Abstract
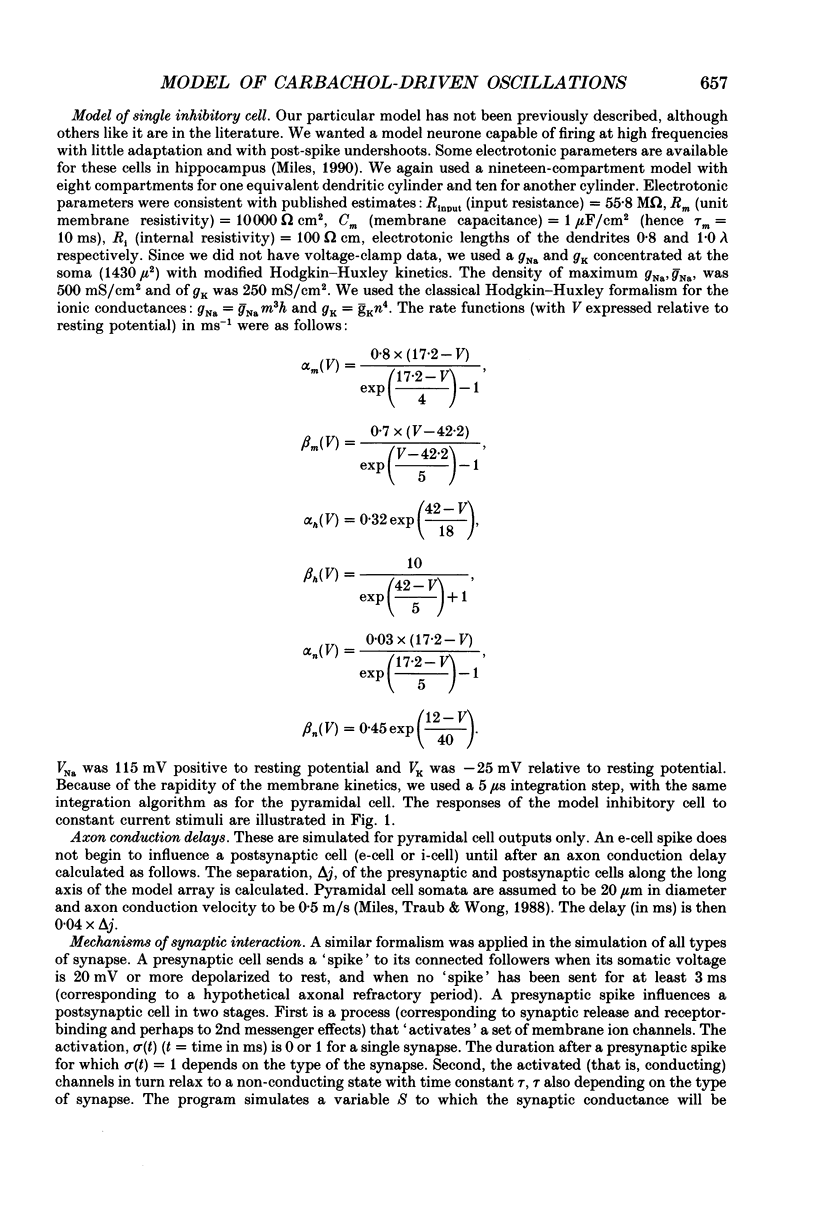
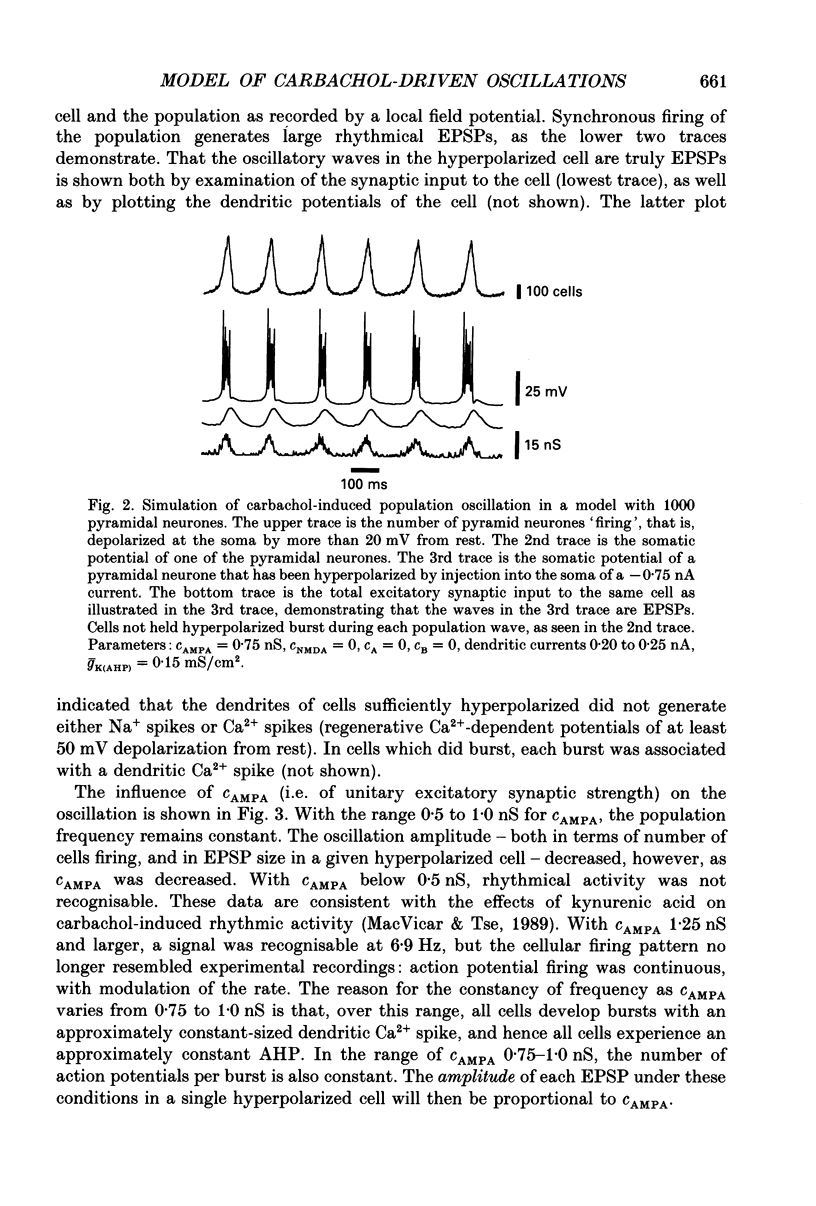
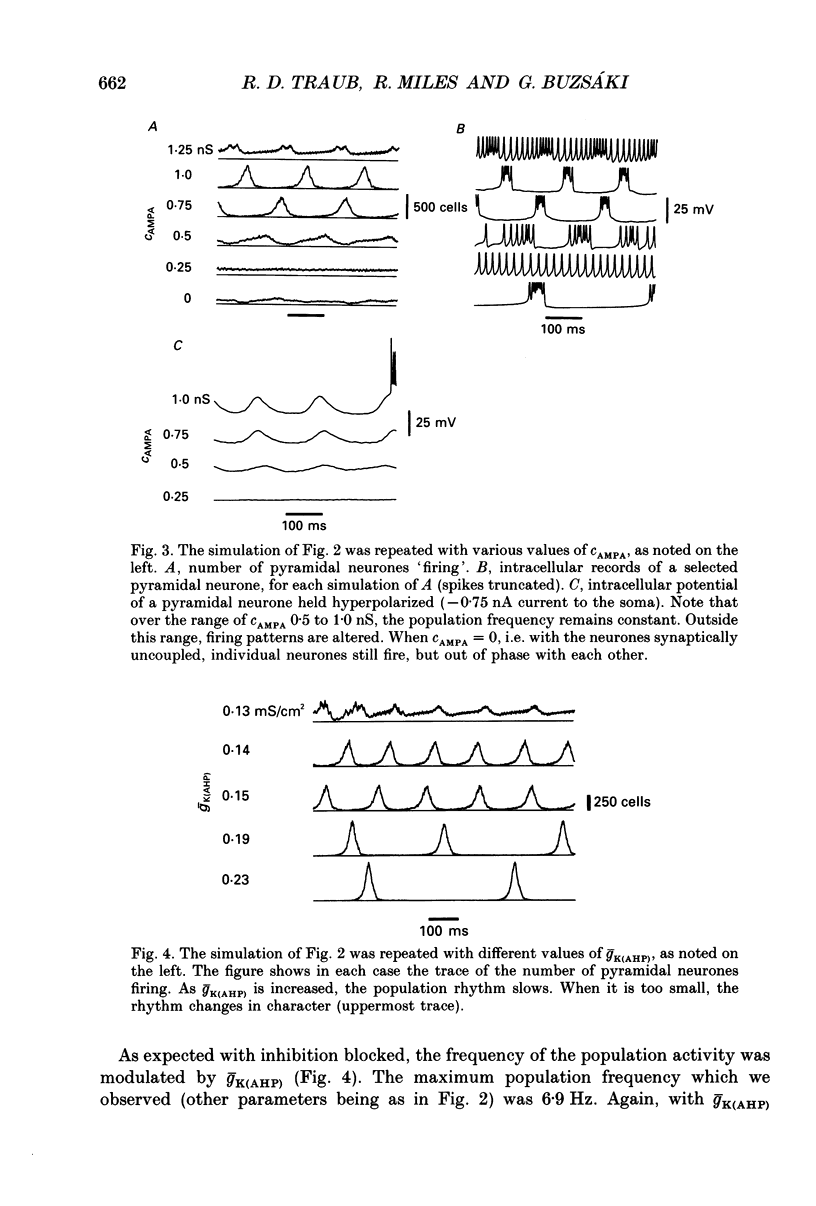
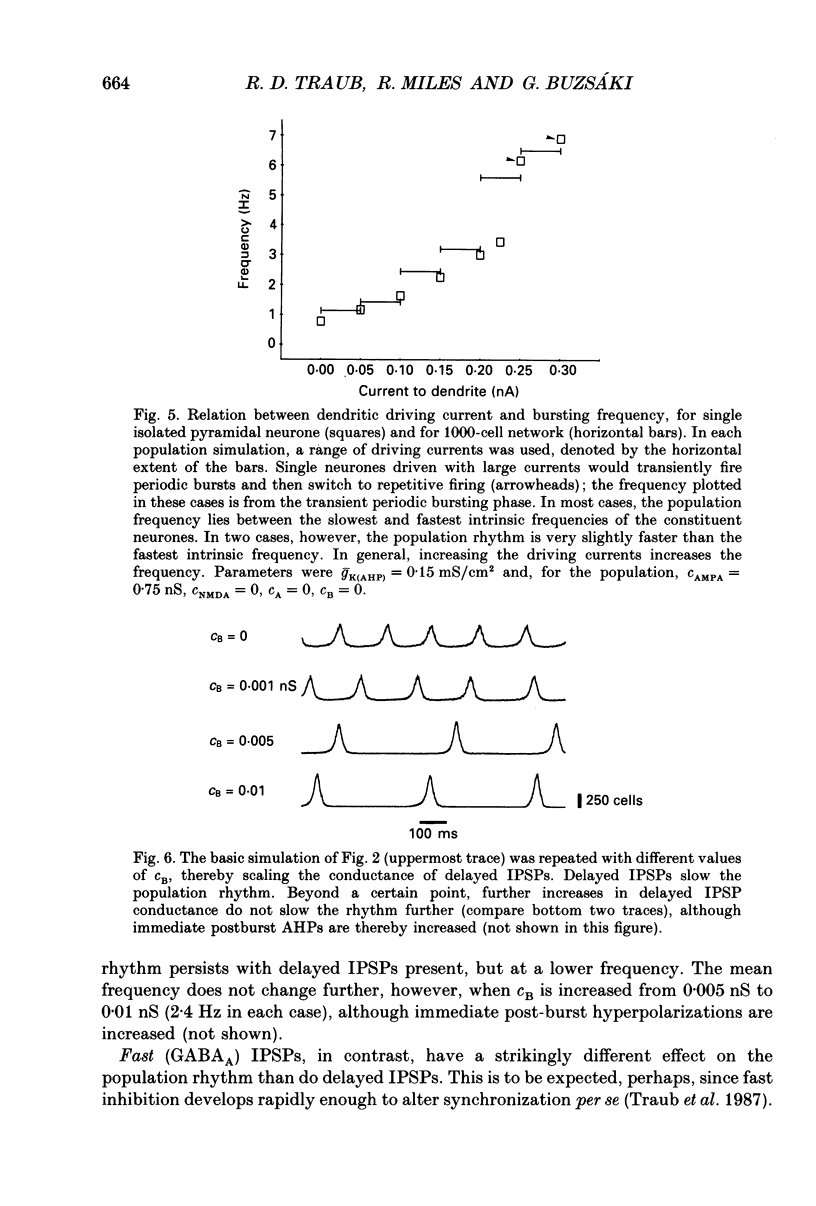
1. We used simulations of the in vitro CA3 region of the hippocampus to analyse the 5 Hz population oscillations recorded experimentally in carbachol. 2. A simulation model of the in vitro CA3 region was constructed with 1000 pyramidal neurones and 200 inhibitory neurones (100 producing fast inhibitory postsynaptic potentials (IPSPs) and 100 producing slow IPSPs of delayed onset). Each neurone contained nineteen soma-dendritic compartments. Pyramidal neurones contained six voltage- and/or calcium-dependent ionic currents, whose kinetics were consistent with voltage-clamp data. The connectivity and waveform of unitary synaptic events for excitatory and fast inhibitory synapses were consistent with dual intracellular recordings. This network was shown to generate previously described network oscillations, including synchronized bursts recorded in the presence of GABAA blockers, and synchronized synaptic potentials observed during partial blockade of GABAA inhibition. 3. The model generated 5 Hz oscillations as recorded in carbachol under the following conditions: (a) excitatory synaptic conductance was within a limited range; (b) there was blockade of fast and slow IPSPs (consistent with the experimental lack of effect of bicuculline and phaclofen on carbachol oscillations and the known depression of IPSPs by acetylcholine); (c) the after hyperpolarization (AHP) conductance was reduced (consistent with the known pharmacology of carbachol); (d) the apical dendrites of the pyramidal cells were depolarized, as suggested by the carbachol-induced depolarization of pyramidal neurones. Each oscillation was associated in pyramidal cells with a burst of action potentials riding on a depolarizing wave. The N-methyl-D-aspartate (NMDA) type of excitatory synapse was not necessary for the oscillations to occur. 