Abstract
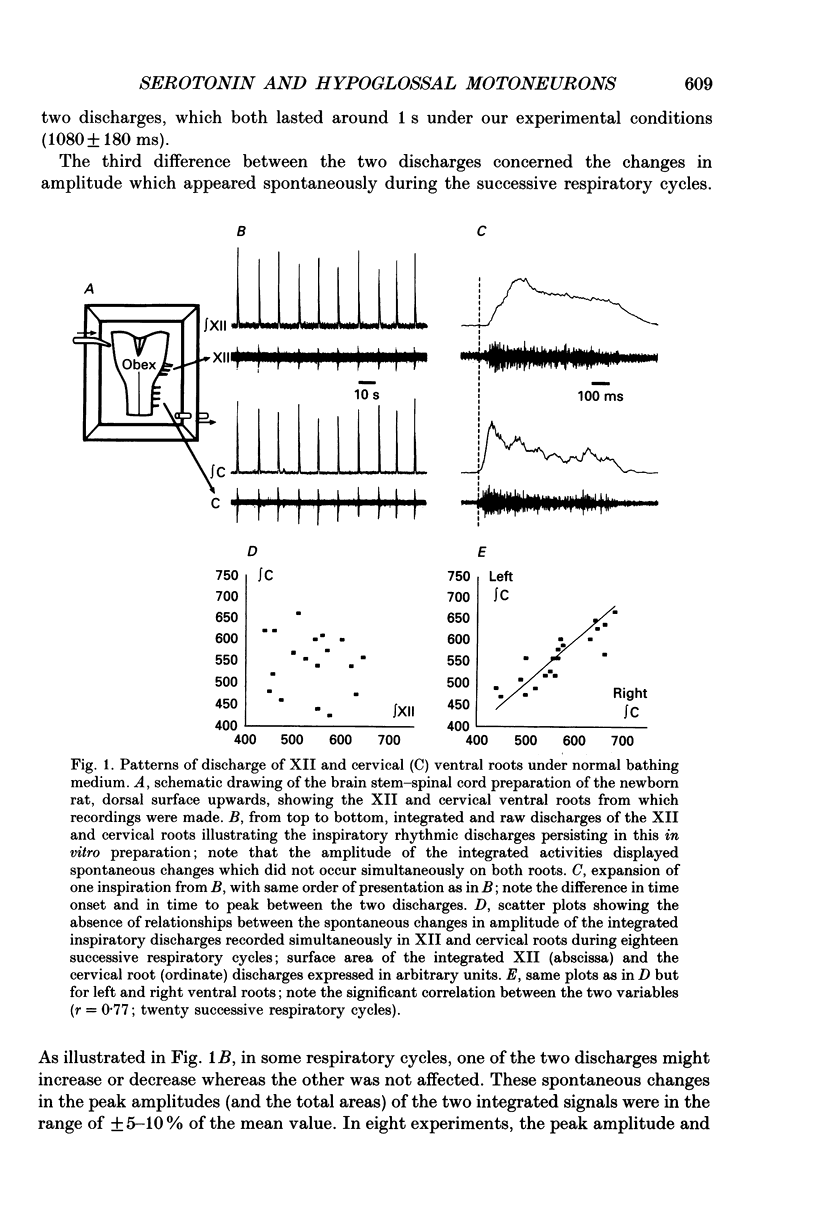
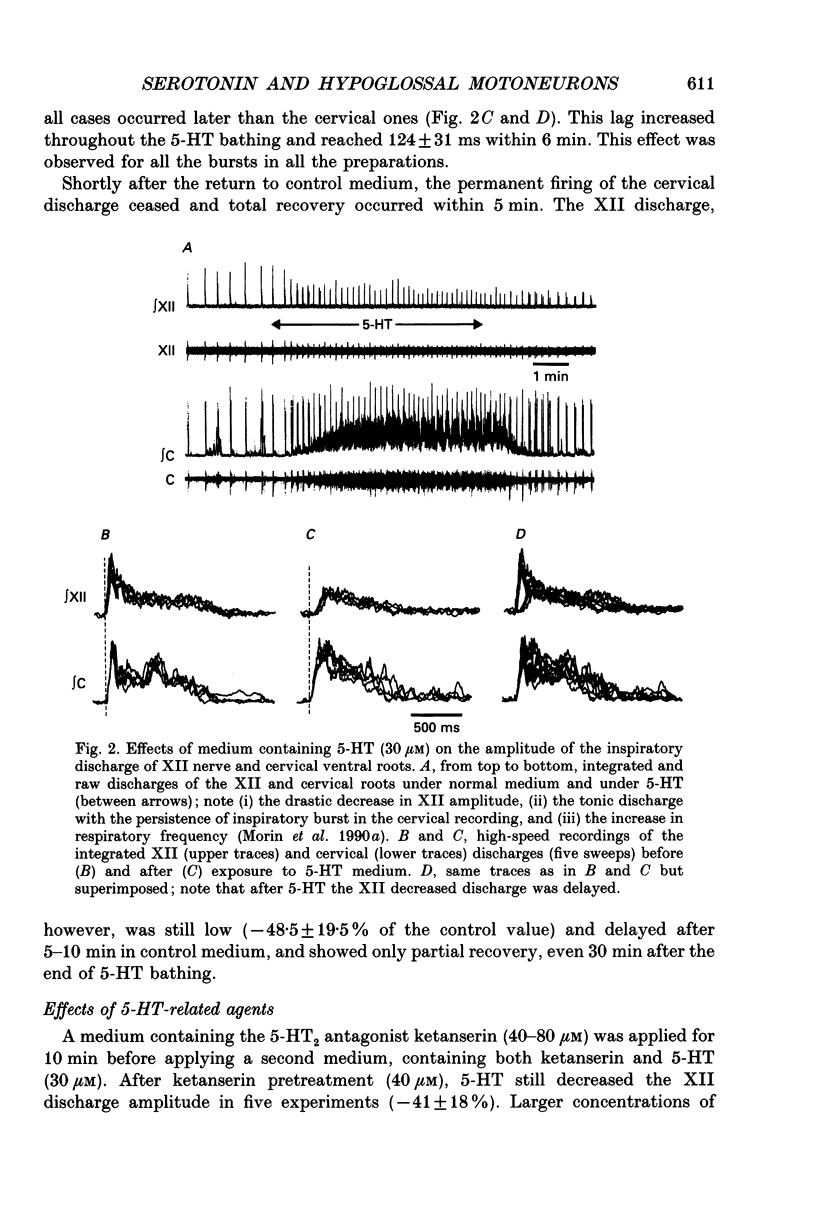
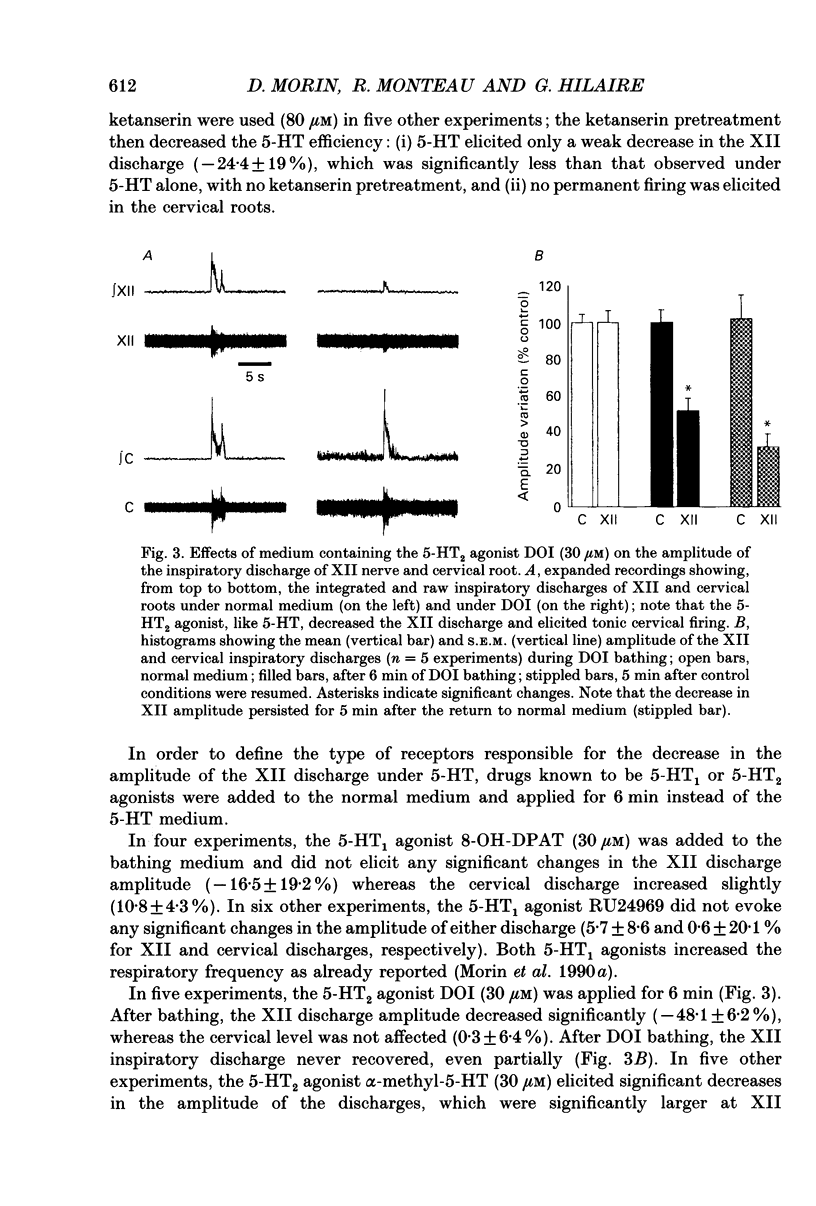
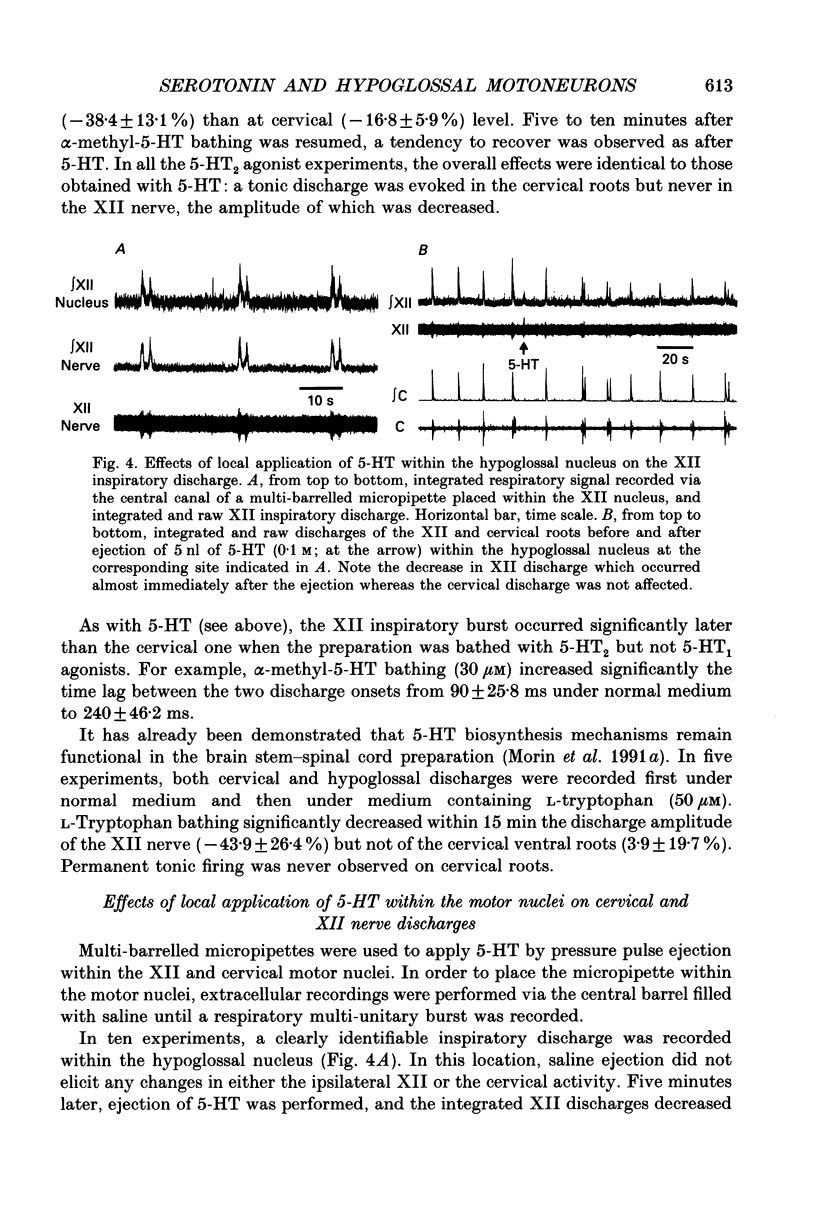
1. Experiments were performed on the brain stem-spinal cord preparation of newborn rats, in which the phrenic and hypoglossal nerves continue to show rhythmic respiratory activity in vitro, in order to compare the effects of serotonin (5-HT) on both activities and to analyse the mechanisms responsible for the depression by 5-HT of the hypoglossal activity. 2. Under control conditions, simultaneous recordings of the inspiratory discharges of hypoglossal and cervical roots showed that the two bursts did not start simultaneously and had different patterns (time-to-peak and peak values); this suggests that both pools of motoneurons did not share the same central drive(s). 3. Adding 5-HT and related agents to the bathing medium delayed and depressed the hypoglossal inspiratory discharge via activation of 5-HT2 receptors since these effects were elicited by 5-HT2 agonists (alpha-methyl-5-HT and 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane-HCl (DOI)) but not by 5-HT1 agonists (RU 24969 and (+/-)-8-hydroxy-2-(di-N-propylamino)tetralin hydrobromide (8-OH-DPAT)). The 5-HT depression of the hypoglossal discharge was prevented by applying a pretreatment with a specific 5-HT2 antagonist (ketanserin). Parallel to the hypoglossal discharge decrease, 5-HT elicited a permanent cervical root discharge along with a persistent inspiratory bursting. Adding the 5-HT precursor L-tryptophan to the bathing medium depressed the hypoglossal (XII) discharge without affecting the cervical one. 4. Local application of 5-HT within the hypoglossal motor nucleus decreased the hypoglossal output, revealing that the 5-HT depression of the hypoglossal discharge was at least partly mediated by the 5-HT effects at the level of the motoneurons. Local application of 5-HT within the cervical motor nucleus elicited a permanent firing in the cervical root with a persistent inspiratory bursting. 