-fortified warm water, dehydrated with graded ethanol and xylene, and cover slipped using permanent mounting media. The procedure was executed on a Ventana Discovery Ultra system (Ventana Medical Systems, Inc., Tucson, AZ).
Statistics
Statistical significance was set at not significant (NS), p > 0.5; *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001. All statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc.).
Results
Optimization of nanodisc for ANC against SARS-CoV-2
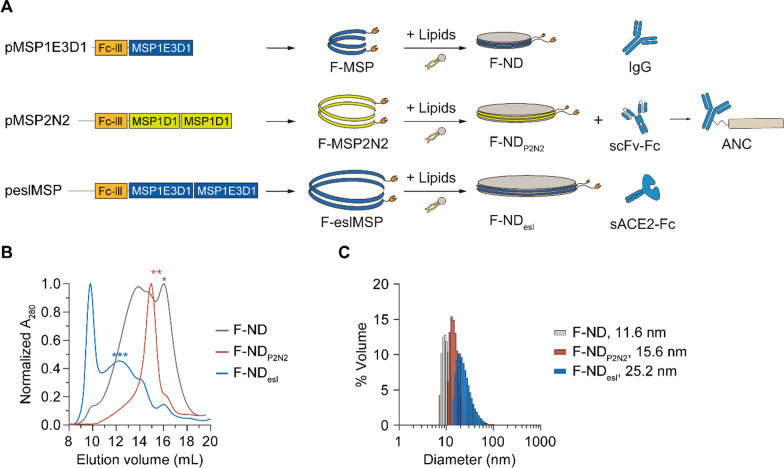
To implement the ANC strategy against SARS-CoV-2, we optimized the size of the nanodiscs by varying the length of the membrane scaffold proteins (MSPs) in the Fc-binding MSPs (F-MSP) (Fig. 1A). In a previous study, we have observed that an increase in nanodisc size enhances antiviral activity but results in reduced thermostability in the presence of trypsin and production yield. The Fc-binding peptide sequence (Fc-III, NH2-DCAWHLGELVWCT-COOH), which has a high affinity for immunoglobulin G (IgG), has been added to the end of various MSP sequences to make the protein Fc-binding MSPs (F-MSPs) [42]. The 12 nm nanodiscs made with F-MSP has been shown to assemble with high yield but exhibits low antiviral activity [37]. In contrast, the 25 nm nanodiscs made with Fc-binding end-spliced and elongated MSPs (F-eslMSP, with two sequentially arranged MSP1E3D1 sequences) displays strong antiviral efficacy but low stability [37]. Therefore, we sought to find a balance between yield and antiviral potency. Unlike F-ND and F-NDesl, F-NDP2N2, where two MSP1D1 sequences were sequentially arranged, resulted in an increase in nanodisc size with a monodisperse population (Fig. 1B). SEC profiles of F-ND displayed distinct peaks, with one population of small F-ND found at an elution volume of 16 mL and another population of larger ND structures at 13.5 mL (Fig. 1B). In contrast, F-ND P2N2 showed a single population at 15 mL, while F-NDesl showed a population of large nanodiscs at 12 mL with a substantial amount of voids. After collecting each peak, particle size was analyzed using dynamic light scattering spectroscopy (DLS). F-ND P2N2 exhibited a diameter of approximately 15.6 nm, F-ND measured 11.6 nm, and F-NDesl was 25.2 nm (Fig. 1C). When the thermal stability of each F-ND fraction was measured by incubating the samples at 37 ℃ and analyzing the amount of soluble fraction by SDS-PAGE, the band intensity did not decrease over 48 h, confirming that the proteins of the NDs remained stable at 37 ℃ (Additional file 1: Fig. S1). To evaluate the binding of NDs and F-NDs to IgG, an ELISA-based assay was conducted using IgG-coated plates with serially diluted NDs. The results demonstrated that all F-NDs exhibited significantly higher binding affinity to IgG compared to NDs lacking the Fc-binding motif (Additional file 1: Fig. S2). The binding affinity increased in the order of F-ND < F-NDP2N2 < F-NDesl, likely due to steric hindrance associated with nanodisc size. Further ELISA tests were conducted to assess whether F-NDP2N2 could bind to various Fc fusion proteins, including IgG, scFv-Fc, and sACE2-Fc, using plates coated with either NDP2N2 or F-NDP2N2. F-NDP2N2 was able to bind to all the tested Fc fusion proteins. To quantify the binding stoichiometry between IgG and F-NDP2N2, we employed SEC using an FPLC system with a Superose column. The elution volumes observed were 16.2 mL for IgG, 15.1 mL for F-NDP2N2, and approximately 14.5 mL for the IgG + F-NDP2N2 complex (Additional file 1: Fig. S3). This elution pattern supports a 1:1 molar binding ratio between IgG and F-NDP2N2.
Fig. 1.
Optimization of Fc-binding nanodisc. A Schematic representation of various types of ANC. Fc-binding nanodiscs (F-NDs) can form ANCs by binding with IgG, single-chain variable fragment-Fc (scFv-Fc), and sACE2-Fc. B SEC chromatograms of F-NDs. Asterisks denote the elution volume of F-NDs. C Particle size distribution of F-NDs measured using dynamic light scattering
We conducted a SARS-CoV-2 pseudovirus inhibition assay using F-NDs of various sizes. The results showed that combinations of sACE2-Fc or scFv-Fc P2B-2F6 [43] with F-NDs significantly reduced pseudovirus infectivity, whereas the combination of an isotype IgG control and F-NDs showed no neutralizing efficacy (Additional file 1: Fig. S4 and Fig. S5). The observed antiviral effects, increasing in the order of F-ND < F-NDesl < F-NDP2N2, suggest that a steric hindrance-based mechanism may be contributing to virus inhibition by the ND. Furthermore, this suggests that virus binding is crucial for the antiviral activity of ANCs, whether through sACE2-Fc or neutralizing antibodies (nAbs). Both ANCs of scFv-Fc P2B-2F6 and sACE2-Fc exhibited enhanced antiviral activity compared to each component alone, with the optimized F-NDP2N2 formulation showing a significant reduction in IC50 compared to the antibody alone. Specifically, scFv-Fc P2B-2F6 alone had an IC50 of 10.6 nM, while the ANC with F-NDP2N2 showed an IC50 of 2.29 nM, with F-ND at 3.94 nM, and with F-NDesl at 4.5 nM. In the case of sACE2-Fc ANCs, sACE2-Fc alone had an IC50 of 152 nM, while the ANC with F-ND showed an IC50 of 31.5 nM, with F-NDP2N2 at 7.87 nM, and with F-NDesl at 7.74 nM. Notably, the sACE2-Fc ANC with F-NDP2N2 achieved complete inhibition of SARS-CoV-2 pseudovirus entry.
Since the phospholipids in nanodiscs significantly influence their physical properties, stability, and interaction with viral membranes, we compared the effects of different phospholipids in the F-NDs on antiviral activity. F-NDP2N2 was prepared using F-MSP2N2 and phospholipids with the same head groups but different acyl tails—POPC, DPPC, and DMPC—resulting in F-NDP2N2/POPC, F-NDP2N2/DPPC, and F-NDP2N2/DMPC, respectively (Fig. 2A). Given that sACE2-Fc exhibits antiviral activity against various SARS-CoV-2 variants, though its efficacy is lower than that of approved neutralizing antibodies (nAbs) [36], we used sACE2-Fc to test the potential of F-NDs with different lipid compositions to enhance antiviral activity. First, we determined the effect of sACE2-Fc on the infection of SARS-CoV-2 pseudovirus (Fig. 2B). Then, after serial dilution of the F-NDs, each F-ND was mixed with either a vehicle, 20 nM sACE2-Fc (corresponding to a 10% effective concentration; EC10) or 100 nM sACE2-Fc (corresponding to EC50) and subjected to a SARS-CoV-2 pseudovirus inhibition assay. The F-NDs alone showed no antiviral efficacy in the absence of sACE2-Fc (Fig. 2C). Interestingly, in the presence of 20 nM sACE2-Fc (EC10), F-NDP2N2/DMPC exhibited very strong antiviral potency (Fig. 2D). The presence of 10 nM F-NDP2N2/DMPC reduced infection by 50%, compared to 10% in the absence of F-NDP2N2/DMPC. This amplification effect increased with higher concentrations of F-NDs. While the effect of lipid composition on the F-NDs was evident under EC10 conditions, no dramatic difference was observed at EC50 conditions (Fig. 2E). However, it was clear that F-NDs could significantly enhance the reduction of infection by sACE2-Fc. Furthermore, the antiviral efficacy shown in the presence of both sACE2-Fc and F-NDs could not be achieved by increasing the concentration of sACE2-Fc alone.
