Abstract
Degradation or “down-regulation” of protease-activated receptor-1 (PAR1), a G protein-coupled receptor for thrombin, is critical for termination of receptor signaling. Toward understanding the molecular mechanisms by which activated PAR1 is internalized, sorted to lysosomes, and degraded, we investigated whether PAR1 interacted with sorting nexin 1 (SNX1). SNX1 is a membrane-associated protein that functions in lysosomal sorting of the epidermal growth factor receptor. In vitro biochemical binding assays revealed a specific interaction between a glutathione S-transferase fusion of SNX1 and PAR1. In HeLa cells, activated PAR1 colocalized with endogenous SNX1 and coimmunoprecipitated SNX1. SNX1 contains a phox homology domain predicted to bind phosphatidylinositol-3-phosphate and a C-terminal coiled-coil region. To assess SNX1 function, we examined the effects of SNX1 deletion mutants on PAR1 trafficking. Neither the N terminus nor phox homology domain of SNX1 affected PAR1 trafficking. By contrast, overexpression of SNX1 C-terminal domain markedly inhibited agonist-induced degradation of PAR1, whereas internalization remained virtually intact. Immunofluorescence microscopy studies revealed substantial PAR1 accumulation in an early endosome antigen-1-positive compartment in agonist-treated cells expressing SNX1 C terminus. By contrast, lysosome-associated membrane protein-1 distribution was unperturbed. Together, these findings strongly suggest a role for SNX1 in sorting of PAR1 from early endosomes to lysosomes. Moreover, this study provides the first example of a protein involved in lysosomal sorting of a G protein-coupled receptor in mammalian cells.
INTRODUCTION
The precise regulation of G protein-coupled receptor (GPCR) signaling is critical for a variety of physiological responses. The β2-adrenergic receptor has served as a model system to elucidate the molecular mechanisms responsible for desensitization and resensitization of GPCR signaling (Krupnick and Benovic, 1998; Lefkowitz et al., 1998). Desensitization, internalization, and down-regulation, three temporarily distinct processes, mediate termination of GPCR signaling. Activated GPCRs are initially desensitized by rapid phosphorylation and binding of arrestins, which uncouples the receptor from G proteins. Arrestins also facilitate GPCR internalization, thereby removing the receptor from the cell surface. Once internalized into endosomes, receptors are either recycled back to the cell surface or sorted to lysosomes and degraded. The molecular mechanisms mediating GPCR degradation or down-regulation by trafficking of internalized receptors from endosomes to lysosomes remains poorly understood.
The importance of down-regulation in the regulation of GPCR signaling is best understood for protease-activated receptor-1 (PAR1). PAR1, a GPCR for the coagulant protease thrombin, elicits a variety of signaling events important for hemostasis, thrombosis, and embryonic development (Coughlin, 2000; Griffin et al., 2001). PAR1 is activated by an unusual proteolytic mechanism. Thrombin binds to and cleaves PAR1's extracellular amino terminus, creating a new amino terminus that functions as a tethered ligand (Vu et al., 1991a). A synthetic peptide, SFLLRN, which represents PAR1's newly formed amino terminus, can fully activate the receptor, independent of thrombin and receptor cleavage (Vu et al., 1991b; Scarborough et al., 1992; Vassallo et al., 1992). Activated PAR1 is then rapidly internalized, sorted to lysosomes, and degraded with a half-life of ∼30 min (Hein et al., 1994; Woolkalis et al., 1995; Trejo and Coughlin, 1999). In fibroblasts, a mutant PAR1 able to internalize and recycle back to the cell surface shows enhanced and prolonged signaling after activation with thrombin (Trejo et al., 1998; Trejo and Coughlin, 1999). This prolonged signaling is apparently due to recycling and continued signaling by receptors that return to the plasma membrane with their tethered ligands intact. These studies strongly suggest that the down-regulation of activated PAR1 by internalization and lysosomal sorting is critical for termination of receptor signaling.
The efficiency by which activated PAR1 is internalized and sorted to lysosomes suggests that it might be a useful system for elucidating the molecular mechanisms responsible for GPCR down-regulation. We previously demonstrated that PAR1 is internalized by a dynamin- and clathrin-dependent pathway like recycling receptors (Trejo et al., 2000). Activated PAR1 is recruited to clathrin-coated pits, where it colocalizes with transferrin receptor. Dominant-negative dynamin and clathrin hub mutants both block agonist-induced PAR1 internalization. Moreover, inhibition of PAR1 internalization by dynamin (K44A) mutant blocks agonist-induced degradation. Interestingly, we recently showed that PAR1 internalization through a dynamin- and clathrin-dependent pathway is independent of arrestins (Paing et al., 2002). The mechanism by which activated PAR1 is recruited to clathrin-coated pits, internalized, and sorted to lysosomes remains unknown.
Sorting nexin 1 (SNX1) was originally identified as a protein that interacts with the epidermal growth factor receptor (EGF-R) (Kurten et al., 1996). EGF-R degradation was enhanced in cells overexpressing SNX1, suggesting a role in endosome-to-lysosome trafficking. Moreover, SNX1 interaction with the EGF-R cytoplasmic tail lysosomal sorting sequence is required for down-regulation. The yeast ortholog of SNX1, Vps5p, is part of a multimeric retromer complex that functions in endosome-to-Golgi retrieval of a sorting receptor that mediates efficient delivery of hydrolases to the vacuole, an organelle analogous structurally and functionally to the mammalian lysosome (Horazdovsky et al., 1997; Nothwehr and Hindes, 1997; Seaman et al., 1998). SNX1 has also been shown to be part of a large multimeric complex in mammalian cells, suggesting a similar function in cargo selection and vesicle formation perhaps as part of the endosome-to-lysosome sorting machinery (Haft et al., 2000). Toward understanding the molecular mechanisms by which PAR1 is down-regulated, we investigated whether PAR1 interacts with SNX1. In this study, we report that SNX1 associates with PAR1 and regulates agonist-induced degradation of the receptor. These studies reveal a new role for SNX1 in sorting of PAR1 from early endosomes to lysosomes and provide the first example of a protein involved in lysosomal sorting of a GPCR in mammalian cells.
MATERIALS AND METHODS
Materials and Antibodies
Agonist peptide SFLLRN was synthesized as the carboxyl amide and was purified by high-pressure liquid chromatography (University of North Carolina, Chapel Hill Peptide Facility). ATP was from Sigma (St. Louis, MO). Monoclonal anti-c-myc-peroxidase antibody was purchased from Roche Molecular Biochemicals (Indianapolis, IN). M1 and M2 anti-FLAG monoclonal antibodies (mAbs) were purchased from Sigma. Anti-mouse immunoglobulin G (IgG) was from Pierce (Rockford, IL). Antihemagglutinin (HA) mAb (HA.11) was purchased from Covance (Richmond, CA). Anti-PAR1 1809 rabbit polyclonal antibody was generously provided by Shaun R. Coughlin (University of California, San Francisco) (Hung et al., 1992). Rabbit polyclonal anti-c-myc antibody (A-14) and mouse anti-myc antibody (9E10) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Chicken anti-c-myc IgY antibody was obtained from Molecular Probes (Eugene, OR). SNX1 antiserum was generated as described (Haft et al., 2000). Mouse antiearly endosome antigen-1 (EEA1) antibody was purchased from Transduction Laboratories (San Diego, CA). Antilysosome-associated membrane protein-1 (lamp1) H4A3 mAb was obtained from the Developmental Studies Hybridoma Bank maintained by the University of Iowa (Department of Biological Sciences, Iowa City). Secondary antibodies goat anti-mouse and anti-rabbit conjugated to horseradish peroxidase were purchased from Bio-Rad (Richmond, CA). Alexa™488- and Alexa™594-conjugated goat anti-mouse and anti-rabbit antibodies, and Alexa™647-conjugated goat anti-chicken antibodies were from Molecular Probes.
cDNAs and Cell Lines
A PAR1 cDNA containing an amino-terminal FLAG sequence (DYKDDDD) has been described (Ishii et al., 1993). The P2Y2 receptor bearing an amino-terminal HA (YPYDVPDYA) tag was generously provided by T. Kendall Harden (University of North Carolina, Chapel Hill) (Sromek and Harden, 1998). N-terminal c-myc- and HA-tagged SNX1 constructs were previously described (Haft et al., 1998). SNX1 deletion mutants were generated by polymerase chain reaction (PCR), cloned into pcDNA3.1 (Invitrogen, Carlsbad, CA), and yielded an N terminus consisting of residues 1–160, N-PX domain contained residues 1–273, and PX-C terminus contained residues 156–522. The C-terminal domain generated by PCR consisted of residues 273–522 and was cloned into pCMV5 (Andersson et al., 1989). All SNX1 deletion mutants contained an N-terminal c-myc epitope tag and were confirmed by dideoxy sequencing. HeLa cells were maintained in DMEM supplemented with 10% fetal bovine serum, 4.5 mg/ml glucose, 100 U/ml penicillin, and 100 μl/ml streptomycin. HeLa cells stably expressing FLAG-tagged PAR1 were previously described (Trejo et al., 2000).