5. Intracellular analysis confirmed the existence of differences in central respiratory drive between cervical and hypoglossal motoneurons under control conditions, as well as differences in response to 5-HT. All the hypoglossal motoneurons became silent under 5-HT bathing, and showed no change in the input membrane resistance, a moderate depolarization, and a delayed central respiratory drive with a decreased amplitude. The cervical motoneurons became more active during inspiration, despite a decrease in the amplitude of the central respiratory drive, which was compensated for by a large depolarization and an increased input membrane resistance. Some cervical motoneurons even fired at a low rate during expiration.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




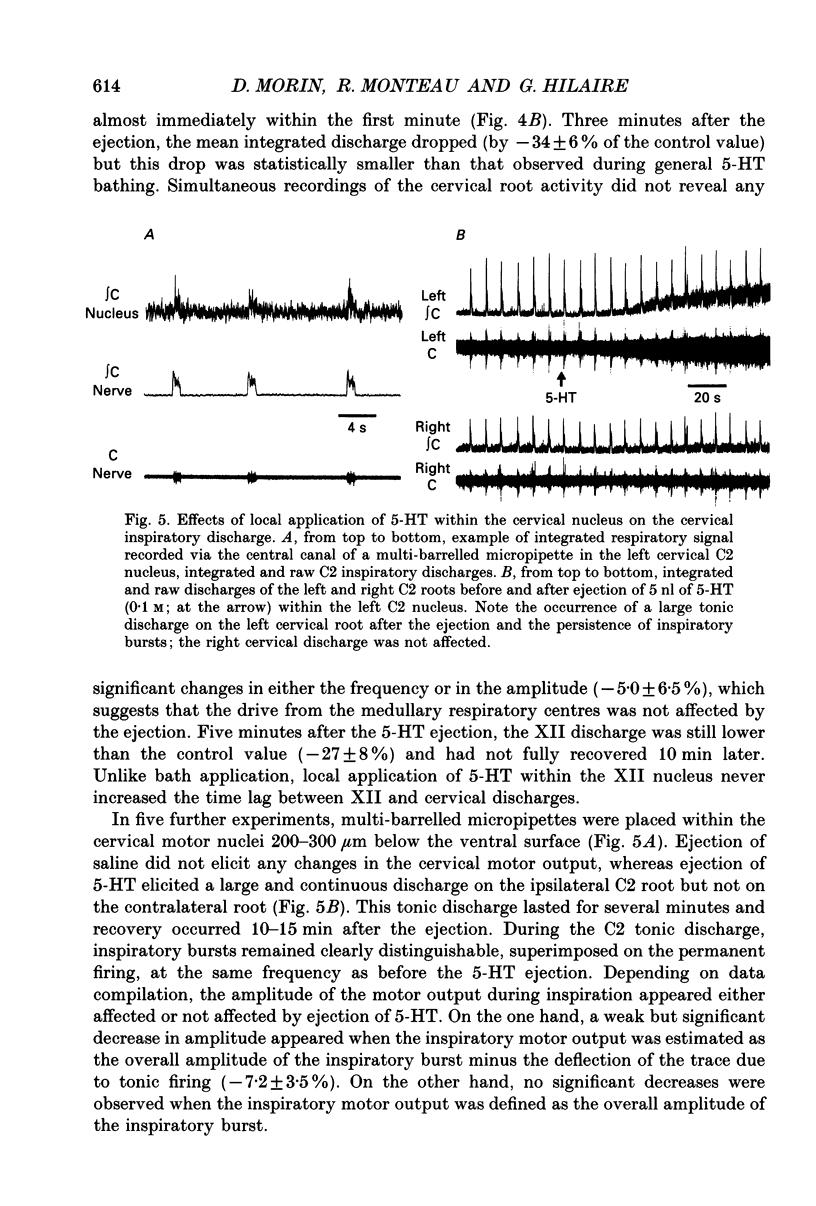
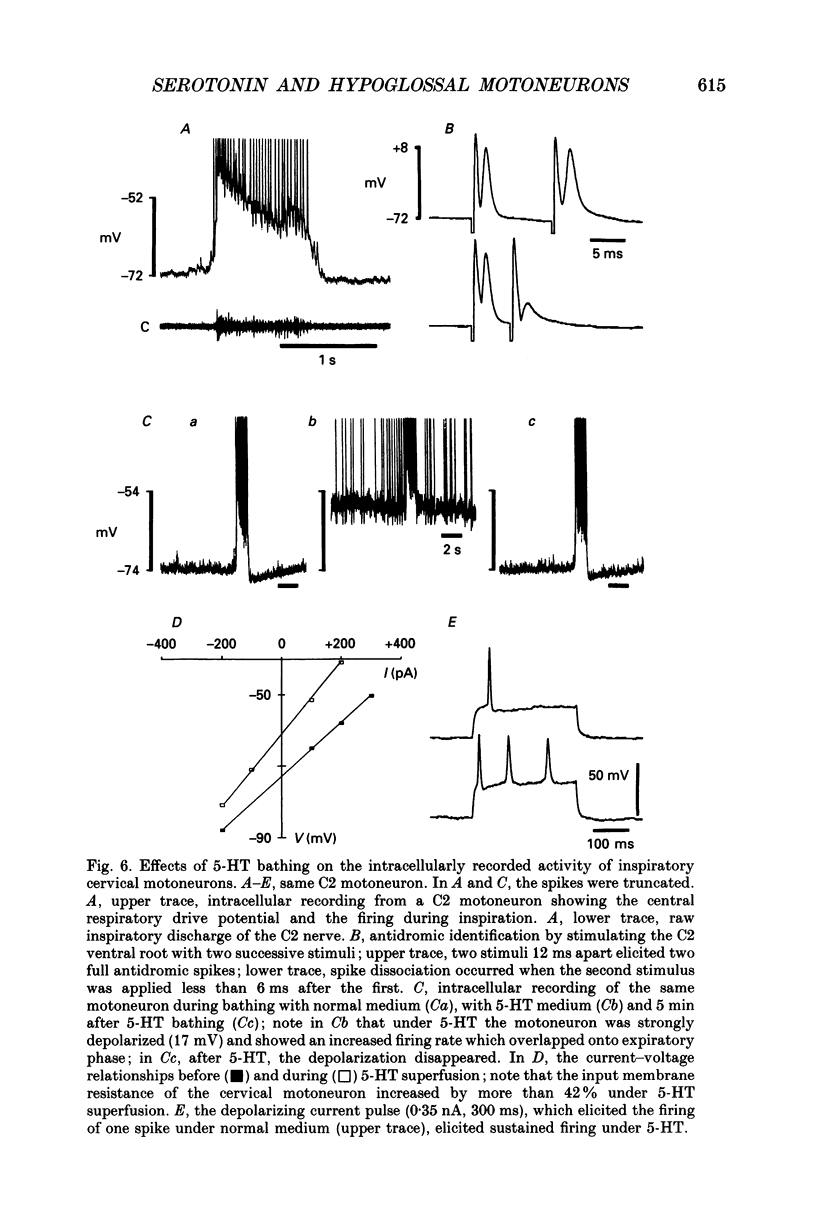
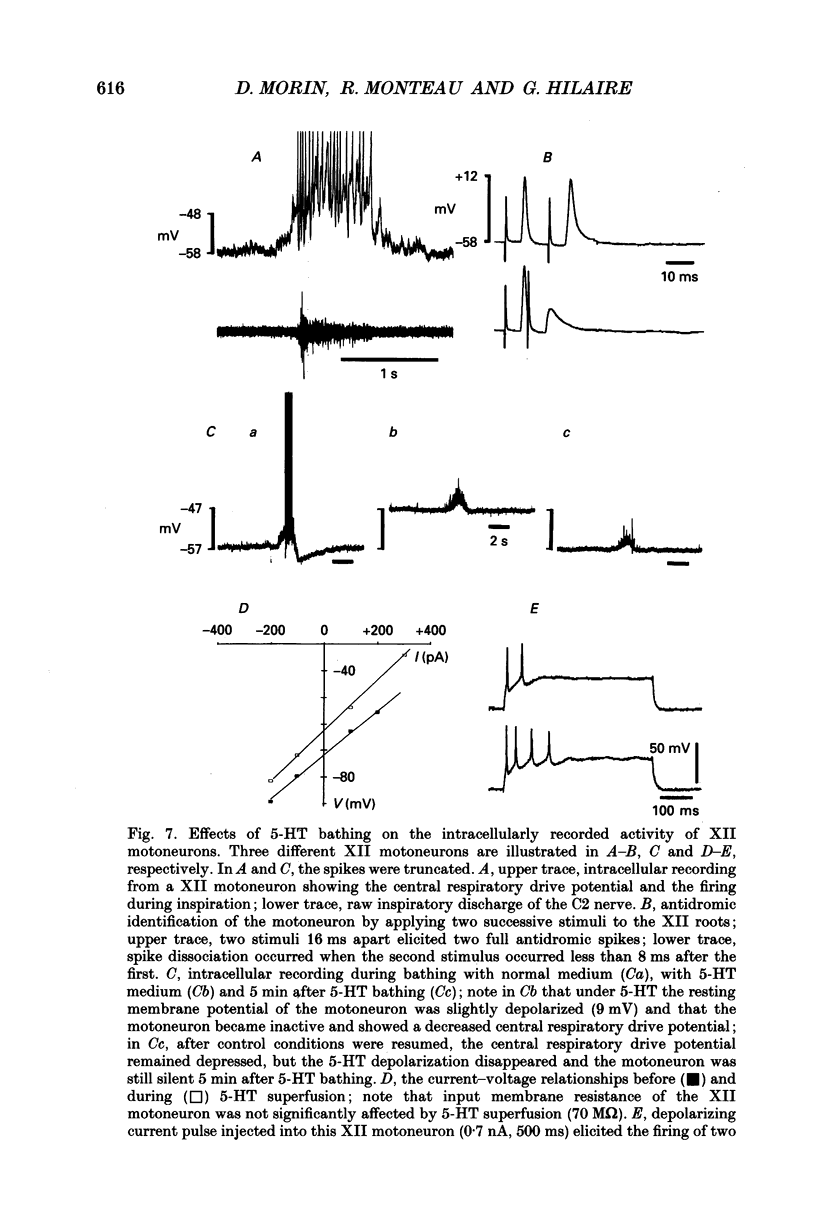

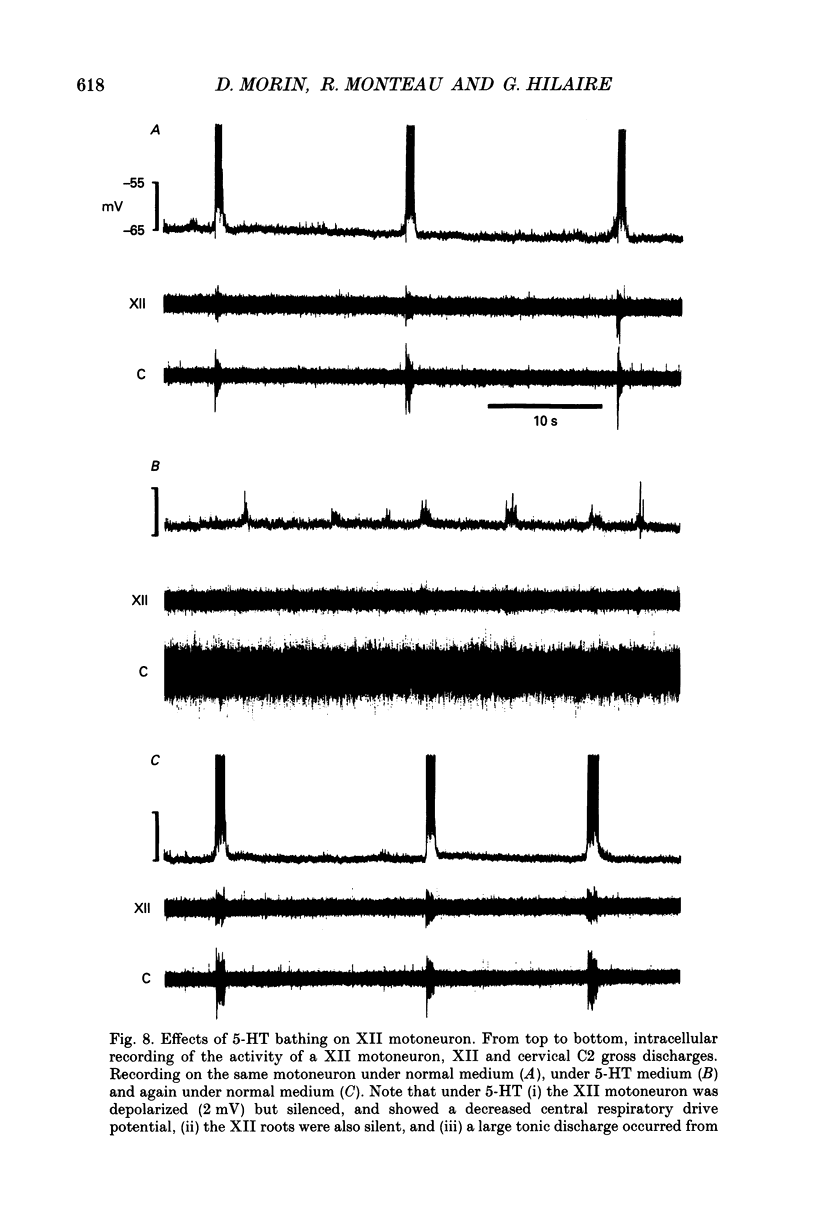











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldes L. D., Chronister R. C., Marco L. A., Haycock J. W., Thibault J. Differential distribution of biogenic amines in the hypoglossal nucleus of the rat. Exp Brain Res. 1988;73(2):305–314. doi: 10.1007/BF00248222. [DOI] [PubMed] [Google Scholar]
- Aldes L. D., Marco L. A., Chronister R. B. Serotonin-containing axon terminals in the hypoglossal nucleus of the rat. An immuno-electronmicroscopic study. Brain Res Bull. 1989 Sep;23(3):249–256. doi: 10.1016/0361-9230(89)90154-8. [DOI] [PubMed] [Google Scholar]
- Amri M., Car A. Projections from the medullary swallowing center to the hypoglossal motor nucleus: a neuroanatomical and electrophysiological study in sheep. Brain Res. 1988 Feb 16;441(1-2):119–126. doi: 10.1016/0006-8993(88)91389-3. [DOI] [PubMed] [Google Scholar]
