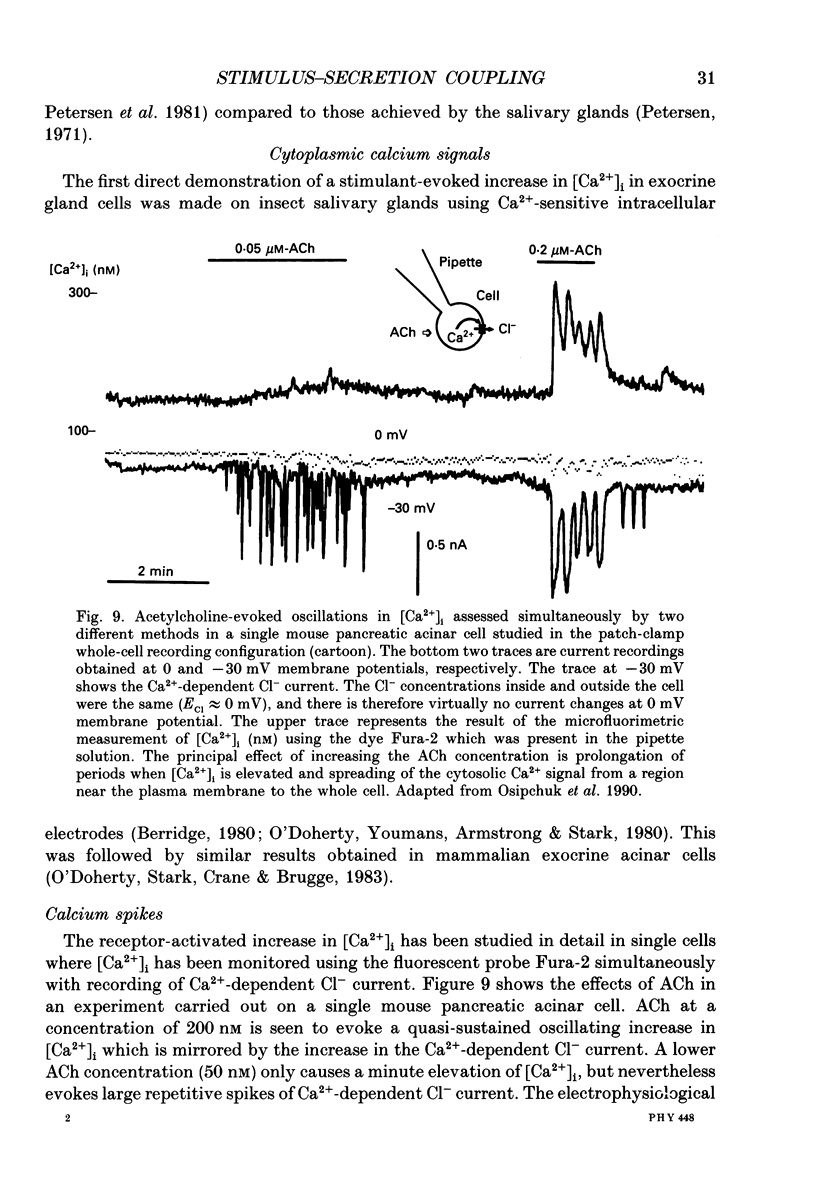
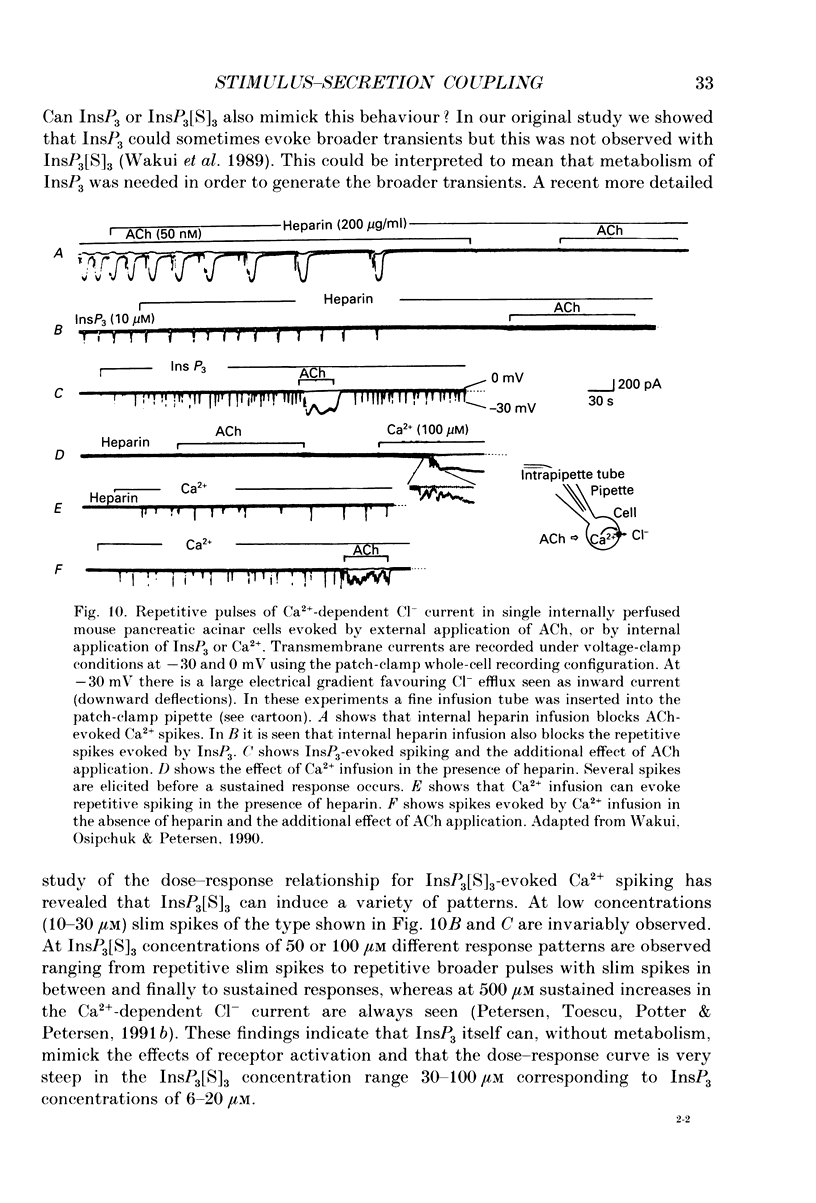