4. Progressive reduction of excitatory synaptic strength led to an oscillation of the same frequency, with bursts riding on smaller EPSPs (consistent with the experiment). Further reduction of excitatory synaptic strength abolished the population oscillation by uncoupling the neurones. When excitatory synaptic conductance was too large, population oscillations were attenuated as the cells switched from a bursting mode to a repetitively firing mode. 5. Increasing the AHP conductance prolonged the interburst interval as expected. Inclusion of slow IPSPs exerted a similar effect. 6. When fast IPSPs were included, an oscillation with different characteristics emerged: a 10 Hz oscillation that was gated by compound GABAA IPSPs. On any oscillatory wave, few pyramidal neurones fired, and the firing of individual neurones was irregular.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Williamson A. A transient calcium-dependent potassium component of the epileptiform burst after-hyperpolarization in rat hippocampus. J Physiol. 1988 May;399:191–205. doi: 10.1113/jphysiol.1988.sp017075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban J. M., Snyder S. H., Alger B. E. Protein kinase C regulates ionic conductance in hippocampal pyramidal neurons: electrophysiological effects of phorbol esters. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2538–2542. doi: 10.1073/pnas.82.8.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benardo L. S., Prince D. A. Cholinergic excitation of mammalian hippocampal pyramidal cells. Brain Res. 1982 Oct 14;249(2):315–331. doi: 10.1016/0006-8993(82)90066-x. [DOI] [PubMed] [Google Scholar]
- Benardo L. S., Prince D. A. Ionic mechanisms of cholinergic excitation in mammalian hippocampal pyramidal cells. Brain Res. 1982 Oct 14;249(2):333–344. doi: 10.1016/0006-8993(82)90067-1. [DOI] [PubMed] [Google Scholar]
- Benson D. M., Blitzer R. D., Landau E. M. An analysis of the depolarization produced in guinea-pig hippocampus by cholinergic receptor stimulation. J Physiol. 1988 Oct;404:479–496. doi: 10.1113/jphysiol.1988.sp017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland B. H., Andersen P., Ganes T., Sveen O. Automated analysis of rhythmicity of physiologically identified hippocampal formation neurons. Exp Brain Res. 1980 Jan;38(2):205–219. doi: 10.1007/BF00236742. [DOI] [PubMed] [Google Scholar]