Fig. 2.
Optimization of lipid composition of F-NDs. A Illustration of the lipid structures within the nanodiscs, including DMPC, DPPC, and POPC. B Inhibition of SARS-CoV-2 pseudovirus by sACE2-Fc, with data presented as mean ± standard deviation (SD; n = 3). C–E SARS-CoV-2 pseudovirus inhibition assay comparing the antiviral efficacy of F-NDs composed of different phospholipids (POPC, DPPC, and DMPC). The assay was conducted with serially diluted F-NDs mixed with vehicle, 20 nM sACE2-Fc (corresponding to a 10% effective concentration; EC10), and 100 nM sACE2-Fc (corresponding to EC50). Data are presented as mean ± SD (n = 3). F Pseudovirus inhibition assay comparing the relative infectivity of SARS-CoV-2 in the presence of 20 nM sACE2-Fc (corresponding to EC10) combined with serially diluted F-NDP2N2/DMPC or F-NDP2N2/DMPC/GM1. G SARS-CoV-2 inhibition assay showing the results of serially diluted S309 mixed with a vehicle, 10 nM F-NDP2N2/DMPC, or 10 nM F-NDP2N2/DMPC/GM1. Data are presented as mean ± SD (n = 3)
To further enhance the antiviral efficacy of F-NDs in augmenting sACE2-Fc, we tested whether the addition of ganglioside GM1 to F-NDs would affect the antiviral potency of sACE2-Fc, given that ganglioside GM1 has been shown to bind to the SARS-CoV-2 spike protein [44–46]. At sACE2-Fc concentration EC10 (20 nM), F-NDP2N2/DMPC/GM1 showed a 2.5-fold reduction in the IC50 compared to F-NDP2N2/DMPC without GM1 (Fig. 2F). Next, we tested whether this enhancement of sACE2-Fc’s antiviral efficacy by F-NDs could also be achieved with neutralizing antibodies (nAbs). We used the S309 nAb, which is the parent monoclonal antibody (mAb) of FDA-approved sotrovimab, whose half-life has been extended by modification of the Fc. A SARS-CoV-2 pseudovirus inhibition assay was conducted using serially diluted nAbs with either 10 nM of F-NDP2N2/DMPC or F-ND P2N2/DMPC/GM1 (Fig. 2G). We observed 100% inhibition with 10 nM of S309 + 10 nM of F-NDP2N2/DMPC/GM1, whereas the same concentration of S309 + 10 nM F-NDP2N2/DMPC achieved 80% inhibition, and S309 alone resulted in approximately 50% inhibition against SARS-CoV-2 pseudovirus. Just 10 nM of F-NDP2N2/DMPC/GM1 reduced the IC50 of S309 from 30 to 2 nM, an enhancement that could not be achieved by increasing the concentration of S309 alone. Based on these results, NDP2N2/DMPC/GM1 comprising F-MSP2N2, DMPC and GM1 were selected for subsequent experiments to achieve the most optimized lipid composition for ANC against SARS-CoV-2.
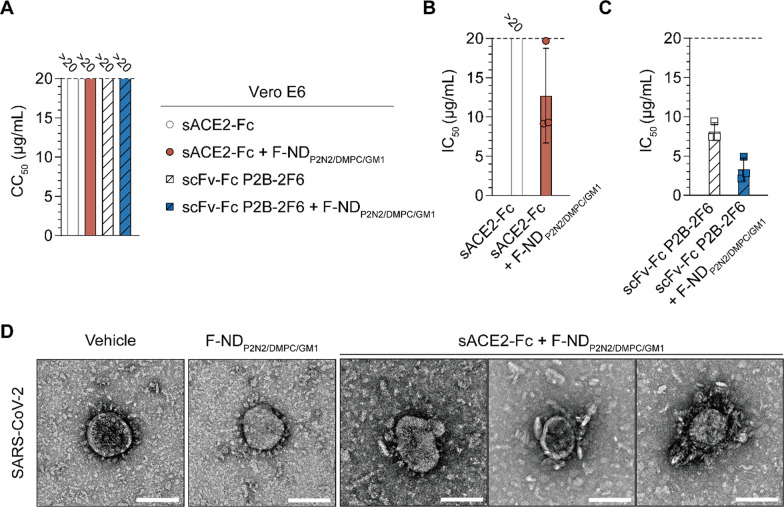
The antiviral activity of ANC was evaluated using authentic SARS-CoV-2 in a multiple-cycle infection assay. A cell viability assay revealed that the 50% cytotoxic concentration (CC50) values for sACE2-Fc, sACE2-Fc complexed with F-NDP2N2/DMPC/GM1, scFv-Fc P2B-2F6, and scFv-Fc P2B-2F6 complexed with F-NDP2N2/DMPC/GM1 were consistently above 20 µg/mL, indicating that these formulations are well-tolerated by the cells (Fig. 3A). In parallel, the antiviral efficacy against 100 × TCID50 of the Beta-CoV/Korea/KCDC03/2020 strain showed significant inhibition of viral infectivity by the ANCs. Serial dilutions of each antiviral were mixed with the virus and then added to the cells. After a 1-h incubation at 37 ℃, the supernatant was replaced with fresh medium containing the respective inhibitor, and the cells were incubated for 96 h. The final cytopathic effect due to viral infection was then assessed. sACE2-Fc alone exhibited an IC50 above 20 µg/mL, while the combination of sACE2-Fc with F-NDP2N2/DMPC/GM1 reduced the IC50 to 12.7 µg/mL (Fig. 3B). Similarly, scFv-Fc P2B-2F6 alone showed moderate antiviral activity with an IC50 of 8.1 µg/mL, but when combined with F-NDP2N2/DMPC/GM1, the IC50 significantly decreased to 3.3 µg/mL (Fig. 3C). These results demonstrate that the antiviral efficacy of nAbs and sACE2-Fc is enhanced by ANCs against authentic virus during multiple infection cycles.
Fig. 3.
In vitro antiviral efficacy against authentic SARS-CoV-2. A Cell viability and antiviral activity in Vero E6 cells exposed to different formulations. (Left) CC50 values, the concentration causing 50% cytotoxicity of Fc fusion proteins and their ANCs. B Antiviral efficacy of sACE2-Fc and its ANC against SARS-CoV-2. C Antiviral efficacy of scFv-Fc P2B-2F6 and its ANC. IC50 values are the concentration required to reduce viral infectivity by 50%. Data are expressed as mean ± SD (n = 3). D Transmission electron microscopy images demonstrating the morphology in SARS-CoV-2 following treatment with vehicle, F-NDP2N2/DMPC/GM1, and sACE2-Fc complexed with F-NDP2N2/DMPC/GM1. Scale bars, 100 nm
Transmission electron microscopy (TEM) provided visual confirmation of the ANCs' mechanism of action, revealing significant morphological changes in SARS-CoV-2 particles upon treatment (Fig. 3D). SARS-CoV-2 particles treated with the vehicle or with F-NDP2N2/DMPC/GM1 alone maintained an intact and well-defined viral morphology. In contrast, SARS-CoV-2 particles treated with sACE2-Fc combined with F-NDP2N2/DMPC/GM1 were surrounded by nanodiscs. The TEM images suggest that the ANC's sACE2-Fc or nAb binds to the spike protein, with the adjacent nanodisc moiety then enveloping the virus particle. This coverage likely prevents cellular infection by the virus, thereby enhancing the antiviral efficacy of the ANC compared to the antibody alone.