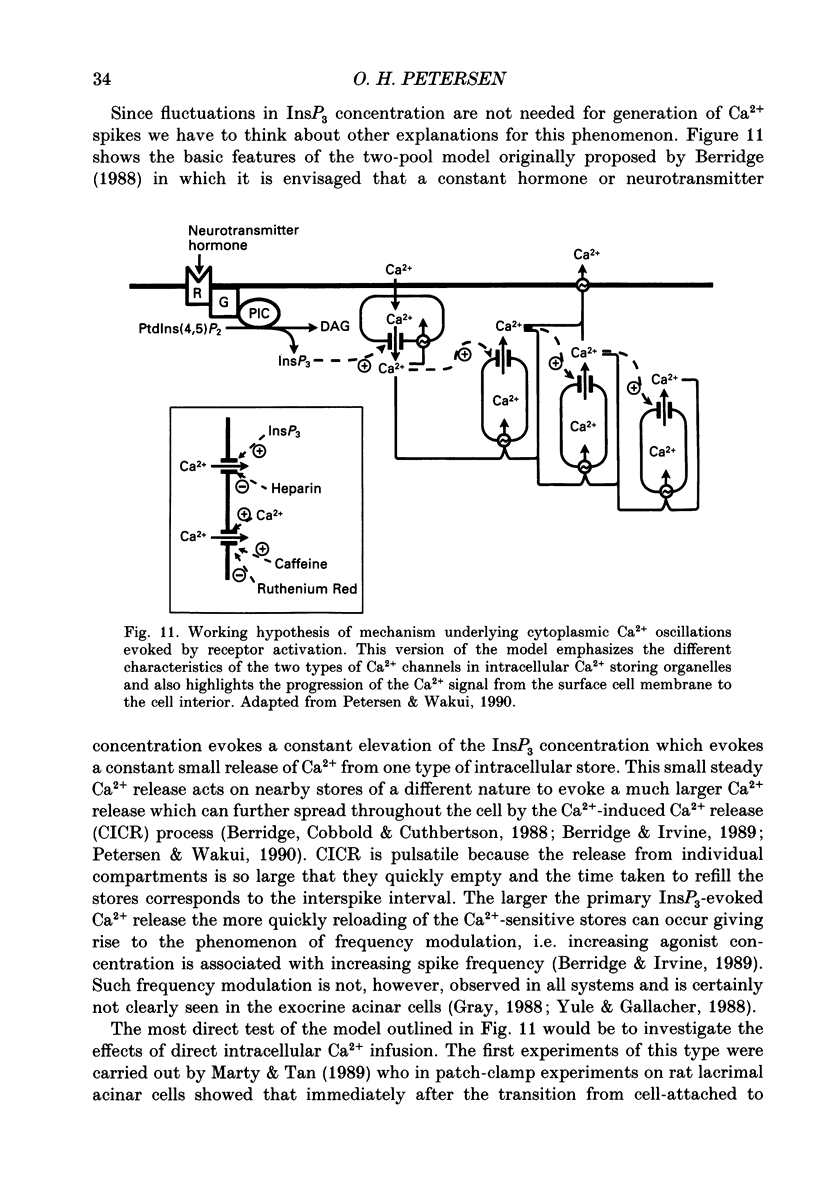