- Amri M., Car A., Roman C. Axonal branching of medullary swallowing neurons projecting on the trigeminal and hypoglossal motor nuclei: demonstration by electrophysiological and fluorescent double labeling techniques. Exp Brain Res. 1990;81(2):384–390. doi: 10.1007/BF00228130. [DOI] [PubMed] [Google Scholar]
- Borke R. C., Nau M. E., Ringler R. L., Jr Brain stem afferents of hypoglossal neurons in the rat. Brain Res. 1983 Jun 13;269(1):47–55. doi: 10.1016/0006-8993(83)90961-7. [DOI] [PubMed] [Google Scholar]
- Bradley P. B., Engel G., Feniuk W., Fozard J. R., Humphrey P. P., Middlemiss D. N., Mylecharane E. J., Richardson B. P., Saxena P. R. Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology. 1986 Jun;25(6):563–576. doi: 10.1016/0028-3908(86)90207-8. [DOI] [PubMed] [Google Scholar]
- Cameron W. E., Fang H., Brozanski B. S., Guthrie R. D. The postnatal growth of motoneurons at three levels of the cat neuraxis. Neurosci Lett. 1989 Oct 9;104(3):274–280. doi: 10.1016/0304-3940(89)90588-0. [DOI] [PubMed] [Google Scholar]
- Card J. P., Rinaman L., Schwaber J. S., Miselis R. R., Whealy M. E., Robbins A. K., Enquist L. W. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990 Jun;10(6):1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer H., Warter J. M., Renaud B., Krieger J., Marescaux C. H., Hammers R. Cerebrospinal fluid adenosine 3',5'-monophosphate, 5-hydroxyindoleacetic acid and homovanillic acid in patients with sleep apnoea syndrome. J Neurol Neurosurg Psychiatry. 1981 Dec;44(12):1165–1167. doi: 10.1136/jnnp.44.12.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie M., Wilkinson L. S., Roberts M. H. Evidence for excitatory 5-HT2-receptors on rat brainstem neurones. Br J Pharmacol. 1988 Jun;94(2):483–491. doi: 10.1111/j.1476-5381.1988.tb11551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L., Millhorn D. E. Central regulation of respiration by endogenous neurotransmitters and neuromodulators. Annu Rev Physiol. 1981;43:121–135. doi: 10.1146/annurev.ph.43.030181.001005. [DOI] [PubMed] [Google Scholar]
