Abstract
Purpose
Myopic choroidal neovascularization (CNV) is a common reason for visual impairment. This study investigated the clinical effects of repeated intravitreal injections of ranibizumab among patients with CNV secondary to pathologic myopia.
Methods
This study involved a single-center, non-randomized clinical prospective cohort research design including 39 patients with myopic CNV and a control group of 10 patients with cataract. Plasma and aqueous humor samples were analyzed to compare cytokine concentrations between the two groups and assess changes after intravitreal ranibizumab injections. Best-corrected visual acuity (BCVA) and central macular thickness (CMT) were also monitored.
Results
BCVA values and CMT varied significantly after intravitreal ranibizumab injections. The study group had significantly higher plasma concentrations of vascular endothelial growth factor (VEGF)-A and significantly lower epidermal growth factor (EGF) and angiopoietin-2 concentrations than the control group. Likewise, in the aqueous humor, the study group had significantly higher concentrations of fibroblast growth factor and significantly lower concentrations of EGF and VEGF-A than the control group. The average VEGF-A content decreased significantly after 1 and 2 months relative to the baseline. Mean VEGF-D and endoglin contents at two months were significantly reduced compared to the baseline and at 1 month. The average EGF contents were significantly higher at 2 months than the baseline.
Conclusion
Ranibizumab could increase the BCVA and lower the CMT and cytokines involved in angiogenesis. This study contributes to further understanding the pathogenesis of myopic CNV and promoting new drug research and development for patients with this condition.
Keywords: Aqueous humor, Choroidal neovascularization, Cytokine, Myopic, Plasma
Introduction
In many countries, myopic choroidal neovascularization (CNV) secondary to pathologic myopia represents a prevalent source of visual impairment, notably among individuals < 50 years of age [1–3]. Complications associated with pathologic myopia encompass posterior staphyloma, retinal pigment epithelium changes, choroidal atrophy, retinal detachment, and CNV [4]. Of these pathologic myopic complications, myopic CNV is one of the most significant threats to vision [5]. This disease decreases productivity and quality of life. Vascular endothelial growth factor (VEGF) has been proposed as the most significant factor regulating new blood vessel growth and vascular leakage during the development of CNV [6, 7]. Ranibizumab (Lucentis; Novartis Pharma AG, Switzerland) is specifically used to stop the active forms of VEGF-A [8] as a humanized, recombinant, monoclonal antibody fragment. Intravitreal injections of ranibizumab have demonstrated a curative effect on visual acuity among patients with myopic CNV [9, 10]. Further investigation is required to elucidate the clinical effects and mechanism of action of ranibizumab in the treatment of myopic CNV. In addition to VEGF, the potential influence of other cytokines on the pathogenesis of myopic CNV warrants further investigation.
In this study, we assessed the cytokine concentrations in the plasma and aqueous humor of patients with myopic CNV or with cataract before and after consecutive intravitreal injections of ranibizumab or cataract operations, respectively. We then assessed the efficacy of ranibizumab in treating myopic CNV by combining this with monitoring the best-corrected visual acuity (BCVA) and central macular thickness (CMT).
Methods and materials
The Institutional Review Ethics Committee of the Affiliated Suzhou Hospital of Nanjing Medical University approved the protocol, and all patients received a thorough explanation of the study design and aims and confirmed informed consent. The study was conducted in compliance with the tenets of the Declaration of Helsinki.
This study used a single-center, non-randomized clinical prospective cohort research design. In this study, the observation group included 39 patients diagnosed with myopic CNV, and all patients were required to receive 3 consecutive intravitreal injections of ranibizumab. In the control group, we enrolled 10 regular cataract patients with normal axial length who received cataract operations. All participants were collected in the Department of Ophthalmology, the Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital from July 2022 to December 2023. The inclusion criteria were age ≥ 18 years, refractive error ≥ − 6.00 diopters, CNV secondary to pathologic myopia and involving the foveal center, and recent disease progression. The exclusion criteria were CNV secondary to age-associated macular degradation, polypoidal choroidal vasculopathy, inflammatory disease, angioid streaks or trauma, subfoveal fibrosis or atrophy, any prior treatment such as photodynamic therapy or retinal laser photocoagulation for CNV secondary to pathologic myopia, and systemic diseases.
Before the first intravitreal injection, all patients received a systematic ophthalmic examination, including a BCVA assessment, slit-lamp examination, fundus examination, optical coherence tomography (OCT; OCT spectral domain, Humphrey Field Analyzer 3 from Zeiss), fundus fluorescein angiography, and indocyanine green angiography. The logarithm of the minimum angle of resolution (logMAR) was used as the BCVA for statistical analysis. The CMT was assessed by OCT centering on the fovea, and the scan length was 6.0 mm, measuring the neural epithelium thickness. The average central foveal thickness (1 mm in diameter) was adopted for comparison. Each eye with myopic CNV received 3 consecutive monthly intravitreal injections. The BCVA and OCT examinations were repeated monthly after the intravitreal ranibizumab injections. The follow-up visit was 2 month after the first injection.