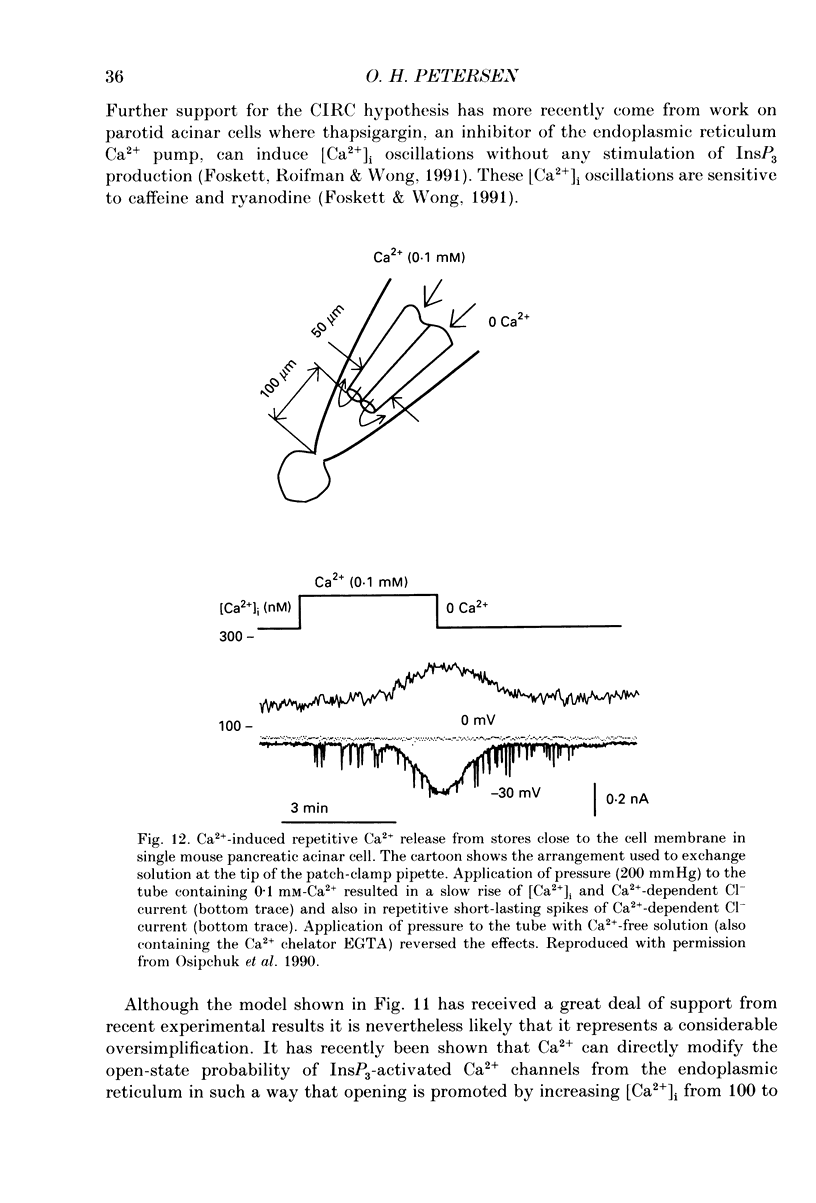
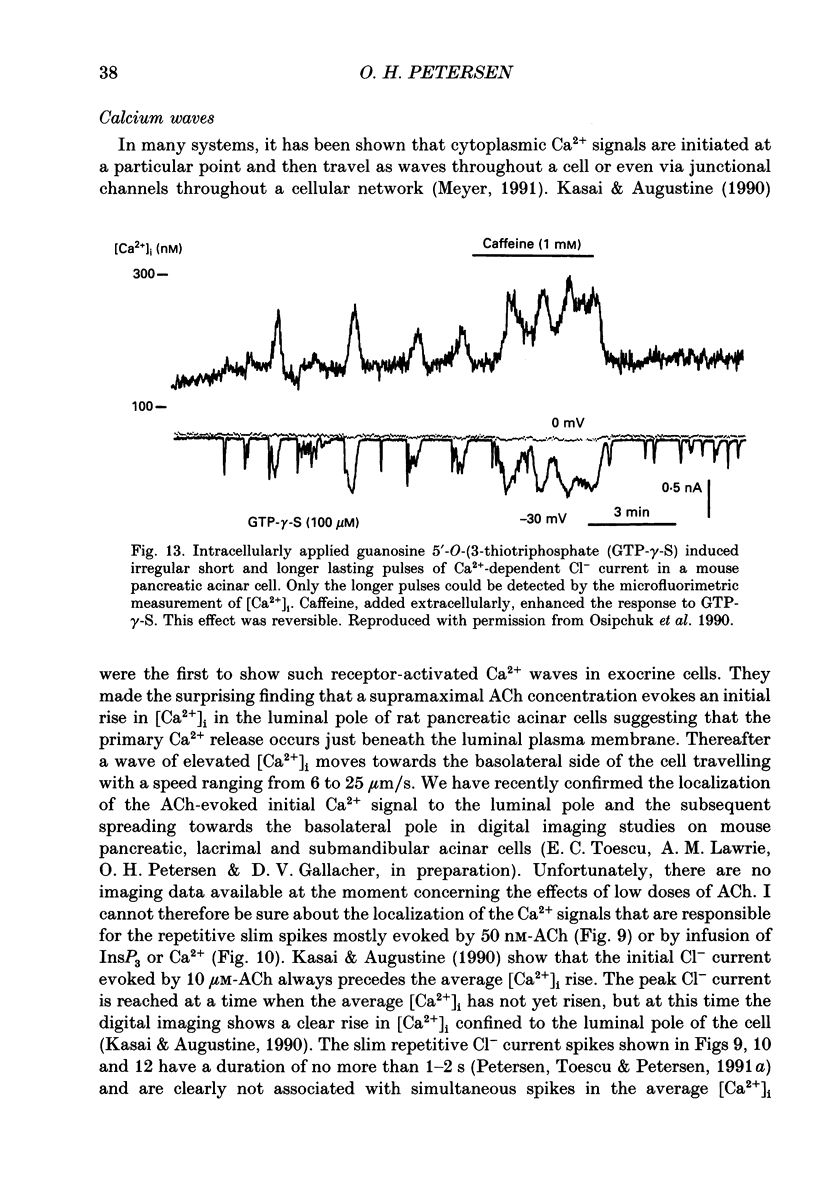