Pharmacokinetics of IgG and its ANC
The pharmacokinetics of IgG and its ANC were evaluated following intranasal (IN) administration to BALB/c mice at a dose of 5 mg/kg to investigate the effect of F-NDP2N2/DMPC/GM1 attachment on IgG half-life. IgG levels in bronchoalveolar lavage fluid (BALF), larynx/trachea, and lung tissues were measured at multiple time points using ELISA to detect the human Fc of IgG. The concentration of IgG and ANC in these tissues was quantified at 2-, 6-, 12-, 24-, and 48-h post-administration. Pharmacokinetic parameters, including time to reach maximum concentration (Tmax), maximum concentration (Cmax), half-life (T1/2), and area under the concentration–time curve (AUCt), were calculated. ANC exhibited a significantly increased half-life and AUCt compared to IgG, particularly in the larynx/trachea, indicating a prolonged retention and potentially enhanced efficacy of ANC in these tissues (Fig. 5). Specifically, the half-life of ANC in the larynx and trachea was approximately 3.4 times longer than that of IgG alone, indicating that the attachment of F-NDP2N2/DMPC/GM1 to IgG significantly extended its half-life in these tissues (Table 1). This suggests that ANCs may provide superior antiviral protection compared to IgG treatments, highlighting their potential as a more effective prophylactic strategy by offering longer-lasting protection in the upper respiratory tract. This advantage is particularly important for combating respiratory infections, such as those caused by Omicron variants, which are known to actively target the upper respiratory tract [17–19].
Fig. 5.
Pharmacokinetics of IgG and IgG-based ANC post-intranasal administration. IgG concentrations in various tissues (bronchoalveolar lavage fluid; BALF, larynx/trachea, and lung tissue) following intranasal administration of IgG and ANC at a dose of 5 mg/kg. Samples were collected from treated mice at the indicated time points post-administration, and antibody concentrations were measured by detecting human Fc regions of IgG using ELISA. Data represent mean ± standard error of the mean (n = 4). Statistical analysis was performed using two-tailed t-tests to compare IgG and ANC concentrations at each time point. Not significant (NS), p > 0.5; *, p ≤ 0.05
Table 1.
Pharmacokinetic parameters following intranasal administration of IgG and IgG-based ANC, including time to reach maximum concentration (Tmax), maximum concentration (Cmax), half-life (T1/2), and area under the concentration–time curve (AUCt)
| Tissue | Tmax (h) | Cmax (ng.mL) | T1/2 (h) | AUCt (h*ng/mL) | |
|---|---|---|---|---|---|
| IgG | BALF | 2 | 357,061.64 | 8.70 | 6,001,573.02 |
| Larynx/Trachea | 2 | 2317.29 | 20.97 | 48,325.33 | |
| Lung | 24 | 115,205.90 | 36.59 | 3,993,899.53 | |
| ANC | BALF | 2 | 157,457.70 | 13.24 | 2,226,325.03 |
| Larynx/Trachea | 12 | 2595.72 | 71.51 | 102,410.47 | |
| Lung | 12 | 79,793.71 | 35.74 | 2,944,785.38 |
Intranasal administration of sACE2-Fc ANCs protects against lethal doses of SARS-CoV-2 in infected mice
Due to the substantial enhancement in efficacy observed with sACE2-Fc combined with F-NDP2N2/DMPC/GM1 in authentic SARS-CoV-2 in vitro experiments (Fig. 3B), we aimed to assess this ND-induced efficacy enhancement in mice model. To evaluate protective effects of sACE2-Fc and its ANC in K18-hACE2 transgenic mice infected with SARS-CoV-2, eight-week-old female C57BL/6 Cg-Tg 2Prlmn/J mice were intranasally challenged with 3 MLD50 of the Beta-CoV/Korea/KCDC03/2020 strain. Prophylactic efficacy was assessed by administering inhibitors intranasally 24 h prior to virus inoculation (Fig. 4A). Specifically, sACE2-Fc and isotype control IgG were administered at a dose of 10 mg/kg, while ANCs were administered at 10 mg/kg with the nanodisc concentration adjusted to achieve twice the molar equivalent of the antibodies.
Fig. 4.
Protective effects of IN administered sACE2-Fc and its ANC in mice infected with SARS-CoV-2. A Schematic representation illustrates the intranasal delivery of IgG (isotype control), sACE2-Fc, and its ANC to K18-hACE2 transgenic mice. Subsequently, mice were inoculated to a 3 MLD50 dose of the SARS-CoV-2 isolate Beta-CoV/Korea/KCDC03/2020 one day post administration. B Kaplan–Meier survival curves (left, n = 5) and changes in body weight (right). Data are expressed as mean ± SD (n = 5). Statistical analysis was performed using ordinary two-way analysis of variance (ANOVA) with Tukey's multiple comparisons test. Not significant (NS), p > 0.5; *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001. C Viral titers in lung tissue at 5 days post-infection. Data are expressed as mean ± SD (n = 6). Statistical analysis was performed using one-way ANOVA with Tukey's multiple comparisons test. Not significant (NS), p > 0.5; *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001. D Histological analysis of lung tissues was conducted using immunohistochemistry (top), and hematoxylin and eosin (bottom) staining at 5 days post-infection. Scale bar, 100 μm
Mice in the isotype IgG control group had an 80% survival rate, while those treated with IgG + F-NDP2N2/DMPC/GM1 had a 60% survival rate (Fig. 4B). In contrast, both the sACE2-Fc and ANC-treated groups achieved 100% survival. Additionally, body weight analysis showed that the control group experienced significant weight loss of approximately 10% or more, whereas the sACE2-Fc and ANC groups maintained body weights above 100% of their initial values, with the ANC group showing a notable improvement compared to the sACE2-Fc group.
At 5 days post-infection (DPI), viral titers in lung tissues were assessed (Fig. 4C). Mice treated with sACE2-Fc and its ANC exhibited significantly lower viral loads compared with the IgG control group and its ANC group, which had viral titers of approximately 106 TCID50/mL. The sACE2-Fc-treated group showed about a 2-log reduction in viral load, while its ANC form showed about a 4-log reduction, highlighting the enhanced antiviral efficacy of the ANC formulation. Histological examination of lung tissues at 5 DPI was performed using IHC and H&E staining (Fig. 4D). The isotype IgG and its corresponding ANC control group exhibited extensive lung injury, characterized by severe alveolar damage and immune cell recruitment due to SARS-CoV-2 infection. In contrast, the sACE2-Fc-treated group showed moderate protection with reduced lung damage. Immunohistochemistry (IHC) results revealed brown-stained regions indicating SARS-CoV-2 infection in the lung tissue, present in all test sample groups except for the sACE2-Fc ANC group. The ANC-treated group demonstrated the most significant protective effect, with lung tissues showing minimal signs of infection and preserved alveolar structures. These findings aligned with the quantitative data, indicating that the sACE2-Fc ANC provided superior protection against SARS-CoV-2-induced lung pathology (Fig. 5).