- Errchidi S., Hilaire G., Monteau R. Permanent release of noradrenaline modulates respiratory frequency in the newborn rat: an in vitro study. J Physiol. 1990 Oct;429:497–510. doi: 10.1113/jphysiol.1990.sp018269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A., Maayani S., Wolfe B. B. Subtypes of receptors for serotonin. Annu Rev Pharmacol Toxicol. 1990;30:307–348. doi: 10.1146/annurev.pa.30.040190.001515. [DOI] [PubMed] [Google Scholar]
- Haddad G. G., Donnelly D. F., Getting P. A. Biophysical properties of hypoglossal neurons in vitro: intracellular studies in adult and neonatal rats. J Appl Physiol (1985) 1990 Oct;69(4):1509–1517. doi: 10.1152/jappl.1990.69.4.1509. [DOI] [PubMed] [Google Scholar]
- Harada Y., Kuno M., Wang Y. Z. Differential effects of carbon dioxide and pH on central chemoreceptors in the rat in vitro. J Physiol. 1985 Nov;368:679–693. doi: 10.1113/jphysiol.1985.sp015883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilaire G., Monteau R., Errchidi S. Possible modulation of the medullary respiratory rhythm generator by the noradrenergic A5 area: an in vitro study in the newborn rat. Brain Res. 1989 Apr 24;485(2):325–332. doi: 10.1016/0006-8993(89)90577-5. [DOI] [PubMed] [Google Scholar]
- Holtman J. R., Jr, Dick T. E., Berger A. J. Involvement of serotonin in the excitation of phrenic motoneurons evoked by stimulation of the raphe obscurus. J Neurosci. 1986 Apr;6(4):1185–1193. doi: 10.1523/JNEUROSCI.06-04-01185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman J. R., Jr, Vascik D. S., Maley B. E. Ultrastructural evidence for serotonin-immunoreactive terminals contacting phrenic motoneurons in the cat. Exp Neurol. 1990 Sep;109(3):269–272. doi: 10.1016/s0014-4886(05)80016-0. [DOI] [PubMed] [Google Scholar]