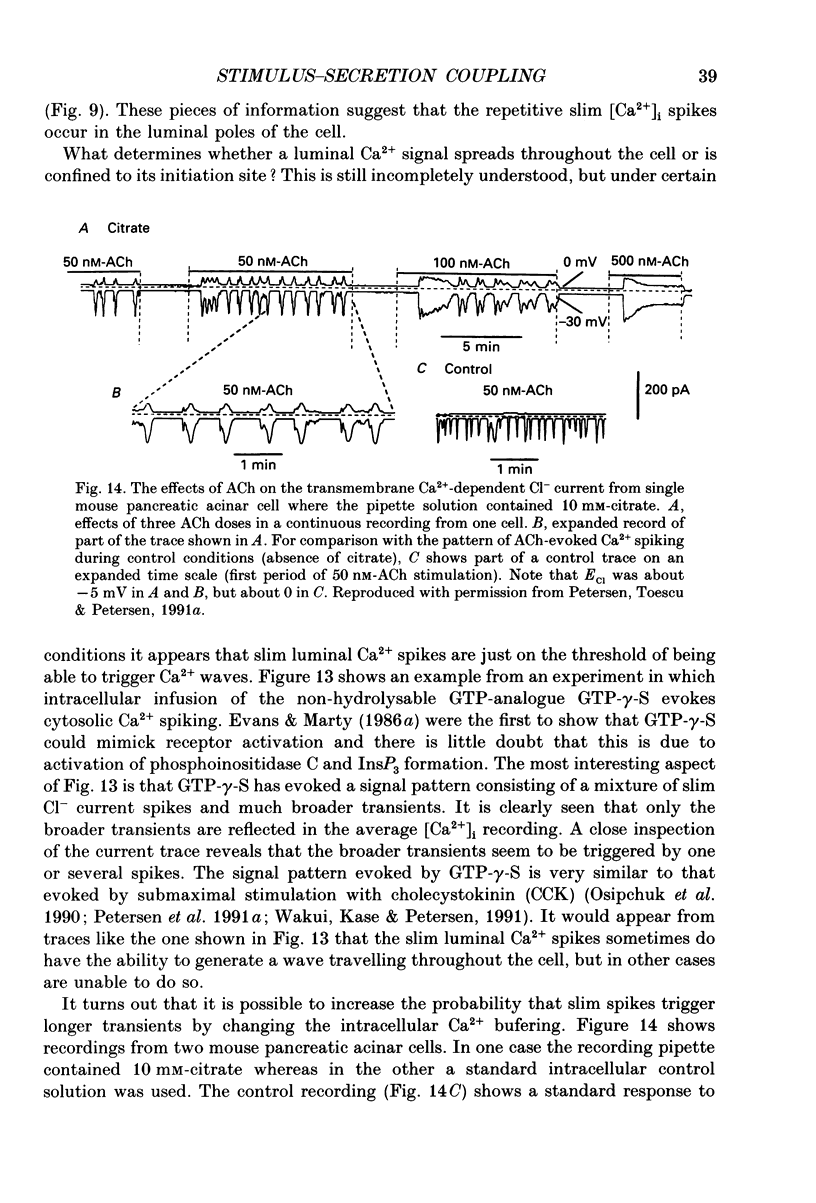
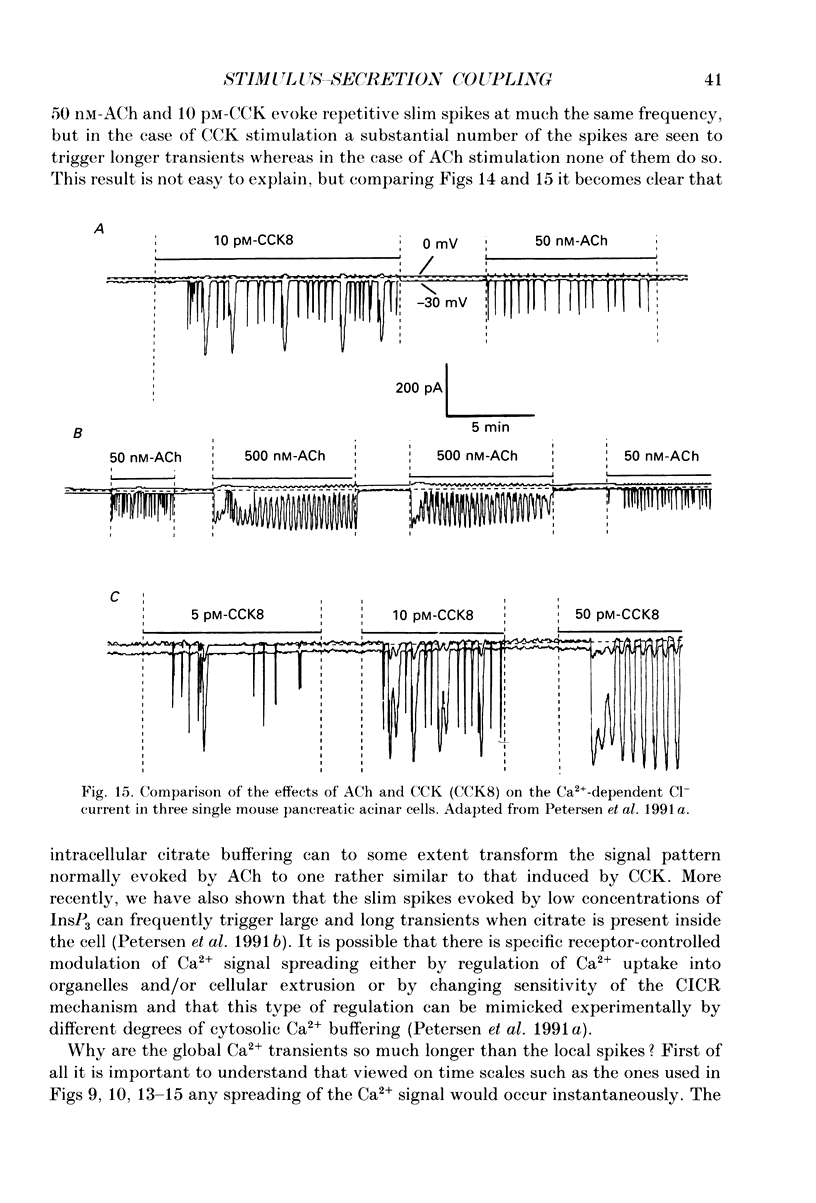