To further assess the efficacy of the treatment, we carried out experiments using a more stringent viral challenge corresponding to a lethal dose of 5 MLD50 of the Beta-CoV/Korea/KCDC03/2020 strain (Additional file 1: Fig. S6). Under these conditions, the vehicle-treated group exhibited 100% mortality. In contrast, the group treated with sACE2-Fc alone demonstrated a 40% survival rate, while the group receiving sACE2-Fc ANCs achieved an 80% survival rate. Notably, body weight loss remained below 5% in the sACE2-Fc ANC-treated group. Viral titration assays further revealed that sACE2-Fc treatment reduced the viral load by 2-log, whereas sACE2-Fc ANC treatment achieved a 4-log reduction. These findings indicate that the sACE2-Fc ANC confers significantly enhanced protection against SARS-CoV-2 infection compared to sACE2-Fc alone in mice.
Evaluation of the efficacy of ANCs with various anti-spike antibodies against SARS-CoV-2
The development of nAbs against SARS-CoV-2 primarily targets the spike protein, which consists of two subunits: S1 and S2. S1 is responsible for receptor binding, while S2 facilitates membrane fusion. Therefore, nAb development focuses on these subunits [47]. Antibodies targeting S1, particularly its RBD, tend to have high antiviral efficacy due to their ability to block virus attachment. Conversely, S2, being a relatively conserved region, is targeted for developing broad-spectrum neutralizing antibodies.
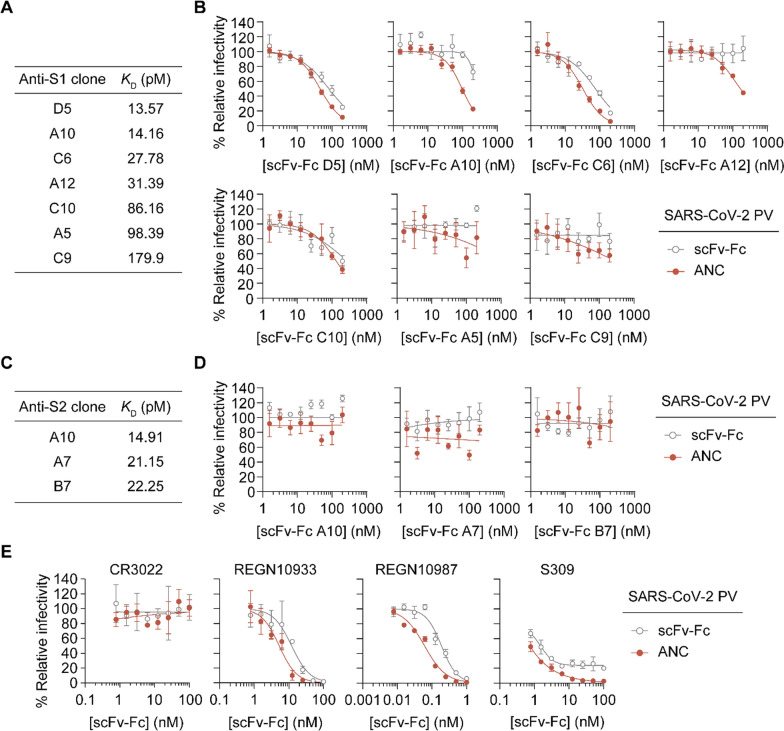
To identify conditions under which F-ND complexation enhances the antiviral efficacy of mAbs, various antibodies (anti-S1 and anti-S2) with different binding affinities were prepared (Additional file 1: Fig. S7), and the antiviral activities of mAbs and their ANCs were tested. The binding affinities of prepared anti-S1 mAbs to the spike protein of SARS-CoV-2 were measured using ELISA. The dissociation constants (KD) for the anti-S1 clones (D5, A10, C6, A12, C10, A5, C9) ranged from 13.57 pM to 179.9 pM. For the anti-S2 clones, the KD values were 14.91 pM for A10, 21.15 pM for A7, and 22.25 pM for B7, indicating a spectrum from high to moderate binding affinity (Fig. 6A, C, and Additional file 1: Fig. S8).
Fig. 6.
Comparison of antiviral effects of anti-S1 and anti-S2 mAbs and their corresponding ANC against SARS-CoV-2 pseudovirus. A Binding affinity of anti-S1 mAbs for the spike protein of SARS-CoV-2, measured using ELISA. The table lists dissociation constants (KD) in picomolar (pM) for selected anti-S1 scFv-Fc (D5, A10, C6, A12, C10, A5, C9), demonstrating a range from high to moderate affinity. B Pseudovirus inhibition assay comparing the relative infectivity of SARS-CoV-2 in the presence of increasing concentrations of each scFv-Fc, and its corresponding ANC. Data are expressed as mean ± SD (n = 3). C Binding affinity of anti-S2 mAbs for the spike protein of SARS-CoV-2, measured using ELISA. The table lists dissociation constants (KD) in picomolar (pM) for selected anti-S1 scFv-F. D Pseudovirus inhibition assay comparing the relative infectivity of SARS-CoV-2 in the presence of increasing concentrations of each scFv-Fcs, and their corresponding ANC. Data are expressed as mean ± SD (n = 3). E Pseudovirus inhibition assay comparing the relative infectivity of SARS-CoV-2 in the presence of increasing concentrations of scFv-Fcs of CR3022, REGN10933, REGN10987 and S309, and their corresponding ANC. Data are expressed as mean ± SD (n = 3)
In the pseudovirus inhibition assay, we compared the relative infectivity of SARS-CoV-2 in the presence of increasing concentrations of each mAb and its corresponding ANC. The results demonstrated that the ANCs with anti-S1 clones D5, A10, C6, and A12 achieved significantly more inhibition of pseudovirus entry compared to the mAbs alone (Fig. 6B and D). In contrast, ANCs with anti-S1 clones C10, A5, and C9, as well as ANCs with anti-S2 clones A10, A7, and B7, showed little difference compared to the mAbs alone. These results suggest that the antibody may require a binding affinity to the S1 subunit below a certain threshold for ANC to be effective against SARS-CoV-2. Specifically, in our ELISA-based assay, ANCs with the dissociation constants higher than approximately 86 pM (such as C10, A5, and C9) did not show enhanced inhibition. Interestingly, even antibodies like A12 and A10, which bind strongly to the spike protein but are weakly neutralizing, could prevent cellular infection when formulated as ANCs.
We further assessed the antiviral efficacy of several anti-S1 nAbs in the form of scFv-Fc such as FDA-approved CR3022 [48, 49], REGN10933, REGN10987 [50], and S309 [51], and their corresponding ANCs against SARS-CoV-2 pseudovirus using a pseudovirus inhibition assay. The inhibition curves demonstrate that the ANCs consistently achieved greater antiviral efficacy compared to the mAbs alone, except for CR3022 (Fig. 6E). Specifically, CR3022, an anti-S1 antibody known to bind the spike protein but lack neutralizing activity against SARS-CoV-2 [49], exhibited no inhibition across a range of concentrations, and neither did the CR3022-ANC. Both REGN10933 and REGN10987 nAbs were effective against pseudovirus infection, but their corresponding ANCs further enhanced the antiviral effect of the nAbs. S309 also demonstrated improved efficacy in its ANC form. Interestingly, while S309 alone had an IC50 of approximately 1 nM, it did not achieve 100% pseudovirus inhibition even at higher concentrations. The S309-ANC, however, achieved a 100% reduction in pseudovirus infectivity, highlighting the potential of ANC to enhance the antiviral potency of existing therapeutic antibodies.
These findings suggest that while ANCs can significantly enhance the antiviral activity of monoclonal antibodies (mAbs) against SARS-CoV-2, this enhancement occurs only when the mAbs possess at least a weak neutralizing effect. Binding alone, however, does not ensure neutralization. For example, although both CR3022 and A12 displayed high binding affinities to the spike protein, only A12-ANC exhibited antiviral effects, whereas CR3022-ANC did not. This indicates that the ANC platform is effective when the antibody engages a neutralization-relevant epitope and has at least some degree of neutralizing effect.