Blood and aqueous humor samples were gathered from the study group before the intravitreal ranibizumab injections were administered and from the control group immediately before the cataract operation was done. Blood samples (5 mL) were collected from the antecubital vein into routine ethylenediamine tetra-acetic acid blood tubes, centrifuged at 4500 × g for 15 min to isolate the plasma, aliquoted, and kept at −80 °C until use. Undiluted aqueous humor samples (100 µL) were acquired using a standard sterilization procedure. Anterior chamber paracentesis was performed before each intravitreal injection to avoid a spike in intraocular pressure after ranibizumab (0.05 mg/0.05 mL) was injected intravitreally. After disinfecting the periorbital skin and conjunctiva with a povidone-iodine solution, sterile draping of the patient, and insertion of a lid speculum, aqueous humor was aspirated with a 30-gauge needle linked to a tuberculin syringe at the temporal limbus. Intravitreal injections or cataract surgeries were then performed. Aqueous humor samples were collected in sterile tubes and kept at − 80 °C until use.
Samples were measured with the Luminex xMAP suspension array. A Milliplex Kit was used to detect epidermal growth factor (EGF) [11], angiopoietin-2 [12], endoglin [13], fibroblast growth factor (FGF)-1 [14], FGF-2 [15], interleukin-8 (IL-8) [16], hepatocyte growth factor (HGF) [17], heparin-binding EGF (HB-EGF) [18], placental growth factor (PLGF) [19], VEGF-C [20], VEGF-D [21], and VEGF-A [22]. This intensive level of study is necessary, as many previous investigations have demonstrated that these cytokines may play a part in the formation and development of myopic CNV.
Before commencing the assay, all reagents were warmed to 20–25 °C. The first step involved adding 25 μL of assay buffer, 25 μL of appropriate matrix solution, 25 μL of plasma sample (1:3 dilution) or undiluted aqueous humor sample, and 25 μL of beads to each well of a 96-well plate. Wrapped with foil, the plate was incubated on a plate shaker overnight at 4 °C or for 2 h at 20–25 °C. Then, after adding 25 μL of detection antibodies and 25 μL of streptavidin–phycoerythrin to each well, the plate was covered with foil and incubated on a plate shaker for 30 min at 20–25 °C. All incubation procedures were conducted in the dark to prevent light exposure to the beads. Finally, the plate was run on a Luminex 200™ with xPONENT software.
The concentrations of cytokines in the plasma and aqueous humor were the primary outcome measures, and the BCVA and CMT were the secondary outcome measures. SPSS (version 27.0; SPSS Inc., Chicago, IL, USA) was used to analyze the data. An independent sample t-test was adopted to compare the two groups’ differences in plasma and aqueous humor cytokine concentrations. The differences in BCVA values, CMT, and cytokine concentrations of the aqueous humor samples collected at baseline, one month, and two months were compared using a one-way analysis of variance. Statistical significance was set at p < 0.05.
Results
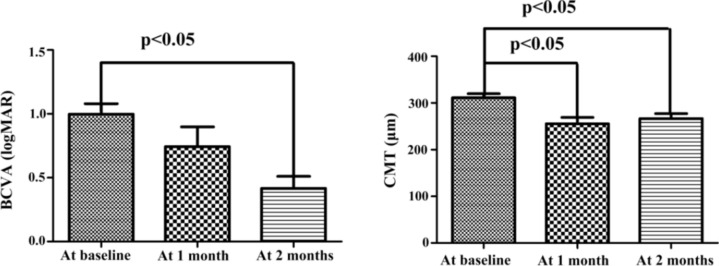
49 plasma samples were collected from 39 patients with myopic CNV and 10 patients with cataract. The average age of the patients with myopic CNV was 54.3 ± 11.7 years (24–75 years), and the average refractive error was − 11.85 ± 3.61 D (− 7 to − 20 D). Every patient in the CNV group received three consecutive intravitreal injections of ranibizumab. 10 patients with age-related cataract (5 males and 5 females) without other ocular or systemic diseases served as the control group. The average patient age in the control group was 70.7 ± 8.0 years (52–80 years). The mean BCVA (logMAR) value and CMT in the CNV group at baseline, at 1 month, and at 2 months are summarized in Table 1. The mean BCVA value at 2 months differed significantly from the baseline (p < 0.05; Fig. 1). The CMT at 1 and 2 months was significantly lower than the baseline (p < 0.05; Fig. 1).
Table 1.
Changes in BCVA and CMT in the myopic choroidal neovascularization (CNV) group after ranibizumab injections
| At baseline | At 1 month | P valuea | At 2 months | P valueb | P valuec | |
|---|---|---|---|---|---|---|
| BCVA (logMAR) | 1.00 ± 0.49 | 0.74 ± 0.58 | 0.411 | 0.42 ± 0.23 | 0.001 | 0.234 |
| CMT (μm) | 311.03 ± 54.32 | 255.57 ± 50.97 | 0.013 | 266.42 ± 37.64 | 0.021 | 0.990 |
BCVA best-corrected visual acuity, logMAR logarithm of the minimum angle of resolution, CMT central macular thickness
aOne-way analysis of variance between baseline and 1 month for BCVA and CMT
bOne-way analysis of variance between baseline and 2 months for BCVA and CMT
cOne-way analysis of variance between 1 and 2 months for BCVA and CMT
Fig. 1.