SNX1 In Vitro Binding Assays
The entire coding region of SNX1 was cloned into pGEX-5X-1 vector (Amersham Pharmacia Biotech, Piscataway, NJ) and was used to generate GST-SNX1 fusion protein. Constructs were transformed into BL21DE3 Escherichia coli, and proteins were induced and purified using standard techniques. Crude membranes were prepared from HeLa cells stably expressing PAR1 or transiently expressing HA-P2Y2 receptor and untransfected (UT) control cells using a previously described procedure (Sarkadi et al., 1992). Briefly, cells plated in 100-mm dishes (Falcon) were collected and Dounce homogenized in 50 mM Tris-HCl, pH 7, 50 mM mannitol, and 2 mM EGTA (TMEP) containing protease inhibitors. The undisrupted cells were removed by centrifugation at 500 × g for 10 min, and the supernatant was then centrifuged at 100,000 × g for 1 h at 4°C. The pelleted membranes were resuspended in TMEP and protein concentrations were determined using BCA Protein Assay Reagent (Pierce). Membrane preparations were stored at −80°C. For binding assays, an ∼10 μg of glutathione S-transferase (GST)-SNX1 fusion protein or GST alone was immobilized on glutathione-Sepharose 4B beads and then incubated with ∼30 μg of crude membranes overnight at 4°C in Binding Buffer-150 (50 mM Tris-HCl, pH 7, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM MgCl2, 0.5 mM CaCl2, and 0.05% Triton X-100). Binding reactions were then washed twice with Binding Buffer-150 and once in Binding Buffer-500 (Binding Buffer-150 supplemented with 500 mM NaCl). Proteins that remained bound were eluted in 2× SDS-gel loading buffer (100 mM Tris-HCl, pH 6.8, 10% SDS, 0.2% bromphenol blue, and 20% glycerol), resolved by SDS-PAGE, transferred to polyvinylidene difluoride membrane (Bio-Rad) and immunoblotted with anti-PAR1 1809 antibody or anti-HA antibody. Immunoblots were developed with ECL-PLUS (Amersham Pharmacia Biotech, Arlington, IL) and imaged by autoradiography. The total amount of GST-SNX1 and GST loaded in each lane was visualized by staining the membrane with Ponceau S (Sigma) according to the manufacturer's instructions.
Transient Transfections
HeLa cells were plated at 5 × 105 cells per well in 6-well dishes (Falcon) or 0.5 × 105 cells per well in 24-well dishes (Falcon) and grown overnight. Cells were then transiently transfected with a total of 2 μg of plasmid DNA per well of a 6-well dish or 0.4 μg of plasmid DNA per well of a 24-well dish using Lipofectamine Reagent according to the manufacturer's instructions (Life Technologies, Grand Island, NY). All assays were performed ∼48 h after transfections.
Coimmunoprecipitation
HeLa cells were transiently cotransfected with FLAG-tagged PAR1 and myc-tagged SNX1 cDNA constructs and grown for ∼48 h. Transfected cells were incubated in the absence or presence of agonist for 10 min at 37°C and were then lysed in 1% Triton X-100, 50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 5 mM EDTA, 50 mM NaF, 10 mM sodium pyrophosphate, and 200 μM sodium orthovanadate containing protease inhibitors. Protein concentrations were determined using BCA Protein Assay Reagent (Pierce), and equal amounts of protein lysates were used for immunoprecipitation with M2 anti-FLAG antibody or isotype-matched IgG control. HeLa cells transiently cotransfected with HA-SNX1 and myc-SNX1 deletion mutants were processed as described above and immunoprecipitated with anti-HA antibody. Immunoprecipitates were resolved by 12% SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and immunoblotted with either anti-PAR1 1809 antibody or anti-c-myc-peroxidase-conjugated antibody. The expression of the various SNX constructs was determined by immunoblotting equivalent amounts of cell lysates with anti-c-myc or anti-HA antibody. Immunoblots were then developed with ECL-PLUS, imaged by autoradiography, and quantitated using a Fluor-S Imager (Bio-Rad).
Cell-Surface ELISA
HeLa cells plated in 24-well dishes were transiently cotransfected, grown for ∼48 h, and then treated in the absence or presence of agonist for various times at 37°C. Following agonist treatment, cells were fixed with 4% paraformaldehyde and the amount of PAR1 or P2Y2 receptor remaining on the cell surface was measured by ELISA as previously described (Paing et al., 2002).
Confocal Microscopy
HeLa cells plated on fibronectin-coated glass coverslips (22 × 22 mm) in 6-well dishes and were grown for ∼48 h. Cells were then incubated with M1 anti-FLAG or anti-PAR1 1809 antibody for 1 h at 4°C; under these conditions, only PAR1 residing on the cell surface bound antibody. Cells were washed and then incubated in the absence or presence of agonist for various times at 37°C. Cells were fixed and processed for microscopy as previously described (Trejo et al., 2000). Endogenous SNX1, lamp1, and EEA1 was detected by incubation with appropriate antibodies for 1 h at 25°C. Myc-tagged SNX1 or SNX1 C terminus was detected with mouse, rabbit, or chicken anti-myc antibodies. Cells were then washed and incubated with species-specific fluorophore-conjugated secondary antibodies for an additional hour at 25°C and were then processed for confocal microscopy. Images were collected using an Fluoview 300 laser scanning confocal imaging system (Olympus, Melville, NY) configured with an IX70 fluorescence microscope fitted with a PlanApo 60 × oil objective (Olympus). Fluorescent images, X-Y section at 0.28 μM, were collected sequentially at 800 × 600 resolution with 2× optical zoom. The final composite image was created using Adobe Photoshop 6.0 (Adobe Systems, Mountain View, CA). The number of cells containing internalized PAR1 was quantitated by counting cells that had greater than 10 PAR1-positive endosomes and costained for either SNX1 or SNX1 C terminus. All pcDNA-transfected cells containing PAR1-positive endosomes were counted. In these experiments, sample conditions were blindly coded and counted by two individuals. The values shown represent at least 25 cells counted per condition in an experiment that was repeated three separate times. The data (mean ± SEM) are expressed as a percentage of PAR1-positive cells that costained for SNX1 or SNX1 C terminus.
RESULTS
Agonist-induced Colocalization of PAR1 with Endogenous SNX1 in HeLa Cells
Toward understanding the molecular mechanisms by which activated PAR1 is internalized and sorted to lysosomes, we investigated whether PAR1 associated with SNX1 in HeLa cells. We have used the HeLa cell line because SNX1 is endogenously expressed (Kurten et al., 1996), and agonist-triggered internalization and lysosomal sorting of PAR1, as seen in fibroblasts and endothelial cells, also occurs in HeLa cells (Trejo et al., 2000). We stably expressed PAR1 containing an amino-terminal FLAG epitope in these cells and assessed agonist-induced colocalization by confocal microscopy using anti-FLAG and anti-SNX1 antibodies. Antiserum raised against SNX1 recognized both endogenous and recombinant SNX1 in HeLa cells (unpublished results).