The observed amplification of antiviral activity may be due to the additional physical blockade provided by the nanodisc component, which could obstruct viral particles from interacting with host cells. These findings suggest that the ANC platform has the potential to enhance the efficacy of antibodies that possess a weak neutralizing effect, positioning ANCs as promising candidates for therapeutic intervention against COVID-19.
Broad-spectrum antiviral activity of S309 ANC against SARS-CoV-2 and its variants
Although many SARS-CoV-2 nAbs have been developed, their neutralizing activity has diminished or even been lost as the virus has evolved into variants like Omicron. For instance, S309, the parental mAb of sotrovimab, has shown reduced neutralizing efficacy against Omicron subvariants. However, it has retained sufficient binding affinity [52, 53]. Leveraging this characteristic of S309, studies have explored its potential as a broad-spectrum antiviral agent with Fc-mediated functions. Similarly, we tested the S309-ANC as a broad-spectrum antiviral capable of effectively combating Omicron variants.
We investigated the broad-spectrum antiviral activity of S309 ANCs against SARS-CoV-2 and its variants, aiming to demonstrate the potential of ANCs in enhancing the efficacy of nAbs. The antiviral efficacy of S309 and its corresponding ANC was evaluated against SARS-CoV-1, SARS-CoV-2, and their variants using pseudovirus inhibition assays (Fig. 7A). The results revealed that while S309 alone achieved about 80% inhibition at the highest tested concentration for both SARS-CoV-1 and SARS-CoV-2, the use of ANCs resulted in 100% inhibition. A similar pattern was observed with the Delta variant. Notably, while S309 alone exhibited reduced neutralizing activity against Omicron variants, the S309 ANC consistently achieved greater inhibition of pseudovirus infectivity across all tested variants, highlighting its potential as a broad-spectrum antiviral strategy. The comparison of IC50 values (Fig. 7B) further emphasized the enhanced efficacy of S309 when formulated as an ANC, with IC50 values consistently lower across all variants compared to S309 alone, indicating a significant improvement in antiviral potency. Importantly, the S309 ANC restored the antiviral efficacy of S309, which had been lost against most Omicron variants. Consequently, given that sotrovimab still retains binding affinity to most Omicron variants, albeit with reduced neutralization efficacy, it is cautiously anticipated that sotrovimab may remain effective against Omicron variants when formulated as an ANC.
Fig. 7.
Broad-spectrum antiviral activity of S309 ANC against SARS-CoV-2 and its variants. A Pseudovirus inhibition assays displaying the efficacy of S309, as both a monoclonal antibody (scFv-Fc form) and an antibody-nanodisc complex (ANC), against SARS-CoV-1, SARS-CoV-2, and its variants. Data represents as mean ± SD (n = 3). B Comparative analysis of half- maximal inhibitory concentration (IC50) values for S309 in its scFv-Fc alone and as ANC. C, D Comparative analysis of IC50 values for romlusevimab and bebtelovimab, in IgG form and ANC
To evaluate the broader applicability of our hypothesis, we included class III antibodies such as romlusevimab and bebtelovimab in our experiments. Previous studies have shown that romlusevimab demonstrates strong neutralizing efficacy against SARS-CoV-2 WT but shows diminished neutralization against the Delta, BA.1, and BA.2 variants. Its neutralizing efficacy is further lost against subsequent variants, including BA.4, BF.7, and BQ.1.1 [6, 54]. In contrast, bebtelovimab exhibits strong neutralizing efficacy against SARS-CoV-2 WT and Omicron subvariants BA.1, BA.2, BA.4, and BF.7, as well as the Delta variant [55]. However, it shows significantly reduced neutralizing efficacy and binding affinity against the BQ.1.1 variant, and from the XBB variant onward, both binding and neutralization are completely lost. Given these observations, we aimed to determine whether ANC could restore antiviral efficacy. Our results demonstrated that romlusevimab ANCs restored potent antiviral efficacy against the Delta, BA.1, BA.2, and BA.4 variants (Fig. 7C and Additional file 1: Fig. S9). Similarly, bebtelovimab ANCs effectively restored antiviral efficacy against the Delta and BQ.1.1 variants, enhancing antiviral potency to levels comparable to those seen with WT (Fig. 7D and Additional file 1: Fig. S9). These findings highlight the potential of ANCs to counteract the loss of neutralizing efficacy caused by mutations in SARS-CoV-2 variants.
Discussion and conclusion
We previously demonstrated the concept of ANCs using the nAb MEDI8852 against influenza viruses. Our observations revealed that the increased surface area of nanodiscs enhances antiviral effects by enabling more effective interactions with viral hemagglutinin trimers [37]. This interaction facilitates membrane fusion and perforation of the viral envelope, thereby amplifying the antiviral efficacy of the nAb. However, the large nanodiscs proved unstable in solution. These findings underscore the importance of optimizing nanodisc size to enhance the antiviral activity of nAbs while maintaining overall stability.
In this study, we further evaluated the efficiency of nanodisc formation, Fc-binding affinity, and antiviral efficacy enhancement by varying the size of Fc-binding nanodiscs through controlling the length of MSP-forming helices. Based on these comprehensive assessments, the F-NDP2N2 formulation emerged as the most effective antiviral F-ND in the ANC platform. Specifically, the smallest F-NDs demonstrated the highest overall assembly yield but tended to exhibit multiple populations (Fig. 1B). In contrast, F-NDP2N2 was produced as a single population, while F-NDesl formed a larger population with a substantial amount of voids. Additionally, smaller Fc-binding nanodiscs demonstrated higher affinity for IgG, likely due to reduced steric hindrance, facilitating more effective antibody binding (Additional file 1: Fig. S2). In vitro results further substantiated this observation, showing that F-NDP2N2 achieved the highest antiviral efficacy among the tested nanodiscs (Additional file 1: Fig. S4). Although the F-NDesl, with its larger size, had the theoretical potential to enhance antiviral activity due to a greater surface area for interacting with viral envelope proteins, its diminished ability to effectively bind antibodies curtailed its overall antiviral performance. This inverse relationship between nanodisc size and antibody binding efficiency underscores the need for a balanced approach in nanodisc design to optimize antiviral efficacy. Furthermore, the overall production yield of NDesl was the lowest among F-NDs. Therefore, the intermediate-sized F-NDP2N2 provided the best balance, resulting in the most effective ANC formulation for antiviral purposes.
The ANC demonstrated a significantly extended half-life in the larynx and trachea compared to IgG alone after intranasal administration (Fig. 5). Although the area under the curve indicated that the ANC levels in the bronchoalveolar lavage fluid (BALF) were lower than those of IgG, this also suggests that a substantial portion of the ANC remains in the nasal wash or nasal turbinates. Indeed, the half-life of ANC in the larynx and trachea was approximately 3.4 times longer than that of IgG alone (Fig. 5). This extended retention enables ANC to maintain antibody levels in the upper respiratory tract for a longer duration, which is crucial for protecting against SARS-CoV-2 Omicron variants, known for their robust replication in the upper airway. Furthermore, the combination of the antibody and nanodisc (i.e., ANC) effectively inhibited viral infection in cells (Fig. 2 and Fig. 3D). The use of ANCs provided significantly greater antiviral protection in mice compared to sACE2-Fc treatment alone (Fig. 4 and Fig. S6).