- Hwang J. C., St John W. M., Bartlett D., Jr Respiratory-related hypoglossal nerve activity: influence of anesthetics. J Appl Physiol Respir Environ Exerc Physiol. 1983 Sep;55(3):785–792. doi: 10.1152/jappl.1983.55.3.785. [DOI] [PubMed] [Google Scholar]
- Idzikowski C., Mills F. J., Glennard R. 5-Hydroxytryptamine-2 antagonist increases human slow wave sleep. Brain Res. 1986 Jul 16;378(1):164–168. doi: 10.1016/0006-8993(86)90299-4. [DOI] [PubMed] [Google Scholar]
- Jansen A. H., Chernick V. Development of respiratory control. Physiol Rev. 1983 Apr;63(2):437–483. doi: 10.1152/physrev.1983.63.2.437. [DOI] [PubMed] [Google Scholar]
- Kessler J. P., Jean A. Effect of catecholamines on the swallowing reflex after pressure microinjections into the lateral solitary complex of the medulla oblongata. Brain Res. 1986 Oct 29;386(1-2):69–77. doi: 10.1016/0006-8993(86)90142-3. [DOI] [PubMed] [Google Scholar]
- Klein M., Camardo J., Kandel E. R. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz D., Krieger J., Stierle J. C. EMG activity of cricothyroid and chin muscles during wakefulness and sleeping in the sleep apnea syndrome. Electroencephalogr Clin Neurophysiol. 1978 Dec;45(6):777–784. doi: 10.1016/0013-4694(78)90145-1. [DOI] [PubMed] [Google Scholar]
- Leysen J. E., Niemegeers C. J., Van Nueten J. M., Laduron P. M. [3H]Ketanserin (R 41 468), a selective 3H-ligand for serotonin2 receptor binding sites. Binding properties, brain distribution, and functional role. Mol Pharmacol. 1982 Mar;21(2):301–314. [PubMed] [Google Scholar]
- Lowe A. A. The tongue and airway. Otolaryngol Clin North Am. 1990 Aug;23(4):677–698. [PubMed] [Google Scholar]
- Maura G., Fedele E., Raiteri M. Acetylcholine release from rat hippocampal slices is modulated by 5-hydroxytryptamine. Eur J Pharmacol. 1989 Jun 20;165(2-3):173–179. doi: 10.1016/0014-2999(89)90710-3. [DOI] [PubMed] [Google Scholar]