Changes of BCVA and CMT in the myopic CNV group after ranibizumab injection
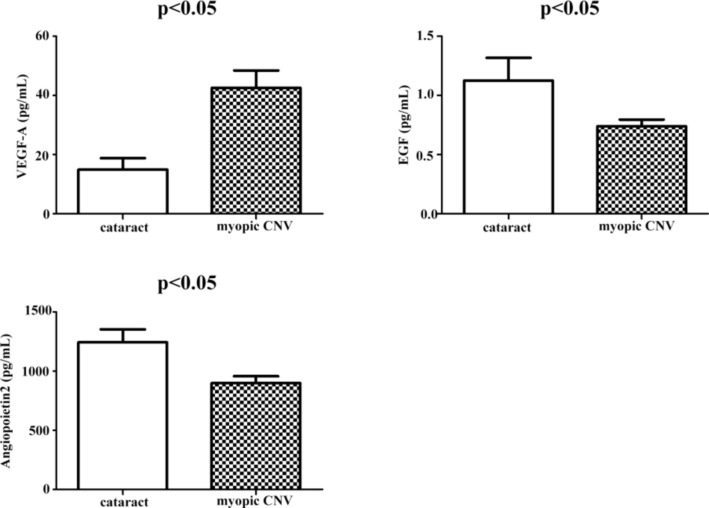
The baseline plasma cytokine concentrations of the CNV and cataract groups are summarized in Table 2. The VEGF-A concentration in the CNV group was significantly higher than in the control group (p < 0.05). In contrast, EGF and angiopoietin-2 concentrations were significantly lower in the CNV than in the control group (p < 0.05; Fig. 2).
Table 2.
Plasma contents of cytokines in myopic CNV and control groups
| Control | CNV | p valued | |
|---|---|---|---|
| VEGF-A (pg/mL) | 14.94 ± 11.57 l | 42.61 ± 34.36 | 0.000 |
| VEGF-C (pg/mL) | 7.50 ± 2.72 | 21.08 ± 79.01 | 0.592 |
| VEGF-D (pg/mL) | 76.15 ± 39.11 | 58.95 ± 46.83 | 0.291 |
| EGF (pg/mL) | 1.13 ± 0.60 | 0.74 ± 0.35 | 0.011 |
| Angiopoietin-2 (pg/mL) | 1242.91 ± 347.91 | 897.71 ± 366.29 | 0.010 |
| Endoglin (pg/mL) | 1222.63 ± 298.57 | 1125.43 ± 497.07 | 0.559 |
| FGF-1 (pg/mL) | 10.42 ± 1.54 | 23.54 ± 70.32 | 0.561 |
| FGF-2 (pg/mL) | 22.84 ± 8.29 | 28.37 ± 20.84 | 0.418 |
| IL-8 (pg/mL) | 3.84 ± 2.87 | 4.60 ± 3.95 | 0.570 |
| HGF (pg/mL) | 200.70 ± 123.95 | 139.34 ± 72.24 | 0.056 |
| HB-EGF (pg/mL) | 9.84 ± 7.18 | 14.10 ± 19.98 | 0.513 |
| PLGF (pg/mL) | 9.44 ± 4.36 | 8.28 ± 6.46 | 0.594 |
VEGF vascular endothelial growth factor, EGF epidermal growth factor, FGF fibroblast growth factor, IL-8 interleukin-8, HGF hepatocyte growth factor, HB-EGF heparin-binding epidermal growth factor, PLGF placental growth factor
dT test between control and myopic CNV groups for plasma contentss of cytokines
Fig. 2.
Plasma cytokine contents in myopic CNV and control groups
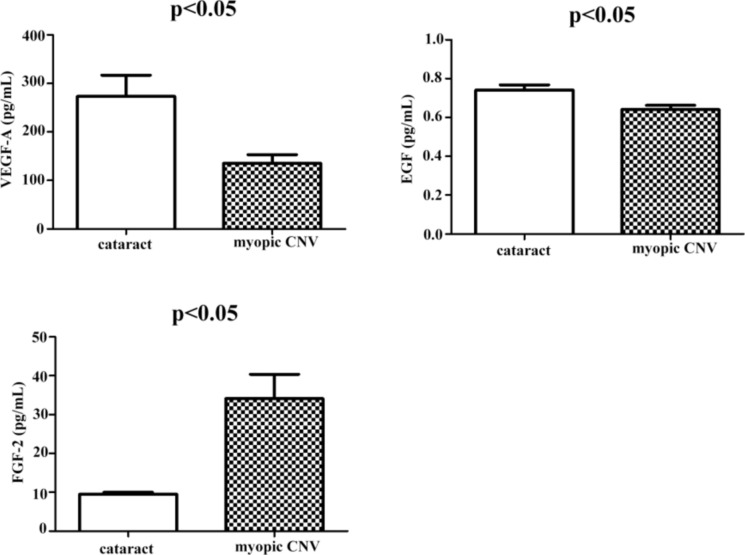
The baseline cytokine concentrations in the aqueous humor of the CNV and control groups are summarized in Table 3. The FGF-2 concentration was significantly higher in the CNV group than in the control group (p < 0.05). In contrast, the EGF and VEGF-A concentrations were significantly lower in the CNV than in the control group (p < 0.05; Fig. 3).