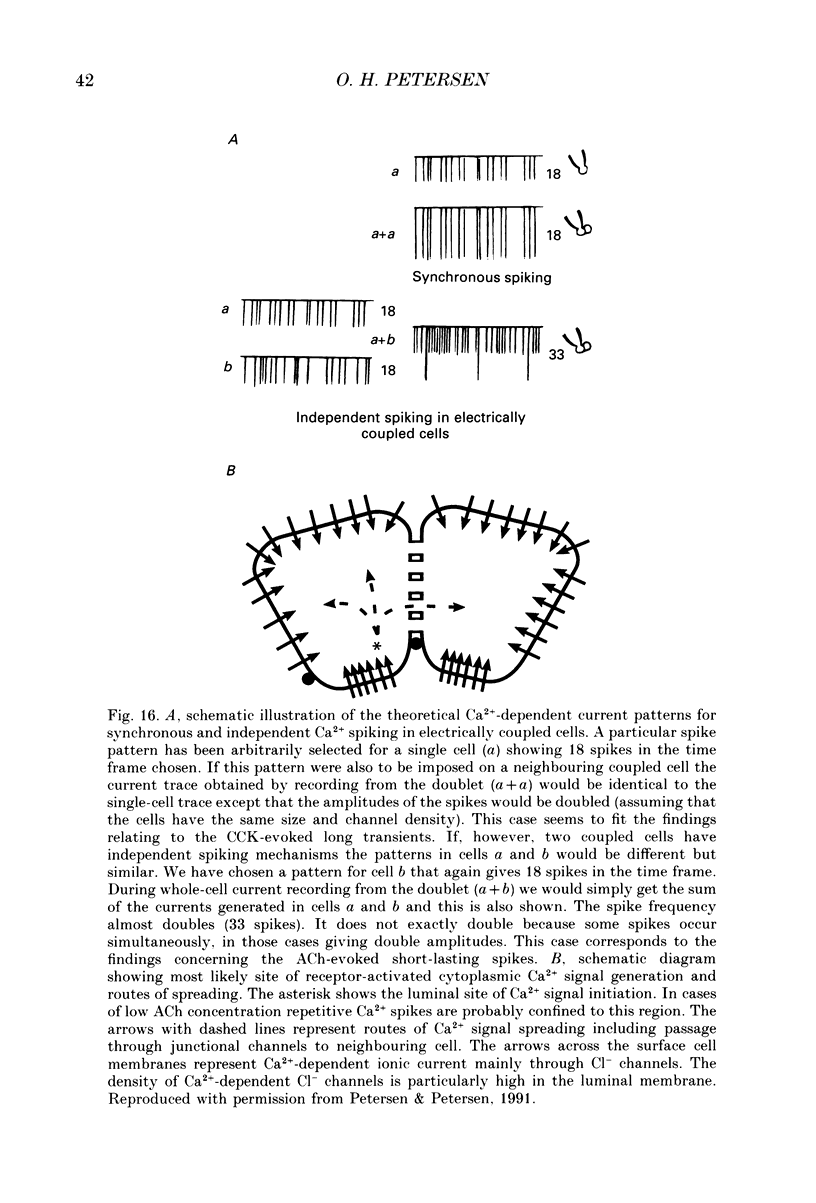
Full text
PDF