- Buzsàki G., Eidelberg E. Phase relations of hippocampal projection cells and interneurons to theta activity in the anesthetized rat. Brain Res. 1983 May 5;266(2):334–339. doi: 10.1016/0006-8993(83)90665-0. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Leung L. W., Vanderwolf C. H. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983 Oct;287(2):139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Chamberlin N. L., Traub R. D., Dingledine R. Role of EPSPs in initiation of spontaneous synchronized burst firing in rat hippocampal neurons bathed in high potassium. J Neurophysiol. 1990 Sep;64(3):1000–1008. doi: 10.1152/jn.1990.64.3.1000. [DOI] [PubMed] [Google Scholar]
- Charpak S., Gähwiler B. H., Do K. Q., Knöpfel T. Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature. 1990 Oct 25;347(6295):765–767. doi: 10.1038/347765a0. [DOI] [PubMed] [Google Scholar]
- Christian E. P., Dudek F. E. Electrophysiological evidence from glutamate microapplications for local excitatory circuits in the CA1 area of rat hippocampal slices. J Neurophysiol. 1988 Jan;59(1):110–123. doi: 10.1152/jn.1988.59.1.110. [DOI] [PubMed] [Google Scholar]
- Cole A. E., Nicoll R. A. Characterization of a slow cholinergic post-synaptic potential recorded in vitro from rat hippocampal pyramidal cells. J Physiol. 1984 Jul;352:173–188. doi: 10.1113/jphysiol.1984.sp015285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S. E. Membrane potential and impedance changes in hippocampal pyramidal cells during theta rhythm. Exp Brain Res. 1989;77(2):283–294. doi: 10.1007/BF00274985. [DOI] [PubMed] [Google Scholar]
- Fox S. E., Wolfson S., Ranck J. B., Jr Hippocampal theta rhythm and the firing of neurons in walking and urethane anesthetized rats. Exp Brain Res. 1986;62(3):495–508. doi: 10.1007/BF00236028. [DOI] [PubMed] [Google Scholar]
- Freund T. F., Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988 Nov 10;336(6195):170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Gulyás A. I., Seress L., Tóth K., Acsády L., Antal M., Freund T. F. Septal GABAergic neurons innervate inhibitory interneurons in the hippocampus of the macaque monkey. Neuroscience. 