Table 3.
Aqueous humor contents of cytokines in myopic CNV and control groups
| Control | CNV | p valuee | |
|---|---|---|---|
| VEGF-A (pg/mL) | 272.71 ± 138.91 | 135.09 ± 70.29 | 0.003 |
| VEGF-C (pg/mL) | 10.13 ± 5.46 | 9.90 ± 4.33 | 0.906 |
| VEGF-D (pg/mL) | 2.92 ± 0.24 | 2.99 ± 0.37 | 0.584 |
| EGF (pg/mL) | 0.74 ± 0.08 | 0.64 ± 0.08 | 0.035 |
| Angiopoietin-2 (pg/mL) | 44.48 ± 17.49 | 31.31 ± 13.10 | 0.055 |
| Endoglin (pg/mL) | 14.59 ± 1.37 | 15.62 ± 1.46 | 0.083 |
| FGF-1 (pg/mL) | 4.54 ± 0.73 | 4.78 ± 1.04 | 0.542 |
| FGF-2 (pg/mL) | 9.49 ± 1.26 | 34.11 ± 23.30 | 0.002 |
| IL-8 (pg/mL) | 5.85 ± 3.62 | 7.00 ± 3.91 | 0.456 |
| HGF (pg/mL) | 1042.51 ± 408.89 | 1326.48 ± 804.78 | 0.311 |
| HB-EGF (pg/mL) | 1.21 ± 0.38 | 1.29 ± 0.36 | 0.575 |
| PLGF (pg/mL) | 2.55 ± 1.06 | 2.97 ± 1.32 | 0.399 |
VEGF vascular endothelial growth factor, EGF epidermal growth factor, FGF fibroblast growth factor, IL-8 interleukin-8, HGF hepatocyte growth factor, HB-EGF heparin-binding epidermal growth factor, PLGF placental growth factor
eT test between control and myopic CNV groups for aqueous humor contents of cytokines
Fig. 3.
Aqueous humor contents of cytokines in myopic CNV and control groups
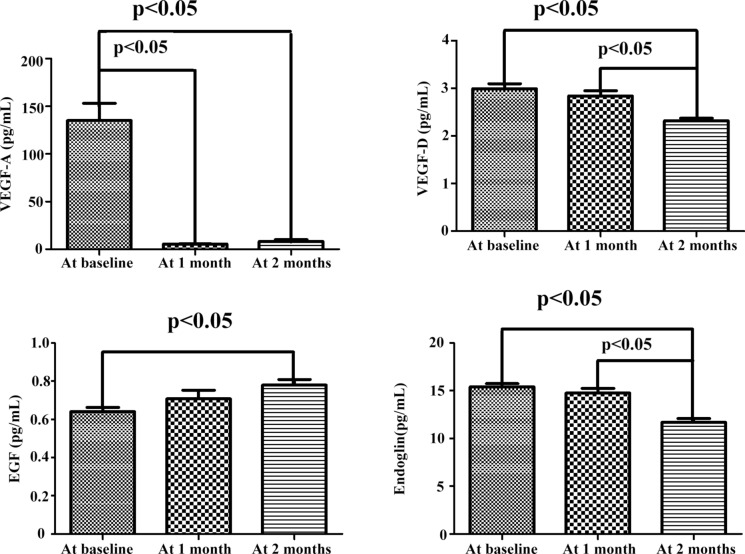
Table 4 summarizes the aqueous humor cytokine concentrations of the CNV group at baseline and 1 and 2 months after intravitreal ranibizumab injections. VEGF-A, VEGF-D, and endoglin concentrations decreased significantly at months 1 and 2 after intravitreal ranibizumab injections. In contrast, the EGF concentration increased significantly at months 1 and 2 after the injections (Fig. 4). The VEGF-A concentration was significantly lower at months one and two than the baseline (p < 0.05). However, the average VEGF-D and endoglin concentrations had significantly decreased from the baseline (and at month 1) by month 2 (p < 0.05). The average EGF contents were significantly higher at 2 months than the baseline only (p < 0.05; Fig. 4). We found no significant differences in the concentrations of the other cytokines after consecutive intravitreal ranibizumab injections.
Table 4.