We successfully enhanced the antiviral efficacy of several weak nAbs using F-NDs (Fig. 6). ANCs could significantly boost the antiviral activity of mAbs against SARS-CoV-2, but this enhancement depends on the mAbs' ability to bind to the virus. However, binding alone does not guarantee neutralization, as shown by CR3022-ANC, which lacked antiviral effects despite high binding affinity. Notably, F-NDs can form a complex with weak neutralizing antibodies, transforming them into potent antiviral agents. This finding is particularly significant in cases of mutational escape of virus, such as with Omicron variants. The nAb S309, which retains binding capacity against Omicron variants despite weakened neutralizing efficacy, could regain antiviral activity when complexed with a nanodisc. S309-based ANCs demonstrated effective inhibition across various SARS-CoV-2 variants, including Omicron subvariants (Fig. 7). This broad-spectrum activity suggests that S309-based ANCs could offer a robust antiviral strategy against Omicron strains, unless sotrovimab becomes a non-binder due to viral escape.
Nanodiscs leverage intrinsic biological components, specifically discoidal high-density lipoprotein (HDL), to enhance antiviral treatments. HDL, commonly known as "good cholesterol," plays a vital role in cardiovascular health by removing cholesterol from artery walls and facilitating its breakdown in the liver [56]. Engineered recombinant HDLs self-assemble into uniform protein-lipid nanoparticles, forming nanodiscs. These nanodiscs have numerous biomedical applications, including mimicking plasma membranes, studying membrane protein structures, and serving as drug carriers for hydrophobic and amphipathic small molecules, offering potential therapeutic applications across various diseases [57]. The clinical potential of nanodiscs is underscored by their safety and efficacy. Extensive clinical research, including Phase I and II studies involving over 800 participants, has demonstrated that HDL-related products are safe, with no significant adverse effects. Trials of the HDL mimetic nanoparticle CER-001 have shown general tolerability without severe reactions. Advances in the manufacturing processes for HDL formulations, such as CSL-111, CSL-112, ETC-216, MDCO-216, and SRC-rHDL, have improved purity and yield standards, making them suitable for clinical trials [58–60]. Additionally, our studies found no cytotoxicity or adverse effects in mice, indicating that nanodiscs are non-toxic antivirals. Their biocompatible and non-immunogenic design minimizes unwanted immune responses, which is crucial for repeated treatments. Furthermore, nanodiscs have demonstrated remarkable stability, maintaining their integrity through repeated freeze–thaw cycles and long-term storage.
Further studies are needed to evaluate the ability of S309-ANC to restore antiviral efficacy in vivo against Omicron, as the antiviral effect of S309 alone is diminished against this variant. Conducting experiments with S309-ANC in an Omicron-challenged mice model could provide critical insights into its potential as an enhanced antiviral strategy. Furthermore, while this study demonstrates the potential of intranasal ANC delivery for prophylactic use, future studies are needed to evaluate their therapeutic efficacy in already infected animals. Future research should focus on further enhancing the properties of nanodiscs to expand their therapeutic applications across a broader range of viral infections. The demonstrated adaptability of this technology to various viral targets underscores its potential as a universal antiviral platform, particularly valuable in combating rapidly evolving viruses such as the severe fever with thrombocytopenia syndrome virus, respiratory syncytial virus, herpes simplex virus, and hepatitis B virus. The ability to tailor the ANC platform for different viral pathogens highlights its versatility and strength as an antiviral therapy approach.
Additionally, the dual mechanism of action provided by ANCs—combining the neutralizing power of antibodies with the physical blockade of viral particles by nanodiscs—offers a promising strategy to overcome the challenges posed by viral mutations and resistance. This dual functionality enhances the likelihood of maintaining high antiviral efficacy, even as viruses rapidly evolve, underscoring the adaptability and effectiveness of the ANC platform in antiviral therapeutics. These findings suggest that by leveraging both antibody-mediated neutralization and nanodisc-induced physical blockade, The ANC platform holds significant promise for developing broad-spectrum antiviral therapies by complementing antibodies with affinity for neutralizing epitopes. This approach not only addresses current viral threats but also equips us to effectively respond to future viral strains, ensuring a robust and adaptable defense against emerging viral pathogens.
Supplementary Information
Acknowledgements
Not applicable.
Author contributions
JH and SC equally designed and contributed to this work. BKK, SS, JHY, KWK, and WP performed experiments. HC, SK, SK, and SY prepared materials and samples. SJ and STJ analyzed the data. MSS, SJK, and DHK supervised the project and wrote the manuscript. All authors read the manuscript and approved the manuscript submission.
Funding
This research was supported by National Research Foundation of Korea (NRF) grant funded by the Korea government (Grant No. 2020R1A2C2101964 and 2022M3E5F1081330). This research was supported by the Samsung Future Technology Center (SRFC-MA1502-53) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI23C0392). This research was also funded by the Korea Research Institute of Bioscience & Biotechnology (KRIBB) Research Initiative Program (KGM9942421).
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Ethics and Consent to Participate declarations: not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jaehyeon Hwang and Soyun Choi contributed equally to this work.
Contributor Information
Min-Suk Song, Email: songminsuk@chungbuk.ac.kr.
Sang Jick Kim, Email: sjick@kribb.re.kr.
Dae-Hyuk Kweon, Email: dhkweon@skku.edu.
References
- 1.Gharbharan A, Jordans CC, GeurtsvanKessel C, den Hollander JG, Karim F, Mollema FP, Stalenhoef-Schukken JE, Dofferhoff A, Ludwig I, Koster A. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat Commun. 2021;12:3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganesh R, Philpot LM, Bierle DM, Anderson RJ, Arndt LL, Arndt RF, Culbertson TL, DestroBorgen MJ, Hanson SN, Kennedy BD. Real-world clinical outcomes of bamlanivimab and casirivimab-imdevimab among high-risk patients with mild to moderate coronavirus disease 2019. J Infect Dis. 2021;224:1278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Obaidi MM, Gungor AB, Nematollahi S, Zangeneh TT, Bedrick EJ, Johnson KM, Low-Adegbija NE, Alam R, Rangan P, WilliamHeise C. Effectiveness of casirivimab-imdevimab monoclonal antibody treatment among high-risk patients with severe acute respiratory syndrome coronavirus 2 B. 1.617.2 (Delta variant) infection. Open Forum Infect Dis. 2022. 10.1093/ofid/ofac186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lv Z, Deng Y-Q, Ye Q, Cao L, Sun C-Y, Fan C, Huang W, Sun S, Sun Y, Zhu L. Structural basis for neutralization of SARS-CoV-2 and SARS-CoV by a potent therapeutic antibody. Science. 