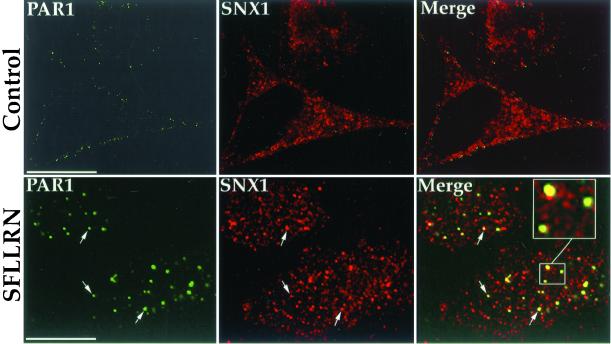
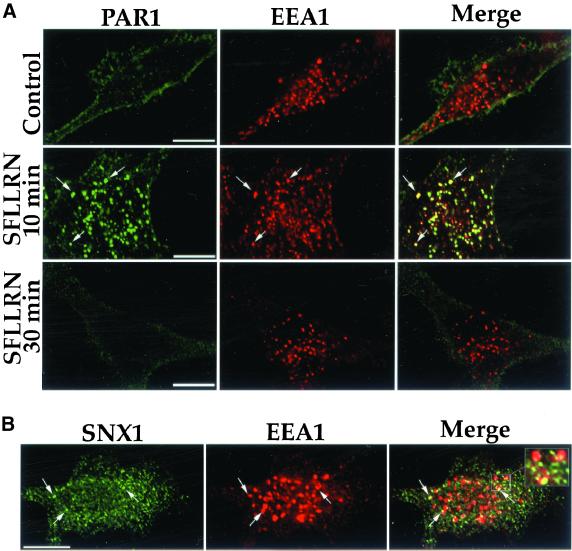
Confocal microscopy studies of PAR1 and endogenous SNX1 revealed an agonist-induced colocalization of these molecules. HeLa cells stably expressing PAR1 were first incubated with anti-PAR1 antibody for 1 h at 4°C; under these conditions, only receptors residing on the cell surface bind antibody (unpublished results). Cells were then warmed to 37°C in the presence or absence of agonist SFLLRN for 10 min, fixed, and immunostained for PAR1 and endogenous SNX1. In untreated control cells, PAR1 was found localized predominantly to the cell surface, whereas endogenous SNX1 was distributed throughout the cytoplasm in punctate structures and failed to colocalize with PAR1 (Figure 1, Control). By contrast, the addition of agonist peptide SFLLRN caused marked redistribution of PAR1 into endocytic vesicles, and endogenous SNX1 showed substantial colocalization with activated PAR1 (Figure 1, SFLLRN); ∼60% of PAR1-positive endosomes costained for SNX1. Thus, after agonist-induced internalization, PAR1 colocalizes with endogenous SNX1 in an endosomal compartment.
Figure 1.
Agonist-induced colocalization of PAR1 with endogenous SNX1 in HeLa cells. HeLa cells stably expressing PAR1 were incubated in the absence (Control) or presence of 50 μM SFLLRN for 10 min at 37°C. Cells were fixed, permeabilized, and immunostained for PAR1 (green) and endogenous SNX1 (red) and were examined by confocal microscopy. These images are representative of many cells examined in three separate experiments. Note the prominent agonist-induced colocalization (yellow) of PAR1 and SNX1 in the merged image. Scale bar represents 10 μm.
In Vitro Interaction of PAR1 and SNX1
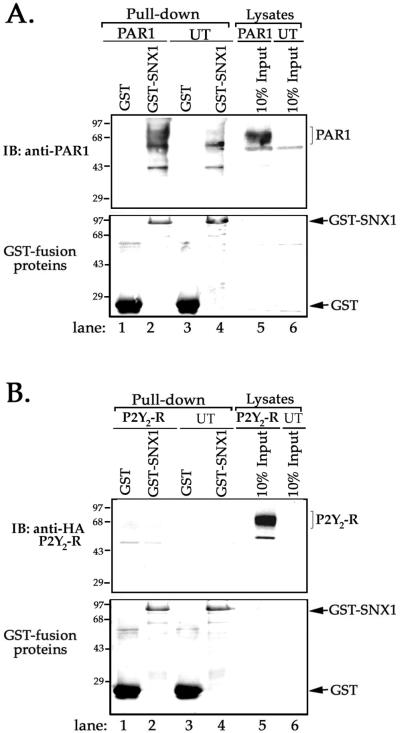
To assess PAR1 interaction with SNX1, we first performed in vitro biochemical binding or “pull-down” assays. In these experiments, equal amounts of GST-SNX1 fusion protein (and GST protein alone) absorbed to glutathione-Sepharose beads were incubated with membranes prepared from HeLa cells stably expressing PAR1 or untransfected control cells. An immunoblot of proteins eluted from GST-SNX1 incubated with membranes prepared from PAR1-expressing cells revealed one major transfection-dependent protein migrating at ∼68 kDa, whereas an immunoblot of proteins eluted from GST alone failed to detect this protein (Figure 2A, lanes 1 and 2). This ∼68 kDa band is consistent with the molecular weight of PAR1 detected in lysates prepared from transfected HeLa cells (Figure 2A, lane 5) and with that previously reported for PAR1 (Ishii et al., 1993; Trejo and Coughlin, 1999). To test whether SNX1 associates only with receptors that efficiently down-regulate, we examined whether GST-SNX1 interacted with the P2Y2 purinergic GPCR. The P2Y2 receptor is activated by extracellular nucleotides, internalized, and efficiently recycled to the cell surface like most classic GPCRs (Sromek and Harden, 1998). Neither GST-SNX1 nor GST alone interacted with the P2Y2 receptor under the same conditions in which GST-SNX1 was associated with PAR1 (Figure 2B, lanes 1 and 2). An aliquot of membrane lysate representing 10% input showed substantial amount of P2Y2 receptor present in the membrane preparations (Figure 2B, lane 5). These observations suggest that in vitro SNX1 is capable of interacting with PAR1, a receptor that undergoes efficient agonist-induced down-regulation.
Figure 2.
SNX1 associates with PAR1 in vitro. (A) GST-SNX1 fusion protein or GST protein alone absorbed to glutathione-Sepharose beads was incubated with equal amounts of membranes prepared from PAR1-transfected HeLa cells or untransfected (UT) control cells. Bound proteins were eluted, resolved by SDS-PAGE, and immunoblotted with anti-PAR1 antibody (upper panel). An aliquot of membranes prepared from PAR1-expressing HeLa cells or UT control cells representing 10% of input is shown in lanes 5 and 6, respectively. The nonspecific bands detected in pull-downs of UT control cells are shown in lanes 3 and 4. Similar findings were observed in four separate experiments. Note the detection of one prominent transfection-dependent ∼68 kDa protein in GST-SNX1 pull-down shown in lane 2. (B) GST-SNX1 or GST protein alone absorbed to glutathione-Sepharose beads was incubated with membranes prepared from HeLa cells expressing HA-tagged P2Y2 receptor or UT control cells. Bound proteins were eluted and immunoblotted with anti-HA antibody (upper panel). An aliquot of membranes representing 10% of input is shown in lane 5 (upper panel). Similar results were observed in three separate experiments. Note the failure of GST-SNX1 to bind the P2Y2 receptor. The total amount of GST-SNX1 and GST protein loaded in various lanes was visualized by Ponceau S staining (bottom panels).
SNX1 Association with PAR1 Is Enhanced following Activation in HeLa Cells
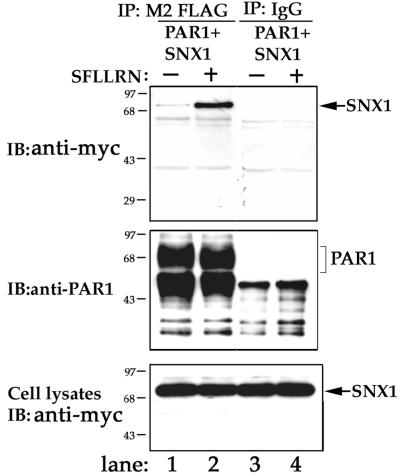
Next, we examined whether activated PAR1 and SNX1 associate in vivo by coimmunoprecipitation. HeLa cells transiently cotransfected with FLAG-tagged PAR1 and myc-tagged SNX1 were incubated with or without agonist peptide SFLLRN for 10 min at 37°C. Cells were lysed and immunoprecipitated with M2 anti-FLAG antibody and the presence of SNX1 was detected by immunoblotting. In untreated control cells expressing myc-SNX1, a substantial amount of PAR1 was immunoprecipitated; however, only a small amount of SNX1 coimmunoprecipitated with the receptor, suggesting that unactivated receptor weakly associates with SNX1 (Figure 3, lane 1). By contrast, immuno-precipitates from agonist-treated cells revealed that substantially more SNX1 associated with activated PAR1 (Figure 3, lane 2). Neither SNX1 nor PAR1 was immunoprecipitated with isotype-matched control IgG (Figure 3, lanes 3 and 4). These observations strongly suggest that in vivo PAR1 activation enhances association with SNX1.