Changes in aqueous humor contents of cytokines in the myopic CNV group after ranibizumab injections
| At baseline | At 1 month | p valuef | At 2 months | p valueg | p valueh | |
|---|---|---|---|---|---|---|
| VEGF-A (pg/mL) | 135.09 ± 70.29 | 5.41 ± 0.58 | 0.000 | 8.33 ± 4.70 | 0.000 | 0.389 |
| VEGF-C (pg/mL) | 2.99 ± 0.39 | 2.84 ± 0.41 | 0.723 | 2.32 ± 0.16 | 0.739 | 0.525 |
| VEGF-D (pg/mL) | 2.99 ± 0.39 | 2.84 ± 0.41 | 0.264 | 2.32 ± 0.16 | 0.000 | 0.002 |
| EGF (pg/mL) | 0.64 ± 0.08 | 0.71 ± 0.17 | 0.150 | 0.78 ± 0.09 | 0.010 | 0.171 |
| Angiopoietin-2 (pg/mL) | 33.04 ± 12.94 | 31.41 ± 8.75 | 0.689 | 30.76 ± 10.22 | 0.624 | 0.891 |
| Endoglin (pg/mL) | 15.41 ± 1.35 | 14.76 ± 1.83 | 0.257 | 11.69 ± 1.19 | 0.000 | 0.000 |
| FGF-1 (pg/mL) | 4.75 ± 1.10 | 4.83 ± 1.35 | 0.848 | 4.05 ± 0.82 | 0.156 | 0.119 |
| FGF-2 (pg/mL) | 46.34 ± 52.41 | 27.77 ± 18.68 | 0.167 | 17.79 ± 10.08 | 0.064 | 0.513 |
| IL-8 (pg/mL) | 6.47 ± 3.81 | 4.86 ± 1.82 | 0.139 | 5.33 ± 2.20 | 0.351 | 0.702 |
| HGF (pg/mL) | 1406.75 ± 820.44 | 1330.27 ± 83.62 | 0.813 | 1218.26 ± 981.87 | 0.608 | 0.764 |
| HB-EGF (pg/mL) | 1.26 ± 0.33 | 1.27 ± 0.25 | 0.978 | 1.33 ± 0.29 | 0.607 | 0.629 |
| PLGF (pg/mL) | 2.74 ± 1.08 | 1.94 ± 1.07 | 0.546 | 1.76 ± 0.89 | 0.531 | 0.674 |
VEGF vascular endothelial growth factor, EGF epidermal growth factor, FGF fibroblast growth factor, IL-8 interleukin-8, HGF hepatocyte growth factor, HB-EGF heparin-binding epidermal growth factor, PLGF placental growth factor
fOne-way analysis of variance (ANOVA) between baseline and 1 month for changes in aqueous humor contents of cytokines
gANOVA between baseline and 2 months for changes in aqueous humor cytokine contents
hANOVA between 1 and 2 months for changes in aqueous humor contents of cytokines
Fig. 4.
Variations in aqueous humor cytokine contents in the myopic CNV group after ranibizumab injection
Discussion
This study examined the therapeutic effects of ranibizumab in patients with myopic CNV. Consecutive monthly intravitreal injections significantly improved BCVA, reduced CMT (measured by OCT), and lowered aqueous humor levels of VEGF-A, VEGF-D, and endoglin while increasing EGF levels. The results are similar to those from a study on the effects of ranibizumab in age-related macular degeneration (AMD) cases [23]. The cytokine concentrations may be quantitative indicators of ranibizumab’s effects in patients with myopic CNV. Ranibizumab significantly affected CNV secondary to pathologic myopia by improving BCVA values, reducing CMT, and modulating cytokines associated with angiogenesis. For different individuals, different treatment plans should be chosen.
The CNV group showed higher plasma concentrations of VEGF-A than the control group, whereas EGF and angiopoietin-2 concentrations were lower in the myopic CNV than in the control group. Moreover, compared to controls, the aqueous humor content of FGF-2 increased, whereas those of EGF and VEGF-A decreased in the eyes of patients with myopic CNV.
Plasma VEGF concentrations are higher in patients with active CNV secondary to AMD than in healthy individuals [24]. VEGF is the most important signaling protein in both vasculogenesis and angiogenesis, and the increase in plasma VEGF is a prerequisite for CNV generation. Therefore, it is not unexpected that a higher plasma concentration of VEGF-A was detected in the CNV than in the control group. However, there is a lack of consensus on whether the VEGF concentration in the aqueous humor is high in myopic CNV. Tong et al. [25] reported that VEGF concentrations in the aqueous humor grew significantly among patients with CNV secondary to AMD, polypoidal choroidal vasculopathy, and CNV secondary to pathologic myopia compared to healthy controls. In contrast, Sawada et al. [26] reported significantly lower VEGF concentrations in the aqueous humor of the myopic CNV group than those in the control group, which is consistent with our results. Vascular endothelial growth factor receptor (VEGFR)-2, which is related to vascular leakage, is mainly found in the retinal blood vessel cells of the leakage area, whereas VEGFR-3 is mainly located in deep retinal capillaries and is associated with VEGFR-2. VEGF-A induces VEGFR-2 and VEGFR-3 expression in the ischemic area, leading to retinal leakage, thereby promoting new blood vessel formation [6]. We hypothesize that VEGF-A may be localized in a small subfoveal area in combination with VEGFR-2 and VEGFR-3, resulting in decreased levels in the aqueous humor during CNV formation. Another possible explanation is that VEGF-A in the anterior chamber and vitreous cavity might be diluted due to the longer axial length and larger intraocular volume in patients with high myopia because of choosing cataract patients with normal axial length as the control group.