- McCall R. B., Aghajanian G. K. Serotonergic facilitation of facial motoneuron excitation. Brain Res. 1979 Jun 15;169(1):11–27. doi: 10.1016/0006-8993(79)90370-6. [DOI] [PubMed] [Google Scholar]
- Mengod G., Pompeiano M., Martínez-Mir M. I., Palacios J. M. Localization of the mRNA for the 5-HT2 receptor by in situ hybridization histochemistry. Correlation with the distribution of receptor sites. Brain Res. 1990 Jul 30;524(1):139–143. doi: 10.1016/0006-8993(90)90502-3. [DOI] [PubMed] [Google Scholar]
- Monteau R., Hilaire G. Spinal respiratory motoneurons. Prog Neurobiol. 1991;37(2):83–144. doi: 10.1016/0301-0082(91)90024-u. [DOI] [PubMed] [Google Scholar]
- Monteau R., Morin D., Hennequin S., Hilaire G. Differential effects of serotonin on respiratory activity of hypoglossal and cervical motoneurons: an in vitro study on the newborn rat. Neurosci Lett. 1990 Mar 26;111(1-2):127–132. doi: 10.1016/0304-3940(90)90356-e. [DOI] [PubMed] [Google Scholar]
- Monteau R., Morin D., Hennequin S., Hilaire G. Differential effects of serotonin on respiratory activity of hypoglossal and cervical motoneurons: an in vitro study on the newborn rat. Neurosci Lett. 1990 Mar 26;111(1-2):127–132. doi: 10.1016/0304-3940(90)90356-e. [DOI] [PubMed] [Google Scholar]
- Monteau R., Morin D., Hilaire G. Acetylcholine and central chemosensitivity: in vitro study in the newborn rat. Respir Physiol. 1990 Aug;81(2):241–253. doi: 10.1016/0034-5687(90)90049-5. [DOI] [PubMed] [Google Scholar]
- Morin D., Hennequin S., Monteau R., Hilaire G. Serotonergic influences on central respiratory activity: an in vitro study in the newborn rat. Brain Res. 1990 Dec 10;535(2):281–287. doi: 10.1016/0006-8993(90)91611-j. [DOI] [PubMed] [Google Scholar]
- Morin D., Monteau R., Hilaire G. 5-Hydroxytryptamine modulates central respiratory activity in the newborn rat: an in vitro study. Eur J Pharmacol. 1991 Jan 3;192(1):89–95. doi: 10.1016/0014-2999(91)90073-y. [DOI] [PubMed] [Google Scholar]