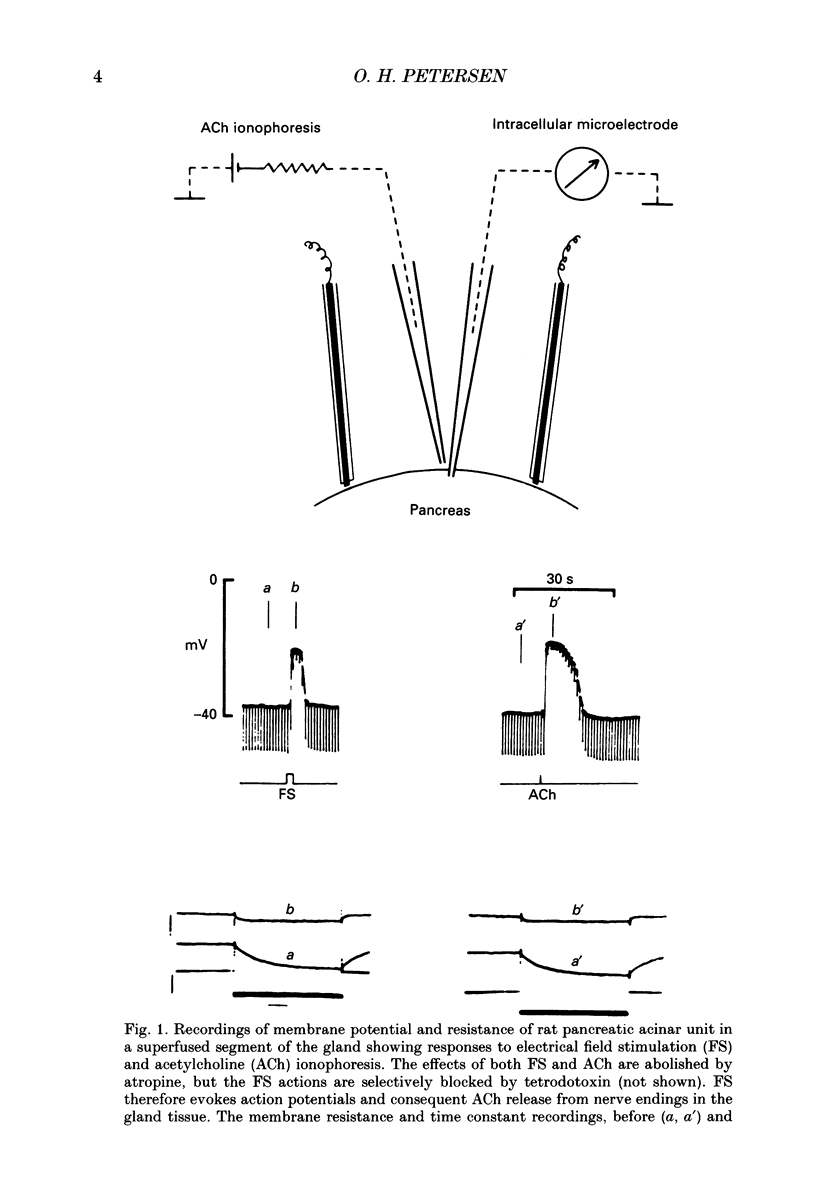

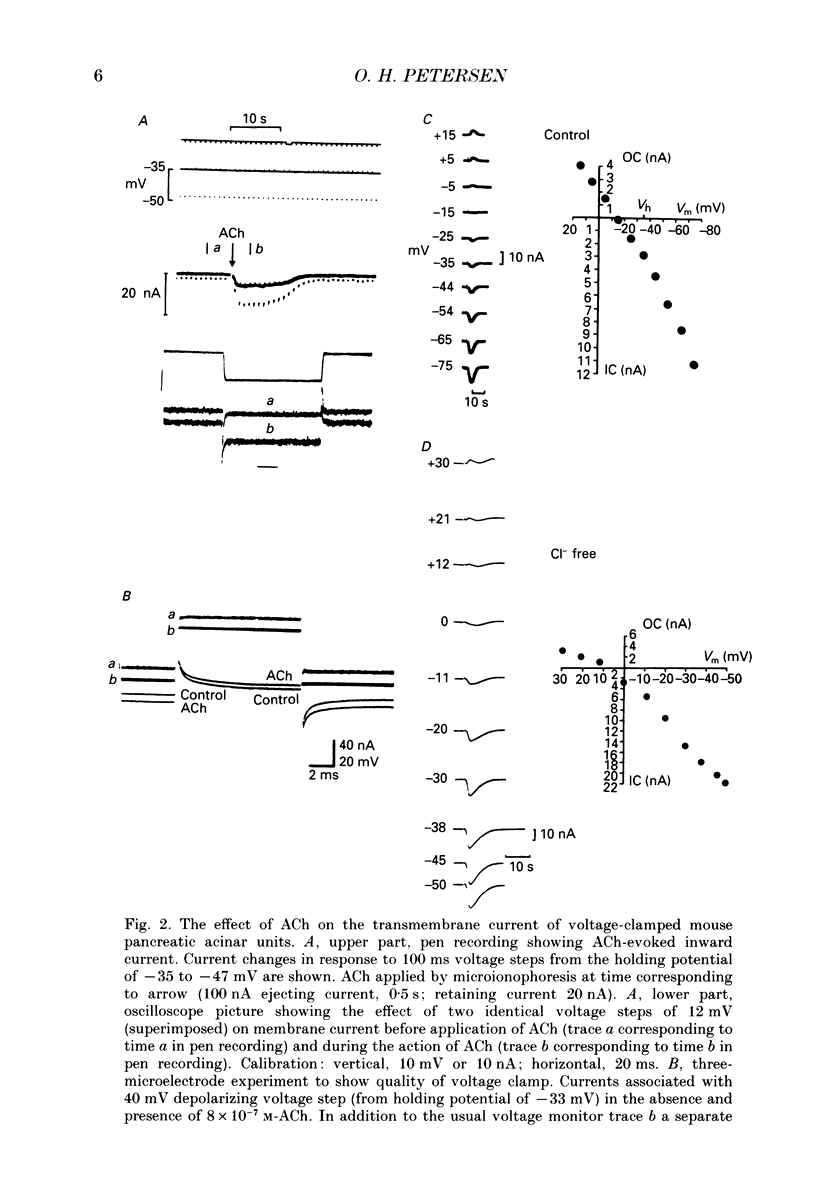