Aqueous humor EGF content is elevated in patients with AMD [11]. However, this was not the case in the patients with myopic CNV in this study. The EGF receptor resides primarily in the cell membrane, where it binds with a significant quantity of EGF. It transduces biological signals by invagination pinocytosis, leading to its internalization into the cytoplasm. Once internalized, lysosomes degrade EGF, and the EGF receptor is recycled back to the membrane. The expression of transforming growth factor-α (TGF-α) increases while CNV progresses [27]. TGF-α and EGF are 33–44% homologous in structure; therefore, TGF-α may competitively inhibit the expression of EGF, leading to a decrease in EGF levels in the plasma and aqueous humor of patients with myopic CNV.
Angiopoietin-2 exerts a key effect on vessel maturation, angiogenesis, and vessel regression. Its expression is predominantly observed in tumor tissue (especially in areas of tumor neovascularization) but not in normal tissue [28]. In myopic CNV, angiopoietin-2 and its receptor are abundantly expressed and localized in CNV lesions. Consequently, our comparison with the control group revealed lower concentrations of angiopoietin-2 in the plasma of patients with myopic CNV.
In a healthy retina, the mRNA of FGF-2 and its receptor are found in the ganglion cell layer and kernel, and transient retinal ischemia induces the synthesis of FGF-2 mRNA [29]. In vitro studies have found that the anti-VEGF antibody/anti-FGF-2 antibody complex can completely inactivate VEGF and FGF-2 in the retinal pigment epithelium containing CNV, better inhibiting the growth of vascular endothelial cells than simply adding the anti-VEGF antibody [30]. Unlike VEGF-A, EGF, and angiopoietin-2, FGF-2 is not expressed in retinal capillary cells and is limited to CNV lesions, which may result in higher aqueous humor levels of FGF-2 in patients with myopic CNV than in controls.
This research demonstrated reduced aqueous humor concentrations of VEGF-A, VEGF-D, and endoglin after consecutive intravitreal injections of ranibizumab, an anti-VEGF-A monoclonal antibody [8]. There is little doubt that the concentration of VEGF-A decreases after intravitreal injections [31]. As a result, CNV atrophy lowers the aqueous humor concentrations of the other two cytokines. VEGF-D works in the processes of angiogenesis and lymphangiogenesis. As there are no lymphatic vessels in the eyes, the VEGF-D expressed by the retinal pigment epithelium is related to ocular angiogenesis [32]. VEGF-D can also upregulate VEGF-A expression and promote angiogenesis [33]. Previous studies have shown that endoglin has strong immunogenicity in the endothelial cells of CNV membranes [13]. However, in this study, the aqueous humor contents of EGF increased after consecutive intravitreal injections of ranibizumab. CNV atrophy led to a decrease in TGF-α contents, weakening the inhibition of EGF. As a result, the aqueous humor content of EGF increased.
This study had some limitations. First, relatively few participants were recruited in the study. Although the outcomes were statistically significant for some detected cytokines, the molecular mechanisms involved should be explored further. Second, because of the restricted volume of the samples, not all cytokines that may be related to myopic CNV were detected. There are still a few molecules potentially associated with the disease that were not examined, such as TGF-β [34], matrix metalloproteinase [35], tumor necrosis factor [36], and pigment epithelium-derived factor [25]. Finally, we selected patients with cataract as the control group, so the refractive errors of the two groups were not equivalent. This was an oversight in the design of the experiment, which might affect the results.
Conclusion
This study contributes to understanding the pathogenesis of myopic CNV by identifying previously unknown cytokines. We hope that this encourages new pharmacological research and development, allowing patients with myopic CNV to receive individualized and targeted treatment.
Author contributions
All authors made contributions to the preparation and design of this study. Material preparation, data collection and analysis were performed by Gu WT and Wang Z. Study design and data interpretation were performed by Peng D and Gu YH. The first draft of the manuscript was written by Gu WT and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding was received for this research.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenting Gu and Zhen Wang made equal contributions to the research and should be seen as co-first authors.
Contributor Information
Duo Peng, Email: pengduosuzhou@163.com.
Yonghui Gu, Email: guyonghuisuzhou@163.com.