1991;41(2-3):381–390. doi: 10.1016/0306-4522(91)90334-k. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. Muscarine affects calcium-currents in rat hippocampal pyramidal cells in vitro. Neurosci Lett. 1987 May 19;76(3):301–306. doi: 10.1016/0304-3940(87)90419-8. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Dreifuss J. J. Multiple actions of acetylcholine on hippocampal pyramidal cells in organotypic explant cultures. Neuroscience. 1982 May;7(5):1243–1256. doi: 10.1016/0306-4522(82)91131-9. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V. Cholinergic responses in human neocortical neurones. EXS. 1989;57:138–149. doi: 10.1007/978-3-0348-9138-7_14. [DOI] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980 Feb;43(2):409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Kay A. R., Wong R. K. Calcium current activation kinetics in isolated pyramidal neurones of the Ca1 region of the mature guinea-pig hippocampus. J Physiol. 1987 Nov;392:603–616. doi: 10.1113/jphysiol.1987.sp016799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöpfel T., Vranesic I., Gähwiler B. H., Brown D. A. Muscarinic and beta-adrenergic depression of the slow Ca2(+)-activated potassium conductance in hippocampal CA3 pyramidal cells is not mediated by a reduction of depolarization-induced cytosolic Ca2+ transients. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4083–4087. doi: 10.1073/pnas.87.11.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopacki J., Bland B. H., Roth S. H. Evidence that activation of in vitro hippocampal theta rhythm only involves muscarinic receptors. Brain Res. 1988 Jul 5;455(1):110–114. doi: 10.1016/0006-8993(88)90119-9. [DOI] [PubMed] [Google Scholar]
- Konopacki J., MacIver M. B., Bland B. H., Roth S. H. Carbachol-induced EEG 'theta' activity in hippocampal brain slices. Brain Res. 1987 Mar 3;405(1):196–198. doi: 10.1016/0006-8993(87)91009-2. [DOI] [PubMed] [Google Scholar]