2020;369:1505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Y, Yisimayi A, Jian F, Song W, Xiao T, Wang L, Du S, Wang J, Li Q, Chen X, Yu Y, Wang P, Zhang Z, Liu P, An R, Hao X, Wang Y, Wang J, Feng R, Sun H, Zhao L, Zhang W, Zhao D, Zheng J, Yu L, Li C, Zhang N, Wang R, Niu X, Yang S, Song X, Chai Y, Hu Y, Shi Y, Zheng L, Li Z, Gu Q, Shao F, Huang W, Jin R, Shen Z, Wang Y, Wang X, Xiao J, Xie XS. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ai J, Wang X, He X, Zhao X, Zhang Y, Jiang Y, Li M, Cui Y, Chen Y, Qiao R, Li L, Yang L, Li Y, Hu Z, Zhang W, Wang P. Antibody evasion of SARS-CoV-2 Omicron BA.1, BA.1.1, BA.2, and BA.3 sub-lineages. Cell Host Microbe. 2022;30:1077-1083.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Y, Song W, Wang L, Liu P, Yue C, Jian F, Yu Y, Yisimayi A, Wang P, Wang Y, Zhu Q, Deng J, Fu W, Yu L, Zhang N, Wang J, Xiao T, An R, Wang J, Liu L, Yang S, Niu X, Gu Q, Shao F, Hao X, Meng B, Gupta RK, Jin R, Wang Y, Xie XS, Wang X. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Cell Host Microbe. 2022;30:1527-1539.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iketani S, Liu L, Guo Y, Liu L, Chan JF, Huang Y, Wang M, Luo Y, Yu J, Chu H, Chik KK, Yuen TT, Yin MT, Sobieszczyk ME, Huang Y, Yuen KY, Wang HH, Sheng Z, Ho DD. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604:553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Iketani S, Li Z, Liu L, Guo Y, Huang Y, Bowen AD, Liu M, Wang M, Yu J, Valdez R, Lauring AS, Sheng Z, Wang HH, Gordon A, Liu L, Ho DD. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023;186:279-286.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yue C, Song W, Wang L, Jian F, Chen X, Gao F, Shen Z, Wang Y, Wang X, Cao Y. ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5. Lancet Infect Dis. 2023;23:278–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockett R, Basile K, Maddocks S, Fong W, Agius JE, Johnson-Mackinnon J, Arnott A, Chandra S, Gall M, Draper J. Resistance mutations in SARS-CoV-2 Delta variant after sotrovimab use. N Engl J Med. 2022;386:1477–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo SH, Kim L, Lu M, Nagoshi K, Namchuk MN. A review of clinical efficacy data supporting emergency use authorization for COVID-19 therapeutics and lessons for future pandemics. Clin Transl Sci. 2022;15:2279–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weidenbacher PA-B, Waltari E, RiosKobara de Los I, Bell BN, Morris MK, Cheng Y-C, Hanson C, Pak JE, Kim PS. Converting non-neutralizing SARS-CoV-2 antibodies into broad-spectrum inhibitors. Nat Chem Biol. 2022;18:1270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desautels TA, Arrildt KT, Zemla AT, Lau EY, Zhu F, Ricci D, Cronin S, Zost SJ, Binshtein E, Scheaffer SM. Computationally restoring the potency of a clinical antibody against Omicron. Nature. 2024;629:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue T, Yamamoto Y, Sato K, Okemoto-Nakamura Y, Shimizu Y, Ogawa M, Onodera T, Takahashi Y, Wakita T, Kaneko MK. Overcoming antibody-resistant SARS-CoV-2 variants with bispecific antibodies constructed using non-neutralizing antibodies. iScience. 2024;27:109363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan Y, Li X, Zhang L, Wan S, Zhang L, Zhou F. SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal Transduct Target Ther. 2022;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng B, Abdullahi A, Ferreira IA, Goonawardane N, Saito A, Kimura I, Yamasoba D, Gerber PP, Fatihi S, Rathore S. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature. 2022;603:706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao H, Lu L, Peng Z, Chen L-L, Meng X, Zhang C, Ip JD, Chan W-M, Chu AW-H, Chan K-H. SARS-CoV-2 Omicron variant shows less efficient replication and fusion activity when compared with Delta variant in TMPRSS2-expressed cells. Emerg Microbes Infect. 2022;11:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow MY, Pan HW, Seow HC, Lam JK. Inhalable neutralizing antibodies–promising approach to combating respiratory viral infections. Trends Pharmacol Sci. 2023;44:85–97. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Peng P, Deng H, Yang Q, Chen S, Li B, He M, Jin A, Yang Z, Tang N. Real-world effectiveness of an intranasal spray A8G6 antibody cocktail in the post-exposure prophylaxis of COVID-19. Signal Transduct Target Ther. 2023;8:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y, Yue S, Yang Y, Yang S, Pan Z, Yang X, Gao L, Zhou J, Li Z, Hu L. Nasal spray of neutralizing monoclonal antibody 35B5 confers potential prophylaxis against severe acute respiratory syndrome coronavirus 2 variants of concern: a small-scale clinical trial. Clin Infect Dis. 2023;76:e336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel A, Rosenke K, Parzych EM, Feldmann F, Bharti S, Griffin AJ, Schouest B, Lewis M, Choi J, Chokkalingam N. In vivo delivery of engineered synthetic DNA-encoded SARS-CoV-2 monoclonal antibodies for pre-exposure prophylaxis in non-human primates. Emerg Microbes Infect. 2024;13:2294860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maisonnasse P, Aldon Y, Marc A, Marlin R, Dereuddre-Bosquet N, Kuzmina NA, Freyn AW, Snitselaar JL, Gonçalves A, Caniels TG. COVA1-18 neutralizing antibody protects against SARS-CoV-2 in three preclinical models. Nat Commun. 2021;12:6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loo Y-M, McTamney PM, Arends RH, Abram ME, Aksyuk AA, Diallo S, Flores DJ, Kelly EJ, Ren K, Roque R. The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans. Sci Transl Med. 2022;14:eabl8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cobb RR, Nkolola J, Gilchuk P, Chandrashekar A, Yu J, House RV, Earnhart CG, Dorsey NM, Hopkins SA, Snow DM. A combination of two human neutralizing antibodies prevents SARS-CoV-2 infection in cynomolgus macaques. Med. 2022;3:188-203.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia J, Yin Z, Zhang X, Li H, Meng D, Liu Q, Wang H, Han M, Suo S, Liu Y. Feasibility studies of nebulized SARS-CoV-2 neutralizing antibody in mice and cynomolgus monkeys. Pharm Res. 2022;39:2191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart TK, Cook RM, Zia-Amirhosseini P, Minthorn E, Sellers TS, Maleeff BE, Eustis S, Schwartz LW, Tsui P, Appelbaum ER. Preclinical efficacy and safety of mepolizumab (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeys. J Allergy Clin Immunol. 2001;108:250–7. [DOI] [PubMed] [Google Scholar]
- 29.Streblow DN, Hirsch AJ, Stanton JJ, Lewis AD, Colgin L, Hessell AJ, Kreklywich CN, Smith JL, Sutton WF, Chauvin D. Aerosol delivery of SARS-CoV-2 human monoclonal antibodies in macaques limits viral replication and lung pathology. Nat Commun. 2023;14:7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cagno V, Andreozzi P, Dalicarnasso M, Jacob Silva P, Mueller M, Galloux M, Le Goffic R, Jones ST, Vallino M, Hodek J, Weber J, Sen S, Janecek ER, Bekdemir A, Sanavio B, Martinelli C, Donalisio M, Rameix Welti MA, Eleouet JF, Han Y, Kaiser L, Vukovic L, Tapparel C, Kral P, Krol S, Lembo D, Stellacci F. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat Mater. 2018;17:195–203. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Xu Y, Huang Y, Wang H, Gong X, Wei K. Spatially patterned neutralizing icosahedral DNA nanocage for efficient SARS-CoV-2 blocking. J Am Chem Soc. 2022;144:13146–53. [DOI] [PubMed] [Google Scholar]