References
- 1.Noble KG, Carr RE (1982) Pathologic myopia. Ophthalmology 89:1099–1100. 10.1016/s0161-6420(82)34677-1 [DOI] [PubMed] [Google Scholar]
- 2.Finger RP, Daien V, Eldem BM et al (2020) Anti-vascular endothelial growth factor in neovascular age-related macular degeneration: a systematic review of the impact of anti-VEGF on patient outcomes and healthcare systems. BMC Ophthalmol 20(1):294. 10.1186/s12886-020-01554-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flitcroft DI, He M, Jonas JB et al (2019) IMI—defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci 60(3):M20–M30. 10.1167/iovs.18-25957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida T, Ohno-Matsui K, Yasuzumi K et al (2023) Myopic choroidal neovascularization: a 10-year follow-up. Ophthalmology 110(7):1297–1305. 10.1016/S0161-6420(03)00461-5 [DOI] [PubMed] [Google Scholar]
- 5.Ohno-Matsui K, Wu PC, Yamashiro K et al (2021) IMI pathologic myopia. Invest Ophthalmol Vis Sci 62(7):17. 10.1167/iovs.62.5.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witmer AN, Blaauwgeers HG, Weich HA et al (2002) Altered expression patterns of VEGF receptors in human diabetic retina and in experimental VEGF-induced retinopathy in monkey. Invest Ophthalmol Vis Sci 43(3):849–857 [PubMed] [Google Scholar]
- 7.Wang SB, Nagasaka Y, Argyle D et al (2023) Targeting the m6A mRNA demethylase FTO suppresses vascular endothelial growth factor release and choroidal neovascularization. Signal Transduct Target Ther 8(1):72. 10.1038/s41392-022-01277-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Wiesmann C, Fuh G et al (1999) Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinitymatured Fab in complex with antigen. Mol Biol 293(4):865–881. 10.1006/jmbi.1999.3192 [DOI] [PubMed] [Google Scholar]
- 9.Sakata R, Miyata M, Ooto S et al (2023) Ten-year visual outcome and change in chorioretinal atrophy after intravitreal ranibizumab for macular neovascularization in pathologic myopia. Retina 43(11):1863–1871. 10.1097/IAE.0000000000003869 [DOI] [PubMed] [Google Scholar]
- 10.Hamilton RD, Clemens A, Minnella AM et al (2020) Real-world effectiveness and safety of ranibizumab for the treatment of myopic choroidal neovascularization: results from the luminous study. PLoS ONE 15(1):e0227557. 10.1371/journal.pone.0227557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonas JB, Tao Y, Neumaier M et al (2012) Cytokine concentration in aqueous humour of eyes with exudative age-related macular degeneration. Acta Ophthalmol 90(5):381–388. 10.1111/j.1755-3768.2012.02414.x [DOI] [PubMed] [Google Scholar]
- 12.Lei W, Xu H, Yao H et al (2023) 5α-hydroxycostic acid inhibits choroidal neovascularization in rats through a dual signalling pathway mediated by VEGF and angiopoietin 2. Mol Med 29(1):151. 10.1186/s10020-023-00674-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasukawa T, Kimura H, Tabata Y et al (2000) Active drug targeting with immunoconjugates to choroidal neovascularization. Curr Eye Res 21(6):952–961. 10.1076/ceyr.21.6.952.6992 [DOI] [PubMed] [Google Scholar]
- 14.Sun T, Wei Q, Gao P, Zhang Y, Peng Q (2021) Cytokine and chemokine profile changes in patients with neovascular age-related macular degeneration after intravitreal ranibizumab injection for choroidal neovascularization. Drug Des Devel Ther 15:2457–2467. 10.2147/DDDT.S307657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang P, Neal SE, Buehne KL et al (2021) Complement-mediated release of fibroblast growth factor 2 from human RPE cells. Exp Eye Res 204:108471. 10.1016/j.exer.2021.108471 [DOI] [PubMed] [Google Scholar]
- 16.Abou Shousha SA, Hussein B, Shahine Y et al (2022) Angiogenic activities of interleukin-8, vascular endothelial growth factor and matrix metalloproteinase-9 in breast cancer. Egypt J Immunol 29(3):54–63 [PubMed] [Google Scholar]
- 17.Lorenc VE, Lima E Silva R, Hackett SF et al (2020) Hepatocyte growth factor is upregulated in ischemic retina and contributes to retinal vascular leakage and neovascularization. FASEB Bioadv 2(4):219–233. 10.1096/fba.2019-00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu J, Qiu Z, Li L et al (2021) Geniposide alleviates choroidal neovascularization by downregulating HB-EGF release from RPE cells by downregulating the miR-145-5p/NF-κB axis. Exp Eye Res 208:108624. 10.1016/j.exer.2021.108624 [DOI] [PubMed] [Google Scholar]
- 19.Ma L, Ng TK, Chen H et al (2019) Identification and characterization of a novel promoter variant in placental growth factor for neovascular age-related macular degeneration. Exp Eye Res 187:107748. 10.1016/j.exer.2019.107748 [DOI] [PubMed] [Google Scholar]