Figure 3.
Coimmunoprecipitation of SNX1 with activated PAR1 in HeLa cells. HeLa cells transiently cotransfected with FLAG-tagged PAR1 and myc-SNX1 were treated in the absence (−) or presence (+) of 50 μM SFLLRN for 10 min at 37°C. Cells were lysed and equal amounts of lysates were immunoprecipitated with M2 anti-FLAG antibody or isotype-matched control IgG. Immunoprecipitates were immunoblotted for SNX1 using anti-myc-peroxidase antibody (upper panel) or for PAR1 using 1809 antibody (middle panel). SNX1 expression in total cell lysates representing 12% of input and was detected with anti-myc antibody (bottom panel). Similar results were observed in six independent experiments. Note the prominent agonist-induced association of SNX1 with PAR1.
Overexpression of SNX1 C-terminal Domain Inhibits Agonist-induced Degradation of PAR1
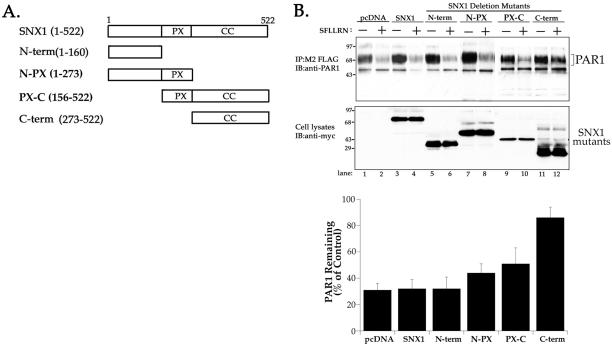
SNX1 contains a phox homology domain predicted to bind phosphatidylinositol-3-phosphate, and a C-terminal coiled-coil region (Kurten et al., 1996; Haft et al., 1998; Wu and Lemmon, 2001). To assess SNX1 function in PAR1 trafficking, we first asked if various deletion mutants of SNX1 would block agonist-induced PAR1 degradation. Accordingly, we generated several SNX1 deletion mutants, as illustrated in Figure 4A. HeLa cells transiently cotransfected with FLAG-PAR1 and myc-tagged SNX1 deletion mutants were treated with agonist peptide SFLLRN for 60 min at 37°C. Cells were lysed, immunoprecipitated with anti-FLAG antibody, and the amount of receptor protein remaining was examined by immunoblotting with anti-PAR1 antibody. In cells cotransfected with SNX1 or empty vector, exposure to agonist decreased PAR1 protein by ∼70% (Figure 4B and C, lanes 1–4). These findings are consistent with the extent of PAR1 degradation previously reported in HeLa and other cell types (Trejo and Coughlin, 1999; Trejo et al., 2000). PAR1 was similarly degraded in cells expressing the SNX1 N terminus (Figure 4B and C, lanes 5 and 6). PAR1 degradation was slightly inhibited in cells expressing the N-PX and PX-C-terminal regions of SNX1 (Figure 4B and C, lanes 7–10). In striking contrast, agonist-induced degradation of PAR1 was markedly inhibited in cells cotransfected with SNX1 C-terminal domain; only a ∼16% decrease in PAR1 receptor protein was detected after 60 min of agonist exposure (Figure 4B and C, lanes 11 and 12). Thus, the ability of SNX1 C terminus to inhibit PAR1 degradation suggests that sorting of activated PAR1 to a degradative pathway is SNX1 dependent.
Figure 4.
Overexpression of SNX C terminus markedly inhibits agonist-induced degradation of PAR1. (A) Domain structure model of SNX1 and various deletion mutants. The phox homology domain is represented as “PX” and the coiled-coiled region is shown as “CC.” (B) HeLa cells transiently cotransfected with PAR1 and SNX1, SNX1 mutants, or pcDNA vector were incubated in the absence (−) or presence (+) of 50 μM SFLLRN for 60 min at 37°C. Cells were lysed, immunoprecipitated with anti-FLAG antibody, and the amount of PAR1 remaining was detected by immunoblot with anti-PAR1 antibody and quantitated by Fluor-S Imager (upper panel). The expression of SNX1 and various deletion mutants in cell lysates was detected with anti-myc anti-body (lower panel). Results in the bar graph are expressed as a percentage of PAR1 measured in untreated control lysates and were determined for each transfection condition. The data are represented as the mean ± SEM of three separate experiments in which duplicate determinations were made. Note the marked inhibition of agonist-induced PAR1 degradation in cells expressing SNX1 C-terminal coiled-coil domain.
SNX1 C-Terminal Coiled-Coil Domain Can Assemble with Full-Length SNX1
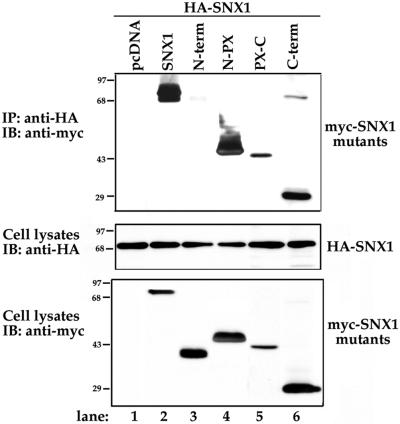
SNX1 C terminus encodes a coiled-coil domain that may assemble with endogenous SNX1 to function as a dominant-negative when expressed in HeLa cells. To determine whether the SNX1 C-terminal coiled-coil domain was sufficient for assembly with full-length SNX1, we tested for association by coimmunoprecipitation. HeLa cells were transiently cotransfected with full-length HA-tagged SNX1 and various myc-tagged SNX1 deletion mutants. Cells were lysed, immunoprecipitated with anti-HA antibody, and the presence of myc-tagged SNX1 deletion mutants was detected by immunoblotting. A full-length myc-tagged SNX1 coimmunoprecipitated with HA-SNX1, suggesting that SNX1 can self associate when expressed in HeLa cells (Figure 5, lane 2). These findings are consistent with previous studies demonstrating SNX1 self-assembly in COS cells (Haft et al., 1998). By contrast, the N terminus of SNX1 failed to coimmunoprecipitate with HA-SNX1 (Figure 5, lane 3). Both the N-PX and PX-C-terminal regions of SNX1 coimmunoprecipitated with full-length SNX1 (Figure 5, lanes 4 and 5), suggesting that the PX domain can mediate assembly with SNX1. Interestingly, the C-terminal coiled-coil domain also was robustly coimmunoprecipitated with full-length HA-SNX1 (Figure 5, lane 6). Thus, SNX1 C-terminal coiled-coil domain alone is sufficient to assemble with full-length SNX1, suggesting that the C terminus may interact with endogenous SNX1 to function in a dominant-negative manner.
Figure 5.
SNX1 C-terminal coiled-coil domain associates with full-length SNX1. HeLa cells transiently cotransfected with HA-SNX1 and myc-SNX1 deletion mutants were lysed and immunoprecipitated with anti-HA antibody. Immunoprecipitates were immunoblotted for full-length myc-SNX1 or deletion mutants using anti-myc-peroxidase antibody (upper panel). The expression of HA-SNX1 and myc-SNX1 deletion mutants in total cell lysates is shown in the middle and lower panels, respectively. Note the prominent association of SNX1 C-terminal coiled-coil domain with full-length SNX1, lane 6. The data are representative of two separate experiments.