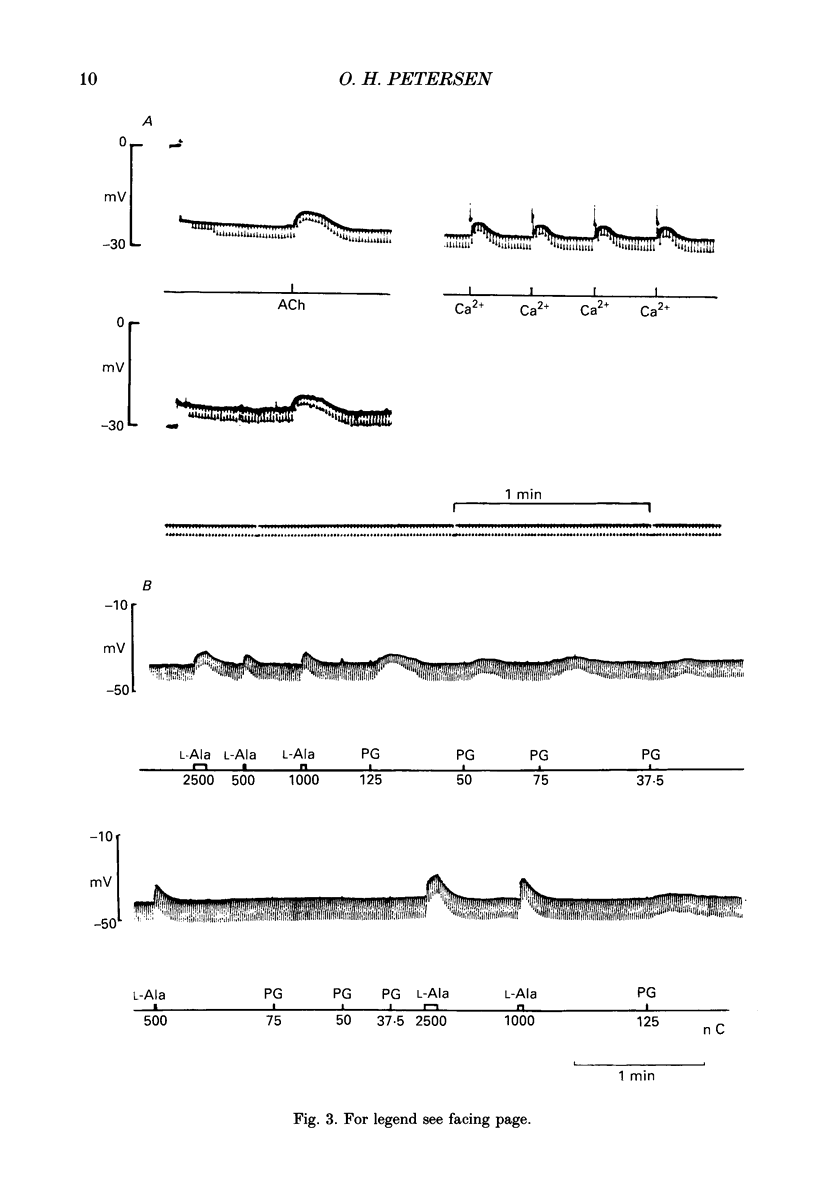