- 32.Gao R, Xu X, Kumar P, Liu Y, Zhang H, Guo X, Sun M, Colombari FM, de Moura AF, Hao C. Tapered chiral nanoparticles as broad-spectrum thermally stable antivirals for SARS-CoV-2 variants. Proc Natl Acad Sci USA. 2024;121: e2310469121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong B, Moon S, Kim Y, Heo P, Jung Y, Yu S-H, Chung J, Ban C, Kim YH, Kim P. Virucidal nano-perforator of viral membrane trapping viral RNAs in the endosome. Nat Commun. 2019;10:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh H, Jung Y, Moon S, Hwang J, Ban C, Chung J, Chung W-J, Kweon D-H. Development of end-spliced dimeric nanodiscs for the improved virucidal activity of a nanoperforator. ACS Appl Mater Interfaces. 2021;13:36757–68. [DOI] [PubMed] [Google Scholar]
- 35.Moon S, Chung J, Kim Y, Hong C, Kim S, Hwang J, Jung Y, Chung W-J, Kweon D-H. Bifunctional hetero di-disc for broad-spectrum influenza neutralization. NBM. 2022;44: 102587. [DOI] [PubMed] [Google Scholar]
- 36.Hwang J, Kim BK, Moon S, Park W, Kim KW, Yoon JH, Oh H, Jung S, Park Y, Kim S. Conversion of host cell receptor into virus destructor by immunodisc to neutralize diverse SARS-CoV-2 variants. Adv Healthc Mater. 2024;13:2470091. [DOI] [PubMed] [Google Scholar]
- 37.Hwang J, Jung Y, Moon S, Yu S, Oh H, Kim S, Kim KW, Yoon JH, Chun J, Kim SJ. Nanodisc-mediated conversion of virustatic antiviral antibody to disrupt virus envelope in infected cells. Small Methods. 2022;6:2101516. [DOI] [PubMed] [Google Scholar]
- 38.Denisov IG, Sligar SG. Nanodiscs in membrane biochemistry and biophysics. Chem Rev. 2017;117:4669–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Son S, Ahn SB, Kim G, Jang Y, Ko C, Kim M, Kim SJ. Identification of broad-spectrum neutralizing antibodies against influenza A virus and evaluation of their prophylactic efficacy in mice. Antiviral Res. 2023;213: 105591. [DOI] [PubMed] [Google Scholar]
- 40.Yoon H, Song JM, Ryu CJ, Kim Y-G, Lee EK, Kang S, Kim SJ. An efficient strategy for cell-based antibody library selection using an integrated vector system. BMC Biotechnol. 2012;12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barinka C, Ptacek J, Richter A, Novakova Z, Morath V, Skerra A. Selection and characterization of Anticalins targeting human prostate-specific membrane antigen (PSMA). Protein Eng Des Sel. 2016;29:105–15. [DOI] [PubMed] [Google Scholar]
- 42.DeLano WL, Ultsch MH, de Vos AM, Wells JA. Convergent solutions to binding at a protein-protein interface. Science. 2000;287:1279–83. [DOI] [PubMed] [Google Scholar]
- 43.Ju B, Zhang Q, Ge J, Wang R, Sun J, Ge X, Yu J, Shan S, Zhou B, Song S. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–9. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen L, McCord KA, Bui DT, Bouwman KM, Kitova EN, Elaish M, Kumawat D, Daskhan GC, Tomris I, Han L. Sialic acid-containing glycolipids mediate binding and viral entry of SARS-CoV-2. Nat Chem Biol. 2022;18:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Negi G, Sharma A, Chaudhary M, Gupta D, Harshan KH, Parveen N. SARS-CoV-2 binding to terminal sialic acid of gangliosides embedded in lipid membranes. ACS Infect Dis. 2023;9:1346–61. [DOI] [PubMed] [Google Scholar]
- 46.Dey M, Sharma A, Dhanawat G, Gupta D, Harshan KH, Parveen N. Synergistic binding of SARS-CoV-2 to ACE2 and gangliosides in native lipid membranes. ACS Infect Dis. 2024;10:907–16. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y, Yang C, Xu X-F, Xu W, Liu S-W. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ter Meulen J, van den Brink EN, Poon LL, Marissen WE, Leung CS, Cox F, Cheung CY, Bakker AQ, Bogaards JA, van Deventer E, Preiser W, Doerr HW, Chow VT, de Kruif J, Peiris JS, Goudsmit J. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3: e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan M, Wu NC, Zhu X, Lee C-CD, So RT, Lv H, Mok CK, Wilson IA. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368:630–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baum A, Fulton BO, Wloga E, Copin R, Pascal KE, Russo V, Giordano S, Lanza K, Negron N, Ni M, Wei Y, Atwal GS, Murphy AJ, Stahl N, Yancopoulos GD, Kyratsous CA. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369:1014–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinto D, Park YJ, Beltramello M, Walls AC, Tortorici MA, Bianchi S, Jaconi S, Culap K, Zatta F, De Marco A, Peter A, Guarino B, Spreafico R, Cameroni E, Case JB, Chen RE, Havenar-Daughton C, Snell G, Telenti A, Virgin HW, Lanzavecchia A, Diamond MS, Fink K, Veesler D, Corti D. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–5. [DOI] [PubMed] [Google Scholar]
- 52.Addetia A, Piccoli L, Case JB, Park YJ, Beltramello M, Guarino B, Dang H, de Melo GD, Pinto D, Sprouse K, Scheaffer SM, Bassi J, Silacci-Fregni C, Muoio F, Dini M, Vincenzetti L, Acosta R, Johnson D, Subramanian S, Saliba C, Giurdanella M, Lombardo G, Leoni G, Culap K, McAlister C, Rajesh A, Dellota E Jr, Zhou J, Farhat N, Bohan D, Noack J, Chen A, Lempp FA, Quispe J, Kergoat L, Larrous F, Cameroni E, Whitener B, Giannini O, Cippa P, Ceschi A, Ferrari P, Franzetti-Pellanda A, Biggiogero M, Garzoni C, Zappi S, Bernasconi L, Kim MJ, Rosen LE, Schnell G, Czudnochowski N, Benigni F, Franko N, Logue JK, Yoshiyama C, Stewart C, Chu H, Bourhy H, Schmid MA, Purcell LA, Snell G, Lanzavecchia A, Diamond MS, Corti D, Veesler D. Neutralization, effector function and immune imprinting of Omicron variants. Nature. 2023;621:592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Planas D, Staropoli I, Michel V, Lemoine F, Donati F, Prot M, Porrot F, Guivel-Benhassine F, Jeyarajah B, Brisebarre A. Distinct evolution of SARS-CoV-2 Omicron XBB and BA 2.86/JN 1 lineages combining increased fitness and antibody evasion. Nat Commun. 2024;15:2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arora P, Kempf A, Nehlmeier I, Schulz SR, Jäck H-M, Pöhlmann S, Hoffmann M. Omicron sublineage BQ 11 resistance to monoclonal antibodies. Lancet Infect Dis. 2023;23:22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Hao A, Ji P, Ma Y, Zhang Z, Chen J, Mao Q, Xiong X, Rehati P, Wang Y. A bispecific antibody exhibits broad neutralization against SARS-CoV-2 Omicron variants XBB. 1.16, BQ. 1.1 and sarbecoviruses. Nat Commun. 2024;15:5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duan Y, Gong K, Xu S, Wang X, Zhang L, Li J, Huang F, Zhao Y, Zhu L, Li W, Huang Y. Regulation of cholesterol homeostasis in health and diseases: from mechanisms to targeted therapeutics. Signal Transduct Target Ther. 2022;7:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuai R, Li D, Chen YE, Moon L, Schwendeman T, Parhami JJ, Knudsen KL, Helmus B, Schnitzer TW. High-density lipoproteins: nature’s multifunctional nanoparticles. ACS Nano. 2016;10:3015–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krause BR, Remaley AT. Reconstituted HDL for the acute treatment of acute coronary syndrome. Curr Opin Lipidol. 2013;24:480–6. [DOI] [PubMed] [Google Scholar]
- 59.MichaelGibson C, Korjian S, Tricoci P, Brown EA, Alexander M, Hernandez AM, Abraham MT, Lytle TJ, Veledar DP, Bhatt DL. Safety and tolerability of CSL112, a reconstituted, infusible, plasma-derived apolipoprotein A-I, after acute myocardial infarction: the AEGIS-I trial (ApoA-I Event Reducing in Ischemic Syndromes I). Circulation. 2016;134:1918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kallend DG, Reijers JAA, Bellibas SE, Bobillier A, Kempen H, Burggraaf J, Moerland M, Wijngaard PLJ. A single infusion of MDCO-216 (ApoA-1 Milano/POPC) increases ABCA1-mediated cholesterol efflux and pre-beta 1 HDL in healthy volunteers and patients with stable coronary artery disease. Eur Heart J Cardiovasc Pharmacother. 2016;2:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.