Effect of SNX1 C Terminus on Agonist-induced PAR1 and P2Y2-Receptor Internalization
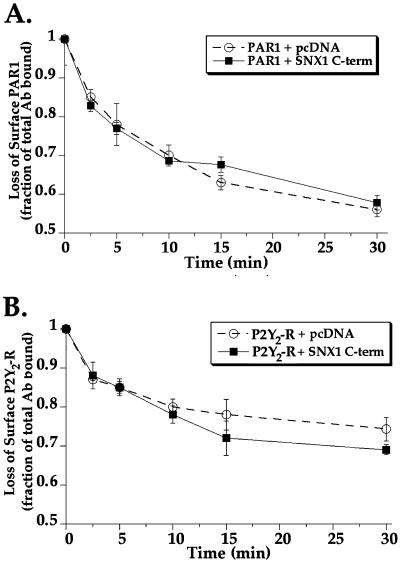
To determine whether SNX1 C-terminal domain blocked PAR1 degradation by inhibiting receptor internalization, we examined agonist-induced loss of cell-surface PAR1. HeLa cells transiently cotransfected with FLAG-tagged PAR1 and SNX1 C terminus or empty vector were treated with agonist peptide SFLLRN for various times at 37°C. After incubations, cells were fixed and the amount of PAR1 remaining on the cell surface was quantitated by cell-surface ELISA and used as a measure of receptor internalization. In these experiments, the initial level of PAR1 detected on the cell surface before incubation, the 0-min time point, was similar for each transfection condition. In cells cotransfected with PAR1 and empty vector, agonist peptide SFLLRN induced rapid internalization of PAR1 from the plasma membrane within 10 min (Figure 6A). PAR1 continued to slowly internalize with an ∼45% loss of surface PAR1 detected after 30 min of agonist exposure (Figure 6A). In cells cotransfected with PAR1 and SNX1 C terminus, the addition of agonist caused a similar decrease in the amount of surface PAR1 over time (Figure 6A). The overexpression of SNX1 also did not effect the rate of agonist-induced PAR1 internalization (unpublished results). In cells cotransfected with P2Y2 receptor and SNX1 C-terminus or empty vector the addition of agonist ATP caused a more modest decrease in cell surface P2Y2 receptor that reached near steady state by 10 min (Figure 6B). The agonist-induced loss of P2Y2 receptor is consistent with internalization and recycling of this receptor. Thus, the marked inhibitory effect of SNX1 C terminus on PAR1 degradation is unlikely to involve regulation at the level of receptor internalization.
Figure 6.
Effect of SNX1 C-terminal domain on agonist-triggered PAR1 and P2Y2 receptor internalization. (A) HeLa cells transiently cotransfected with FLAG-tagged PAR1 and either SNX1 C terminus or pcDNA vector were incubated in the absence or presence of 50 μM SFLLRN for various times at 37°C. (B) HeLa cells transiently cotransfected with P2Y2 receptor and either SNX1 C terminus or pcDNA vector were treated in the absence or presence of 100 μM ATP for various times at 37°C. Cells were then fixed and the amount of PAR1 or P2Y2 receptor remaining on the cell surface was detected by cell surface ELISA. The initial level of PAR1 or P2Y2 receptor expressed on the cell surface before incubation at 37°C (0-min time point) was similar for each transfection condition. Results are expressed as a fraction of total antibody bound to untreated controls. The data (mean ± SEM) are representative of three separate experiments in which triplicate determinations were made.
Overexpression of SNX1 C-terminal Domain Causes Agonist-induced PAR1 Accumulation in Endosomes
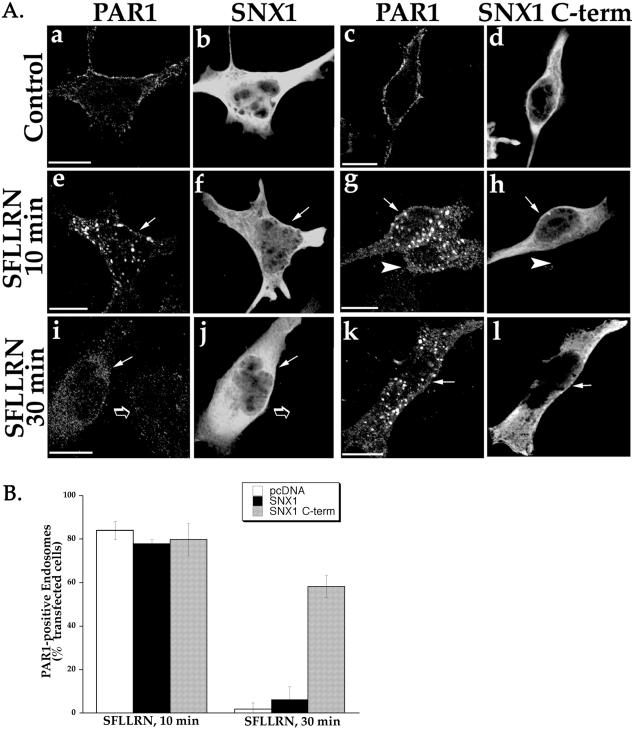
To test whether SNX1 C terminus blocked delivery of PAR1 from endosomes to lysosomes, we examined PAR1 accumulation after prolonged agonist treatment. HeLa cells stably expressing FLAG-tagged PAR1 were transiently transfected with SNX1 or SNX1 C terminus, and then treated with agonist SFLLRN for either 10 or 30 min at 37°C. Cells were fixed, immunostained for PAR1 and SNX1, and examined by confocal microscopy. In cells transfected with SNX1, agonist treatment induced substantial redistribution of PAR1 into endocytic vesicles at 10 min (Figure 7A, e and f). Activated PAR1 was similarly internalized into endosomes in SNX1 C terminus transfected cells after 10 min of agonist exposure (Figure 7A, g and h), consistent with the ELISA experiments shown in Figure 6. An adjacent cell that lacked SNX1 C terminus expression also showed substantial agonist-induced PAR1 internalization at 10 min (Figure 7A, g and h, arrowhead). However, after 30 min of agonist exposure, PAR1-positive endosomes were no longer apparent in SNX1-transfected cells (Figure 7A, i and j), consistent with agonist-induced internalization and sorting of PAR1 to a degradative pathway. PAR1 was similarly degraded after 30 min of agonist exposure in an adjacent cell lacking SNX1 expression (Figure 7A, i and j, open arrow). By contrast, in SNX1 C terminus-transfected cells, PAR1-positive endosomes were apparent and easily detected even after 30 min of agonist incubation (Figure 7A, k and l). Quantitative analysis of hundreds of examined cells was consistent with an inhibitory effect of SNX1 C terminus on lysosomal sorting of PAR1. After 10 min of agonist incubation, ∼80% of cells transfected with SNX1, SNX1 C terminus, or empty vector showed substantial amount of PAR1-positive endosomes (Figure 7B). However, after 30 min of agonist exposure, SNX1- and mock-transfected cells showed almost complete loss of PAR1 endosomes; only ∼2 and 6% of transfected cells costained for PAR1, respectively (Figure 7B). In striking contrast, a significant fraction of cells expressing SNX1 C terminus showed marked accumulation of PAR1-positive endosomes even after 30 min of agonist exposure; ∼58% of SNX1 C terminus-expressing cells costained for PAR1 (Figure 7B). Thus, PAR1 accumulates in endosomes and fails to efficiently sort to a degradative pathway in a significant fraction of cells overexpressing SNX1 C-terminal domain.
Figure 7.
Agonist-induced PAR1 accumulation in endosomes in SNX1 C terminus transfected cells. (A) PAR1-expressing HeLa cells transiently transfected with SNX1 or SNX1 C terminus were treated in the absence (Control) or presence of 50 μM SFLLRN for either 10 or 30 min at 37°C. Cells were fixed, immunostained for PAR1 and SNX1 or SNX1 C terminus, and imaged by confocal microscopy. Note the presence of PAR1-positive endosomes in SNX1 C terminus-transfected cells even after 30 min of agonist exposure (k and l), but the absence of such endosomes in SNX1 transfected cells (i and j). (B) The results of quantitative analysis are expressed as a percentage of cells that showed PAR1-positive endosomes and costained for either SNX1 or SNX1 C terminus. All cells containing PAR1-positive endosomes were counted in pcDNA vector-transfected controls. The data (mean ± SEM) are representative of three separate experiments in which at least 25 determinations were made. Scale bar represents 10 μm.