- Kramis R., Vanderwolf C. H., Bland B. H. Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: relations to behavior and effects of atropine, diethyl ether, urethane, and pentobarbital. Exp Neurol. 1975 Oct;49(1 Pt 1):58–85. doi: 10.1016/0014-4886(75)90195-8. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Nicoll R. A. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol. 1987 Aug;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeda F. J., Hablitz J. J., Johnston D. Antagonism of GABA-mediated responses by d-tubocurarine in hippocampal neurons. J Neurophysiol. 1982 Sep;48(3):622–632. doi: 10.1152/jn.1982.48.3.622. [DOI] [PubMed] [Google Scholar]
- Leung L. S., Yim C. Y. Intracellular records of theta rhythm in hippocampal CA1 cells of the rat. Brain Res. 1986 Mar 5;367(1-2):323–327. doi: 10.1016/0006-8993(86)91611-2. [DOI] [PubMed] [Google Scholar]
- Léránth C., Frotscher M. Cholinergic innervation of hippocampal GAD- and somatostatin-immunoreactive commissural neurons. J Comp Neurol. 1987 Jul 1;261(1):33–47. doi: 10.1002/cne.902610104. [DOI] [PubMed] [Google Scholar]
- MacVicar B. A., Tse F. W. Local neuronal circuitry underlying cholinergic rhythmical slow activity in CA3 area of rat hippocampal slices. J Physiol. 1989 Oct;417:197–212. doi: 10.1113/jphysiol.1989.sp017797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Lancaster B., Nicoll R. A. Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci. 1987 Mar;7(3):733–741. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H., Segal M. Long-lasting facilitation of excitatory postsynaptic potentials in the rat hippocampus by acetylcholine. J Physiol. 1990 Aug;427:381–393. doi: 10.1113/jphysiol.1990.sp018177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R. Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea-pig in vitro. J Physiol. 1990 Sep;428:61–77. doi: 10.1113/jphysiol.1990.sp018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Traub R. D., Wong R. K. Spread of synchronous firing in longitudinal slices from the CA3 region of the hippocampus. J Neurophysiol. 1988 Oct;60(4):1481–1496. doi: 10.1152/jn.1988.60.4.1481. [DOI] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Excitatory synaptic interactions between CA3 neurones in the guinea-pig hippocampus. J Physiol. 1986 Apr;373:397–418. doi: 10.1113/jphysiol.1986.sp016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Inhibitory control of local excitatory circuits in the guinea-pig hippocampus. J Physiol. 