- Morin D., Monteau R., Hilaire G. Serotonin and cervical respiratory motoneurones: intracellular study in the newborn rat brainstem-spinal cord preparation. Exp Brain Res. 1991;84(1):229–232. doi: 10.1007/BF00231779. [DOI] [PubMed] [Google Scholar]
- Moss I. R., Denavit-Saubié M., Eldridge F. L., Gillis R. A., Herkenham M., Lahiri S. Neuromodulators and transmitters in respiratory control. Fed Proc. 1986 Jun;45(7):2133–2147. [PubMed] [Google Scholar]
- Murakoshi T., Otsuka M. Respiratory reflexes in an isolated brainstem-lung preparation of the newborn rat: possible involvement of gamma-aminobutyric acid and glycine. Neurosci Lett. 1985 Nov 20;62(1):63–68. doi: 10.1016/0304-3940(85)90285-x. [DOI] [PubMed] [Google Scholar]
- Murakoshi T., Suzue T., Tamai S. A pharmacological study on respiratory rhythm in the isolated brainstem-spinal cord preparation of the newborn rat. Br J Pharmacol. 1985 Sep;86(1):95–104. doi: 10.1111/j.1476-5381.1985.tb09439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myslinski N. R., Anderson E. G. The effect of serotonin precursors on alpha- and gamma-motoneuron activity. J Pharmacol Exp Ther. 1978 Jan;204(1):19–26. [PubMed] [Google Scholar]
- Onimaru H., Arata A., Homma I. Primary respiratory rhythm generator in the medulla of brainstem-spinal cord preparation from newborn rat. Brain Res. 1988 Apr 5;445(2):314–324. doi: 10.1016/0006-8993(88)91194-8. [DOI] [PubMed] [Google Scholar]
- Pazos A., Cortés R., Palacios J. M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res. 1985 Nov 4;346(2):231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- Remmers J. E. Obstructive sleep apnea. A common disorder exacerbated by alcohol. Am Rev Respir Dis. 1984 Aug;130(2):153–155. doi: 10.1164/arrd.1984.130.2.153. [DOI] [PubMed] [Google Scholar]