SNX1 C-Terminal Coiled-Coil Domain Does Not Generally Inhibit Lysosomal Transport
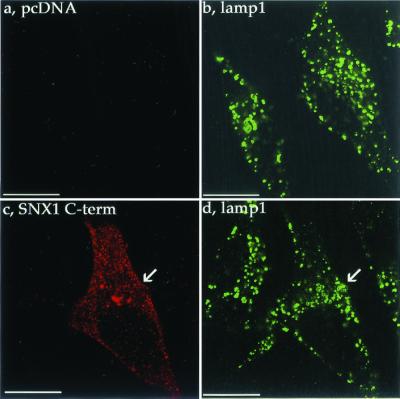
Lamp1 is transported to lysosomes directly via the trans-Golgi network or indirectly via the cell surface within hours (Fukuda, 1991). To exclude the possibility that overexpression of SNX1 C terminus had pleiotrophic inhibitory effects on vesicular transport to lysosomes, we examined the distribution of lamp1. HeLa cells transiently transfected with SNX1 C terminus or empty vector were immunostained for lamp1 and SNX1 C terminus and were examined by confocal microscopy. In mock-transfected cells, lamp1 localized predominantly to a heterogeneous population vesicles distributed throughout the cytoplasm (Figure 8, a and b), consistent with lamp1 distribution previously reported (Futter et al., 1996). SNX1 C terminus was detected predominantly in a punctate pattern (Figure 8, c), similar to that observed for endogenous SNX1 (Figure 1). The distribution of lamp1 was strikingly similar in SNX1 C terminus- and mock-transfected cells (Figure 8, compare b and d). Moreover, incubation with LysoTracker Red, a membrane-permeable probe that accumulates in acidic organelles, revealed a distinct population of vesicles in SNX1 C terminus-expressing cells similar to that observed in mock-transfected control cells (unpublished results). Thus, overexpression of SNX1 C terminus does not appear to inhibit normal biogenesis or maintenance of the lysosomal compartment, suggesting that the SNX1 C-terminal domain does not have general inhibitory effects on vesicular transport to lysosomes.
Figure 8.
Effect of SNX1 C terminus overexpression on lamp1 distribution. HeLa cells were transiently transfected with myc-tagged SNX1 C terminus or pcDNA vector. At ∼48 h after transfection, cells were fixed, immunostained for SNX1 C terminus (red) and lamp1 (green), and examined by confocal microscopy. Note the similar distribution of lamp1 in SNX1 C terminus and pcDNA-transfected cells shown in b and d. The images are representative of many cells examined in two separate experiments. Scale bar denotes 10 μm.
SNX1 C Terminus Overexpression Leads to Accumulation of Agonist-induced Internalized PAR1 in EEA1-Positive Early Endosomes
To begin to understand the mechanism by which SNX1 C terminus disrupts sorting of PAR1 from endosomes to lysosomes, we examined PAR1 colocalization with EEA1. EEA1 is a core component of the endosome docking and fusion machinery and a specific marker of early endosomes (Christoforidis et al., 1999). In untreated control cells, PAR1 was found predominantly on the cell surface by confocal microscopy and did not colocalize with EEA1 (Figure 9A, Control). After 10 min of agonist treatment, PAR1 was redistributed to endocytic vesicles and markedly colocalized with EEA1; ∼80% of PAR1-positive endosomes costained for EEA1 (Figure 9A, SFLLRN 10 min). By contrast, after 30 min of agonist treatment, PAR1-containing endosomes were no longer apparent (Figure 9A, SFLLRN 30 min), whereas EEA1 localization was unperturbed. These findings are consistent with sorting of PAR1 from early endosomes to a degradative compartment after prolonged agonist exposure. Interestingly, endogenous SNX1 also showed substantial colocalization with EEA1 (Figure 9B). Together, these findings suggest that after agonist-induced internalization, PAR1 and SNX1 colocalize in an EEA1-positive endosomal compartment, PAR1 is then sorted away from this compartment, delivered to lysosomes, and degraded.
Figure 9.
PAR1 and endogenous SNX1 colocalize with EEA1-positive early endosomes. (A) HeLa cells expressing PAR1 were treated in the absence (Control) or presence of 50 μM SFLLRN for 10 or 30 min at 37°C. Cells were fixed, immunostained for PAR1 (green) and EEA1 (red), and imaged by confocal microscopy. Note the prominent agonist-induced colocalization (yellow) of PAR1 and EEA1 after 10 min of agonist exposure shown in the merged image and the loss of PAR1-positive endosomes after 30 min of agonist exposure. (B) HeLa cells were immunostained for endogenous SNX1 (green) and EEA1 (red) and were examined by confocal microscopy. Endogenous SNX1 and EEA1 colocalization (yellow) is shown in the merged image. The cells imaged are representative of many cells examined in four separate experiments. Scale bar denotes 10 μm.
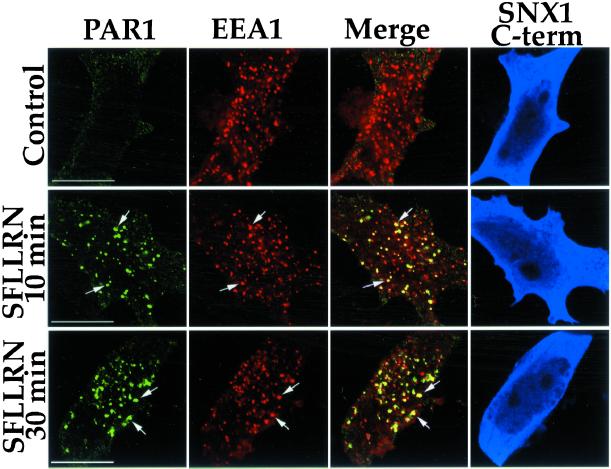
Next, we examined colocalization of internalized PAR1 and EEA1 in cells transfected with SNX1 C terminus after prolonged agonist treatment. In untreated control cells expressing SNX1 C terminus, PAR1 was predominantly localized to the cell surface and failed to colocalize with EEA1 (Figure 10, Control). After 10 min of agonist exposure, PAR1 and EEA1 showed substantial colocalization in cells expressing SNX1 C terminus (Figure 10, SFLLRN 10 min), consistent with the results described above. Strikingly, after 30 min of agonist exposure, PAR1-positive endosomes were easily detected and showed marked colocalization with EEA1 in SNX1 C terminus-expressing cells (Figure 10, SFLLRN 30 min). Thus, after activation, PAR1 is internalized to an EEA1-positive early endosomal compartment and fails to efficiently transit this compartment in cells overexpressing SNX1 C-terminal domain.
Figure 10.
Agonist-induced PAR1 accumulation in EEA1-positive early endosomes in SNX1 C terminus-expressing cells. PAR1-expressing HeLa cells were treated in the absence (Control) or presence of 50 μM SFLLRN for either 10 or 30 min at 37°C. Cells were fixed and immunostained for PAR1 (green), EEA1 (red), and SNX1 C terminus (blue), and were imaged by confocal microscopy. Note the prominent colocalization (yellow) of PAR1 and EEA1 even after 30 min of agonist exposure in SNX1 C terminus-expressing cells (blue). The imaged cells are representative of many cells examined in three separate experiments. The scale bar represents 10 μm.
DISCUSSION
The internalization and lysosomal degradation of activated PAR1 is critical for termination of receptor signaling (Trejo et al., 1998; Trejo and Coughlin, 1999). The molecular mechanisms responsible for intracellular targeting of PAR1 to lysosomes are not known. In this study, we demonstrate that SNX1 associates with PAR1 and regulates lysosomal sorting of the receptor. Our biochemical binding assays demonstrate that SNX1 can associate with PAR1 in vitro. Moreover, confocal microscopy studies of HeLa cells revealed that after agonist-induced internalization, PAR1 colocalizes with endogenous SNX1 in an intracellular endosomal compartment. Consistent with these results, analysis of coimmunoprecipitates showed an enhanced agonist-induced association of PAR1 with SNX1 in vivo. By contrast, the P2Y2 receptor, a classic GPCR that internalizes and efficiently recycles, failed to interact with SNX1. Whether these findings suggest specificity for SNX1 binding to receptors that efficiently down-regulate versus those that efficiently recycle and only slowly down-regulate remains to be determined.