1987 Jul;388:611–629. doi: 10.1113/jphysiol.1987.sp016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Unitary inhibitory synaptic potentials in the guinea-pig hippocampus in vitro. J Physiol. 1984 Nov;356:97–113. doi: 10.1113/jphysiol.1984.sp015455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núez A., García-Austt E., Buño W., Jr Intracellular theta-rhythm generation in identified hippocampal pyramids. Brain Res. 1987 Jul 28;416(2):289–300. doi: 10.1016/0006-8993(87)90909-7. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Alger B. E. Single calcium channels in rat and guinea-pig hippocampal neurons. J Physiol. 1991 May;436:739–767. doi: 10.1113/jphysiol.1991.sp018577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman J. H. Low concentrations of penicillin reveal rhythmic, synchronous synaptic potentials in hippocampal slice. Brain Res. 1986 Nov 29;398(2):231–241. doi: 10.1016/0006-8993(86)91482-4. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Haglund M. M. Spontaneous rhythmic synchronous activity in epileptic human and normal monkey temporal lobe. Epilepsia. 1986 Sep-Oct;27(5):523–533. doi: 10.1111/j.1528-1157.1986.tb03578.x. [DOI] [PubMed] [Google Scholar]
- Stewart M., Fox S. E. Do septal neurons pace the hippocampal theta rhythm? Trends Neurosci. 1990 May;13(5):163–168. doi: 10.1016/0166-2236(90)90040-h. [DOI] [PubMed] [Google Scholar]
- Thompson L. T., Best P. J. Place cells and silent cells in the hippocampus of freely-behaving rats. J Neurosci. 1989 Jul;9(7):2382–2390. doi: 10.1523/JNEUROSCI.09-07-02382.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. M., Wong R. K. Development of calcium current subtypes in isolated rat hippocampal pyramidal cells. J Physiol. 1991 Aug;439:671–689. doi: 10.1113/jphysiol.1991.sp018687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub R. D., Dingledine R. Model of synchronized epileptiform bursts induced by high potassium in CA3 region of rat hippocampal slice. Role of spontaneous EPSPs in initiation. J Neurophysiol. 1990 Sep;64(3):1009–1018. doi: 10.1152/jn.1990.64.3.1009. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Miles R. Multiple modes of neuronal population activity emerge after modifying specific synapses in a model of the CA3 region of the hippocampus. Ann N Y Acad Sci. 1991;627:277–290. doi: 10.1111/j.1749-6632.1991.tb25931.x. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Miles R., Wong R. K. Model of the origin of rhythmic population oscillations in the hippocampal slice. Science. 1989 Mar 10;243(4896):1319–1325. doi: 10.1126/science.2646715. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Miles R., Wong R. K. Models of synchronized hippocampal bursts in the presence of inhibition. I. Single population events. J Neurophysiol. 1987 Oct;58(4):739–751. doi: 10.1152/jn.1987.58.4.739. [DOI] [PubMed] [Google Scholar]
- Traub R. D. Simulation of intrinsic bursting in CA3 hippocampal neurons. Neuroscience. 1982 May;7(5):1233–1242. doi: 10.1016/0306-4522(82)91130-7. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Wong R. K. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982 May 14;216(4547):745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Wong R. K., Miles R., Michelson H. A model of a CA3 hippocampal pyramidal neuron incorporating voltage-clamp data on intrinsic conductances. J Neurophysiol. 1991 Aug;66(2):635–650. doi: 10.1152/jn.1991.66.2.635. [DOI] [PubMed] [Google Scholar]
- Valentino R. J., Dingledine R. Presynaptic inhibitory effect of acetylcholine in the hippocampus. J Neurosci. 1981 Jul;1(7):784–792. doi: 10.1523/JNEUROSCI.01-07-00784.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A., Alger B. E. Characterization of an early afterhyperpolarization after a brief train of action potentials in rat hippocampal neurons in vitro. J Neurophysiol. 1990 Jan;63(1):72–81. doi: 10.1152/jn.1990.63.1.72. [DOI] [PubMed] [Google Scholar]
- Winson J. Patterns of hippocampal theta rhythm in the freely moving rat. Electroencephalogr Clin Neurophysiol. 1974 Mar;36(3):291–301. doi: 10.1016/0013-4694(74)90171-0. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Prince D. A. Afterpotential generation in hippocampal pyramidal cells. J Neurophysiol. 1981 Jan;45(1):86–97. doi: 10.1152/jn.1981.45.1.86. [DOI] [PubMed] [Google Scholar]