- 20.Zhao JF, Hua HR, Chen QB et al (2018) Impact of fenofibrate on choroidal neovascularization formation and VEGF-C plus VEGFR-3 in Brown Norway rats. Exp Eye Res 174:152–160. 10.1016/j.exer.2018.05.030 [DOI] [PubMed] [Google Scholar]
- 21.Jauhiainen S, Häkkinen SK, Toivanen PI et al (2011) Vascular endothelial growth factor (VEGF)-D stimulates VEGF-A, stanniocalcin-1 and neuropilin-2 and has potent angiogenic effects. Arterioscler Thromb Vasc Biol 31(7):1617–1624. 10.1161/ATVBAHA.111.225961 [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Feng M, Cai J et al (2021) Repurposing bortezomib for choroidal neovascularization treatment via antagonizing VEGF-A and PDGF-D mediated signaling. Exp Eye Res 204:108446. 10.1016/j.exer.2021.108446 [DOI] [PubMed] [Google Scholar]
- 23.Gungel H, Osmanbasoglu OA, Altan C et al (2014) The effects of ranibizumab injections on fluorescein angiographic findings and visual acuity recovery in age-related macular degeneration. Clin Ophthalmol 19(8):981–988. 10.2147/OPTH.S61871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carneiro AM, Costa R, Falcao MS et al (2012) Vascular endothelial growth factor plasma contents before and after treatment of neovascular age-related macular degeneration with bevacizumab or ranibizumab. Acta Ophthalmol 90(1):e25-30. 10.1111/j.1755-3768.2011.02240.x [DOI] [PubMed] [Google Scholar]
- 25.Tong JP, Chan WM, Liu DT et al (2006) Aqueous humor contents of vascular endothelial growth factor and pigment epitheliumderived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol 141(3):456–462. 10.1016/j.ajo.2005.10.012 [DOI] [PubMed] [Google Scholar]
- 26.Sawada O, Kawamura H, Kakinoki M et al (2011) Vascular endothelial growth factor in the aqueous humour in eyes with myopic choroidal neovascularization. Acta Ophthalmol 89(5):459–462. 10.1111/j.1755-3768.2009.01717.x [DOI] [PubMed] [Google Scholar]
- 27.Yamanaka R, Ogata N, Yamamoto C et al (2002) Expression of transforming growth factor-beta receptors in normal rat retina and experimental choroidal neovascularization. Jpn J Ophthalmol 46(5):525–532. 10.1016/s0021-5155(02)00536-1 [DOI] [PubMed] [Google Scholar]
- 28.Yoodee S, Peerapen P, Plumworasawat S, Thongboonkerd V (2021) ARID1A knockdown in human endothelial cells directly induces angiogenesis by regulating angiopoietin-2 secretion and endothelial cell activity. Int J Biol Macromol 180:1–13. 10.1016/j.ijbiomac.2021.02.218 [DOI] [PubMed] [Google Scholar]
- 29.Miyashiro M, Ogata N, Takahashi K et al (1998) Expression of basic fibroblast growth factor and its receptor mRNA in retinal tissue following ischemic injury in the rat. Graefes Arch Clin Exp Ophthalmol 236(4):295–300. 10.1007/s004170050081 [DOI] [PubMed] [Google Scholar]
- 30.Stahl A, Paschek L, Martin G et al (2009) Combinatory inhibition of VEGF and FGF2 is superior to solitary VEGF inhibition in an in vitro model of RPE-induced angiogenesis. Graefes Arch Clin Exp Ophthalmol 247(6):767–773. 10.1007/s00417-009-1058-x [DOI] [PubMed] [Google Scholar]
- 31.Hata M, Yamashiro K, Ooto S et al (2017) Intraocular vascular endothelial growth factor contents in pachychoroid neovasculopathy and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 58(1):292–298. 10.1167/iovs.16-20967 [DOI] [PubMed] [Google Scholar]
- 32.Ikeda Y, Yonemitsu Y, Onimaru M et al (2006) The regulation of vascular endothelial growth factors(VEGF-A, -C, and -D) expression in the retinal pigment epithelium. Exp Eye Res 83(5):1031–1040. 10.1016/j.exer.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 33.Jauhiainen S, Häkkinen SK, Toivanen PI et al (2011) Vascular endothelial growth factor(VEGF)-D stimulates VEGF-A, stanniocalcin-1, and neuropilin-2 and has potent angiogenic effects. Arterioscler Thromb Vasc Biol 31(7):1617–1624. 10.1161/ATVBAHA.111.225961 [DOI] [PubMed] [Google Scholar]
- 34.Yang F, Sun Y, Bai Y, Li S, Huang L, Li X (2022) Asthma promotes choroidal neovascularization via the transforming growth factor Beta1/Smad signalling pathway in a mouse model. Ophthalmic Res 65(1):14–29. 10.1159/000510778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J, Kim JH, Do JY et al (2021) Key role of microglial matrix metalloproteinases in choroidal neovascularization. Front Cell Neurosci 15:638098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagbi-Levi S, Tiosano L, Rinsky B et al (2021) Anti-tumor necrosis factor alpha reduces the proangiogenic effects of activated macrophages derived from patients with age-related macular degeneration. Mol Vis 27:622–631 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.