The association of SNX1 with PAR1 is enhanced following activation. This may be due to an agonist-induced modification of PAR1 or redistribution of PAR1 to an intracellular compartment containing SNX1. Phosphorylation of PAR1's cytoplasmic carboxyl tail serine and threonine residues is required for internalization and down-regulation of the receptor in many cell types (Shapiro et al., 1996; Paing et al., 2002). Thus, it is possible that PAR1 phosphorylation per se is sufficient to facilitate association with SNX1 and thereby regulates sorting of the receptor. PAR1 phosphorylation could also mark the receptor for ubiquitination and this may mediate lysosomal sorting of the receptor by facilitating interaction with SNX1. It was recently shown that ubiquitination of the β2-adrenergic and CXCR4-chemokine GPCR is required for lysosomal degradation in mammalian cells (Marchese and Benovic, 2001; Shenoy et al., 2001). It is not known whether activated PAR1 is modified to facilitate association with SNX1 or whether activated PAR1 is simply targeted to an intracellular compartment in which SNX1 resides. Regardless, our findings strongly suggest that after agonist-induced internalization, association of PAR1 with SNX1 is enhanced.
SNX1 was previously demonstrated to function in the degradation of the EGF-R (Kurten et al., 1996), a receptor that undergoes efficient down-regulation. This finding strongly suggests a role for SNX1 in endosome-to-lysosome trafficking and raised the possibility that SNX1 might function in sorting of PAR1 to lysosomes. A SNX1 deletion mutant inhibited EGF-R degradation, suggesting that deletion mutants could function in a dominant-negative manner (Kurten et al., 1996). We found that a deletion mutant of SNX1 encoding the C-terminal coiled-coil domain markedly inhibited agonist-induced PAR1 degradation, whereas internalization remained virtually intact. Consistent with these results, sorting of internalized PAR1 from EEA1-positive early endosomes to lysosomes is inhibited in a significant fraction of cells overexpressing SNX1 C terminus. The localization of PAR1 and endogenous SNX1 to EEA1-positive endosomes provides further evidence in support of a role for SNX1 in trafficking of PAR1 at an early endosomal stage. In cells overexpressing SNX1 C terminus, the distribution of lamp1 was unperturbed, suggesting that general vesicular transport to lysosomes remained intact. Moreover, the EEA1-positive early endosomal compartment was also unaltered in cells overexpressing SNX1 C terminus, further excluding the possibility that SNX1 C terminus had pleiotrophic inhibitory effects on vesicular transport. Thus, the ability of SNX1 C-terminal coiled-coil domain to inhibit sorting of internalized PAR1 to a degradative pathway strongly suggests that lysosomal sorting of the receptor is SNX1 dependent.
SNX1 is a peripheral membrane protein that can self-assemble or assemble with SNX2, a closely related homolog, to form homo-oligomers or hetero-oligomers both in vitro and in vivo (Haft et al., 1998; Kurten et al., 2001). Interestingly, Vps5p, the yeast ortholog of SNX1, also displays self-assembly activity (Seaman et al., 1998). The SNX1 C-terminal coiled-coil domain was recently shown to be important for the localization of SNX1 to endosomes in HeLa cells (Teasdale et al., 2001). Moreover, we demonstrate that the C-terminal coiled-coil domain is sufficient to assemble with full-length SNX1. Indeed, coiled-coils are one of the principal subunits for oligomerization of a variety of proteins (Burkhard et al., 2001). These findings suggest that the C-terminal coiled-coil region of SNX1 might specify interaction with itself or another membrane-associated protein to mediate assembly and perhaps endosomal localization. Thus, it is conceivable that SNX1 C terminus coiled-coil domain acts as a dominant-negative of endogenous SNX1 function by disrupting such interactions and thereby inhibits sorting of internalized PAR1 from endosomes to lysosomes.
The function of SNX1 in intracellular protein trafficking in mammalian cells is poorly understood. However, many membrane trafficking pathways are conserved throughout evolution, suggesting that studies of yeast Vps5p, an ortholog of SNX1 and SNX2, could offer insight regarding the function of SNX1 and SNX2 in mammalian cells. Indeed, SNX1, SNX2, and Vps5p display self-assembly activity, which may provide the mechanical force necessary to drive vesicle budding (Haft et al., 1998; Seaman et al., 1998; Kurten et al., 2001). In support of this idea, Vps5p has been localized to the rims of budding vesicles on prevacuolar endosomes by electron microscopy (Seaman et al., 1998). Moreover, biochemical and genetic evidence suggest that Vps5p is part of a multimeric complex, termed “retromer,” that functions in retrieval of sorting proteins from prevacuolar endosomes to the Golgi (Horazdovsky et al., 1997; Nothwehr and Hindes, 1997; Seaman et al., 1998). This process is required for efficient sorting to the vacuole because the loss of any component of the retromer complex leads to mislocalization of sorting receptors and mistargeting of proteins destined to the vacuole. SNX1 has also been shown to be part of a large multimeric complex in mammalian cells, suggesting a similar function in cargo selection and vesicle formation perhaps as part of the endosome-to-lysosome sorting machinery (Haft et al., 2000). Consistent with this idea, we demonstrate endogenous SNX1 localization to EEA1-positive early endosomes, and others have shown that ectopically expressed SNX1 localizes to EEA1 endosomes (Kurten et al., 2001; Teasdale et al., 2001). Moreover, SNX1 contains a phox homology domain that is predicted to bind phosphatidylinositol-3-phosphate, which is highly enriched in endosomal membranes (Wu and Lemmon, 2001). Components of the yeast retromer complex can directly associate with the cytoplasmic tail of cargo proteins (Nothwehr et al., 2000); whether SNX1 or another component of the mammalian “retromer” complex interacts directly with PAR1 remains to be determined.
In summary, we used PAR1 as a model system to investigate the molecular mechanisms responsible for GPCR down-regulation. Activated PAR1 is internalized via an arrestin-independent but dynamin- and clathrin-dependent pathway and is then efficiently sorted to lysosomes (Trejo et al., 2000; Paing et al., 2002). SNX1 was originally identified as a protein that interacted with the EGF-R cytoplasmic tail and was shown to mediate EGF-R down-regulation (Kurten et al., 1996). In these studies, we demonstrate that SNX1 associates with PAR1 and is involved in down-regulation of the receptor. These studies provide the first example of a protein involved in lysosomal sorting of a GPCR in mammalian cells. In contrast, β2-adrenergic receptor interaction with ezrin-radixin-moesin-binding phosphoprotein-50/Na+-H+ exchanger regulator factor (EBP50/NHERF) family proteins has been suggested to regulate efficient recycling of GPCRs (Hall et al., 1998; Cao et al., 1999). Moreover, we demonstrate that endogenous SNX1 is localized to early endosomes and a dominant-negative SNX1 C terminus blocks sorting of PAR1 from early endosomes to lysosomes. These findings bring new insight into how SNX1 might function in protein trafficking in mammalian cells. The challenge now becomes to elucidate the mechanism by which SNX1 regulates sorting of PAR1 from early endosomes to lysosomes, an event critical for termination of receptor signaling (Trejo et al., 1998; Trejo and Coughlin, 1999).
ACKNOWLEDGMENTS
We thank Drs. Ann Erickson, Sharon L. Milgram, and David P. Siderovski for helpful suggestions and critical review of this manuscript. This work was supported by National Institutes of Health Grant HL67697 (to J.T.).
Abbreviations used:
- BSA
bovine serum albumin
- EEA1
early endosome antigen-1
- EGF-R
epidermal growth factor receptor
- ELISA
enzyme-linked immunosorbent assay
- GPCR
G protein-coupled receptor
- GST
glutathione S-transferase
- HA
hemagglutinin
- lamp1
lysosome-associated membrane protein-1
- PAR1
protease-activated receptor-1
- PBS
phosphate-buffered saline
- SNX1
sorting nexin 1
- UT
untransfected
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E01–11–0131. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E01–11–0131.