- Remmers J. E., deGroot W. J., Sauerland E. K., Anch A. M. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jun;44(6):931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. THE SLOW POTENTIALS OF THORACIC RESPIRATORY MOTONEURONES AND THEIR RELATION TO BREATHING. J Physiol. 1964 Dec;175:404–424. doi: 10.1113/jphysiol.1964.sp007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerland E. K., Harper R. M. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976 Apr;51(1):160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- Sica A. L., Steele A. M., Gandhi M. R., Prasad N. Factors affecting central inspiratory modulation of hypoglossal motoneuron activity in newborn pigs. J Dev Physiol. 1988 Aug;10(4):285–295. [PubMed] [Google Scholar]
- Smith J. C., Greer J. J., Liu G. S., Feldman J. L. Neural mechanisms generating respiratory pattern in mammalian brain stem-spinal cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. J Neurophysiol. 1990 Oct;64(4):1149–1169. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984 Sep;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Berger A. J. Direct excitation of rat spinal motoneurones by serotonin. J Physiol. 1990 Apr;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Kojima M., Matsuura T., Sano Y. Serotonergic innervation on the motoneurons in the mammalian brainstem. Light and electron microscopic immunohistochemistry. Anat Embryol (Berl) 1983;167(3):321–333. doi: 10.1007/BF00315670. [DOI] [PubMed] [Google Scholar]
- Viana F., Gibbs L., Berger A. J. Double- and triple-labeling of functionally characterized central neurons projecting to peripheral targets studied in vitro. Neuroscience. 1990;38(3):829–841. doi: 10.1016/0306-4522(90)90075-f. [DOI] [PubMed] [Google Scholar]
- Wang M. Y., Dun N. J. 5-Hydroxytryptamine responses in neonate rat motoneurones in vitro. J Physiol. 1990 Nov;430:87–103. doi: 10.1113/jphysiol.1990.sp018283. [DOI] [PMC free article] [PubMed] [Google Scholar]