REFERENCES
- Andersson S, Bishop RW, Russell DW. Expression cloning and regulation of steroid 5 α-reductase, an enzyme essential for male sexual differentiation. J Biol Chem. 1989;264:16249–16255. [PMC free article] [PubMed] [Google Scholar]
- Burkhard P, Stetfeld J, Strelkov SV. Coiled-coils: a highly versatile protein folding motif. Trends Cell Biol. 2001;11:82–87. doi: 10.1016/s0962-8924(00)01898-5. [DOI] [PubMed] [Google Scholar]
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- Christoforidis S, McBride H, Burgoyne R, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Coughlin SR. Thrombin signaling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Lysosomal membrane glycoproteins. J Biol Chem. 1991;266:21327–21330. [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin CT, Srinivasan Y, Zheng Y-W, Huang W, Coughlin SR. A role for thrombin receptor signaling in endothelial cells during embryonic development. Science. 2001;293:1666–1670. doi: 10.1126/science.1061259. [DOI] [PubMed] [Google Scholar]
- Haft CR, Sierra M, Bafford R, Lesniak MA, Barr VA, Taylor SI. Human orthologs of yeast vacuolar protein sorting proteins Vps26,29, and 35. Assembly into multimeric complexes. Mol Biol Cell. 2000;11:4105–4116. doi: 10.1091/mbc.11.12.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft CR, Sierra M, Barr VA, Haft DH, Taylor SI. Identification of a family of sorting nexin molecules and characterization of their association with receptors. Mol Cell Biol. 1998;18:7278–7287. doi: 10.1128/mcb.18.12.7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RA, Premont RT, Chow C-W, Blitzer JT, Pitcher JA, Claing A, Stoffel RH, Barak LS, Shenolikar S, Weinman EJ, Grinstein S, Lefkowitz RJ. The β2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- Hein L, Ishii K, Coughlin SR, Kobilka BK. Intracellular targeting and trafficking of thrombin receptors: a novel mechanism for resensitization of a G protein-coupled receptor. J Biol Chem. 1994;269:27719–27726. [PubMed] [Google Scholar]
- Horazdovsky BF, Davies BA, Seaman MNJ, McLaughin SA, Yoon S-H, Emr SD. A sorting nexin-1 homologue, Vps5p, forms a complex with Vps17p and is required for recycling the vacuolar protein-sorting receptor. Mol Biol Cell. 1997;8:1529–1541. doi: 10.1091/mbc.8.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung DT, Vu TK, Wheaton VI, Ishii K, Coughlin SR. Cloned platelet thrombin receptor is necessary for thrombin-induced platelet activation. J Clin Invest. 1992;89:1350–1353. doi: 10.1172/JCI115721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Hein L, Kobilka B, Coughlin SR. Kinetics of thrombin receptor cleavage on intact cells. Relation to signaling. J Biol Chem. 1993;268:9780–9786. [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Kurten RC, Cadena DL, Gill GN. Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science. 1996;272:1008–1010. doi: 10.1126/science.272.5264.1008. [DOI] [PubMed] [Google Scholar]
- Kurten RC, Eddington AD, Chowdhury P, Smith RD, Davidson AD, Shank BB. Self-assembly and binding of a sorting nexin to sorting endosomes. J Cell Sci. 2001;114:1743–1756. doi: 10.1242/jcs.114.9.1743. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Pitcher J, Krueger K, Daaka Y. Mechanisms of β-adrenergic receptor desensitization and resensitization. Adv Pharmacol. 1998;42:416–420. doi: 10.1016/s1054-3589(08)60777-2. [DOI] [PubMed] [Google Scholar]
- Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509–45512. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- Nothwehr SF, Ha S-A, Bruinsma P. Sorting of yeast membrane proteins into an endosome-to-Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J Cell Biol. 2000;151:297–309. doi: 10.1083/jcb.151.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr SF, Hindes AE. The yeast VPS5/GRD2 gene encodes a sorting nexin-1 like protein required for localizing membrane proteins to the late Golgi. J Cell Sci. 1997;110:1063–1072. doi: 10.1242/jcs.110.9.1063. [DOI] [PubMed] [Google Scholar]
- Paing MM, Stutts AB, Kohout TA, Lefkowitz RJ, Trejo J. β-Arrestins regulate protease-activated receptor-1 desensitization but not internalization or down-regulation. J Biol Chem. 2002;277:1292–1300. doi: 10.1074/jbc.M109160200. [DOI] [PubMed] [Google Scholar]
- Sarkadi B, Bauzon D, Huckle WR, Earp SH, Berry A, Suchindran H, Price EM, Olsen JC, Boucher RC, Scarborough GA. Biochemical characterization of the cystic fibrosis transmembrane conductance regulator in normal and cystic fibrosis epithelial cells. J Biol Chem. 1992;267:2087–2095. [PubMed] [Google Scholar]
- Scarborough RM, Naughton MA, Teng W, Hung DT, Rose J, Vu TK, Wheaton VI, Turck CW, Coughlin SR. Tethered ligand agonist peptides. Structural requirements for thrombin receptor activation reveal mechanism of proteolytic unmasking of agonist function. J Biol Chem. 1992;267:13146–13149. [PubMed] [Google Scholar]
- Seaman MNJ, McCaffery JM, Emr SD. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol. 1998;142:665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MJ, Trejo J, Zeng D, Coughlin SR. Role of the thrombin receptor's cytoplasmic tail in intracellular trafficking. Distinct determinants for agonist-triggered versus tonic internalization and intracellular localization. J Biol Chem. 1996;271:32874–32880. doi: 10.1074/jbc.271.51.32874. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- Sromek SM, Harden TK. Agonist-induced internalization of the P2Y2 receptor. Mol Pharmacol. 1998;54:485–494. doi: 10.1124/mol.54.3.485. [DOI] [PubMed] [Google Scholar]
- Teasdale RD, Loci D, Houghton F, Karlsson L, Gleeson PA. A large family of endosome-localized proteins related to sorting nexin 1. Biochem J. 2001;358:7–16. doi: 10.1042/0264-6021:3580007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo J, Altschuler Y, Fu H-W, Mostov KE, Coughlin SR. Protease-activated receptor-1 down-regulation: a mutant HeLa cell line suggests novel requirements for PAR1 phosphorylation and recruitment to clathrin-coated pits. J Biol Chem. 2000;275:31255–31265. doi: 10.1074/jbc.M003770200. [DOI] [PubMed] [Google Scholar]
- Trejo J, Coughlin SR. The cytoplasmic tails of protease-activated receptor-1 and substance P receptor specify sorting to lysosomes versus recycling. J Biol Chem. 1999;274:2216–2224. doi: 10.1074/jbc.274.4.2216. [DOI] [PubMed] [Google Scholar]
- Trejo J, Hammes SR, Coughlin SR. Termination of signaling by protease-activated receptor-1 is linked to lysosomal sorting. Proc Natl Acad Sci USA. 1998;95:13698–13702. doi: 10.1073/pnas.95.23.13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassallo RJ, Kieber ET, Cichowski K, Brass LF. Structure-function relationships in the activation of platelet thrombin receptors by receptor-derived peptides. J Biol Chem. 1992;267:6081–6085. [PubMed] [Google Scholar]
- Vu T-KH, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991a;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Vu T-KH, Wheaton VI, Hung DT, Coughlin SR. Domains specifying thrombin-receptor interaction. Nature. 1991b;353:674–677. doi: 10.1038/353674a0. [DOI] [PubMed] [Google Scholar]
- Woolkalis MJ, DeMelfi TJ, Blanchard N, Hoxie JA, Brass LF. Regulation of thrombin receptors on human umbilical vein endothelial cells. J Biol Chem. 1995;270:9868–9875. doi: 10.1074/jbc.270.17.9868. [DOI] [PubMed] [Google Scholar]
- Wu JW, Lemmon MA. All phox homology (PX) domains from Saccharomyces cerevisiae specifically recognize phosphatidylinositol 3-phosphate. J Biol Chem. 2001;276:44179–44184. doi: 10.1074/jbc.M108811200. [DOI] [PubMed] [Google Scholar]