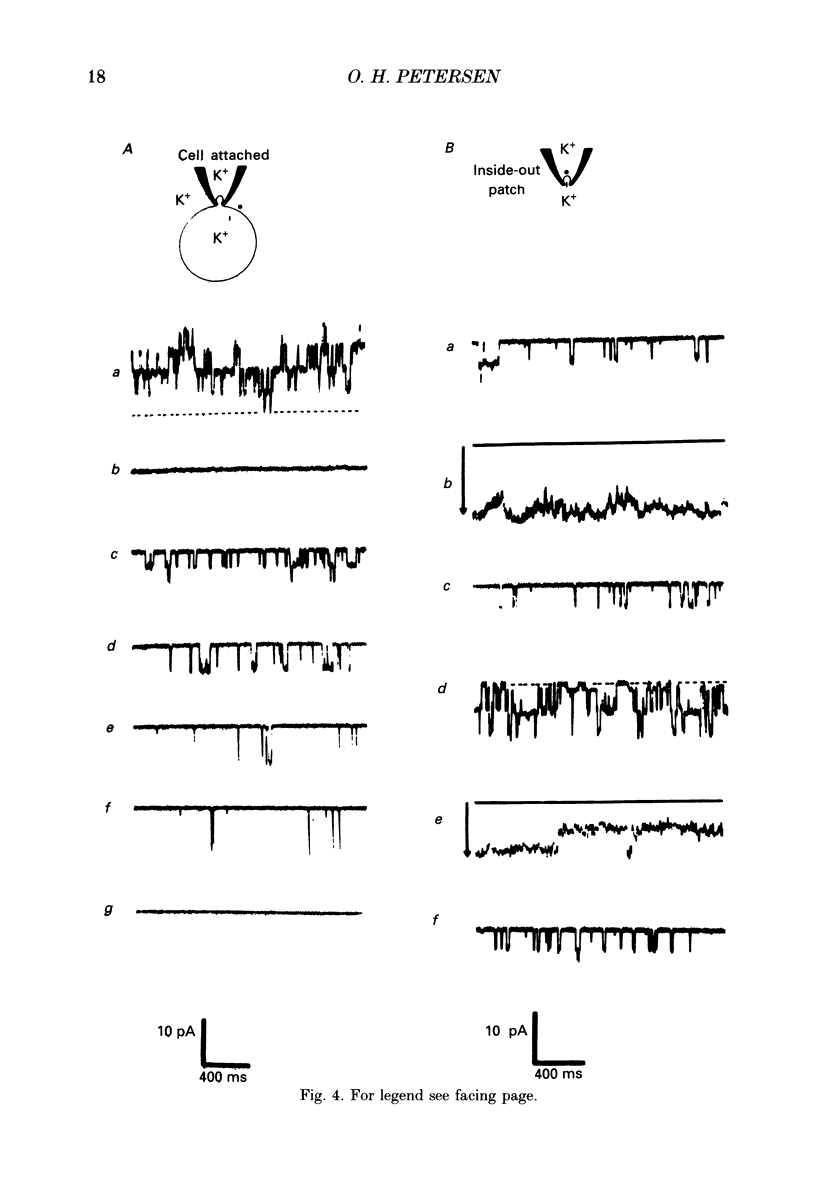

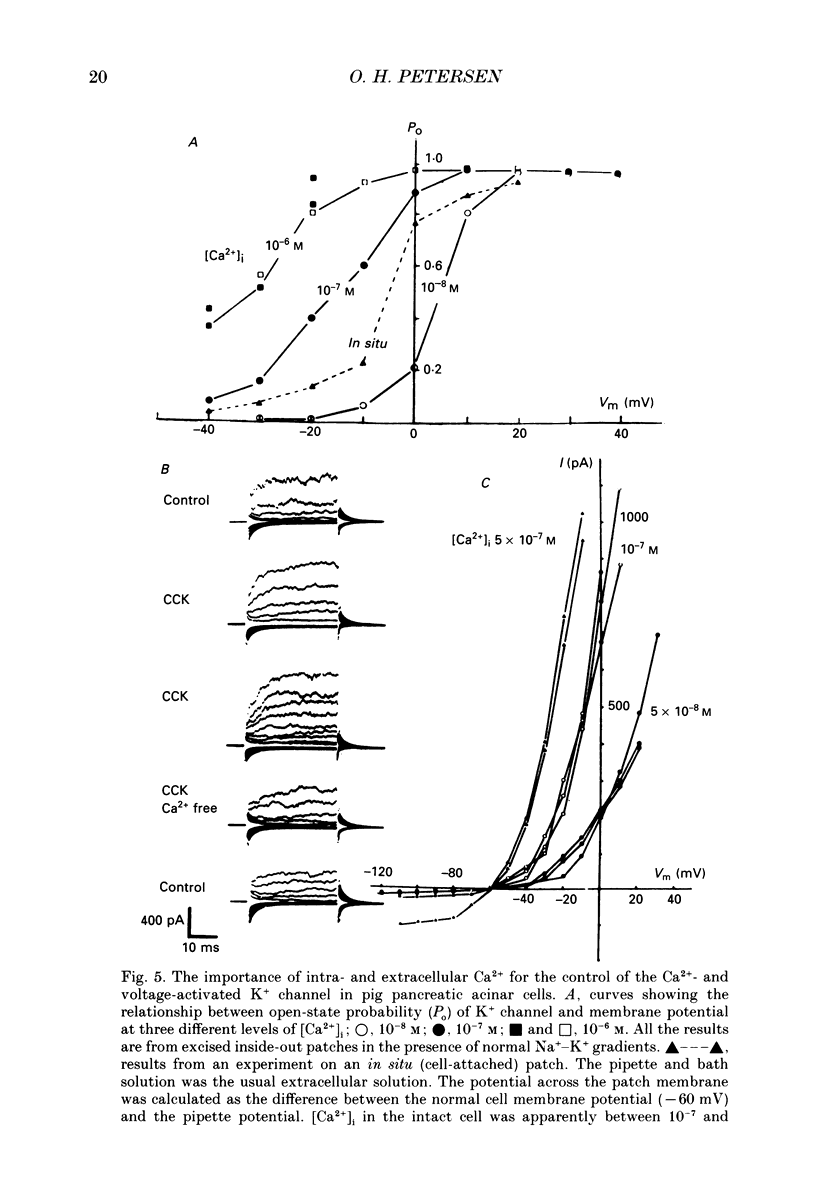

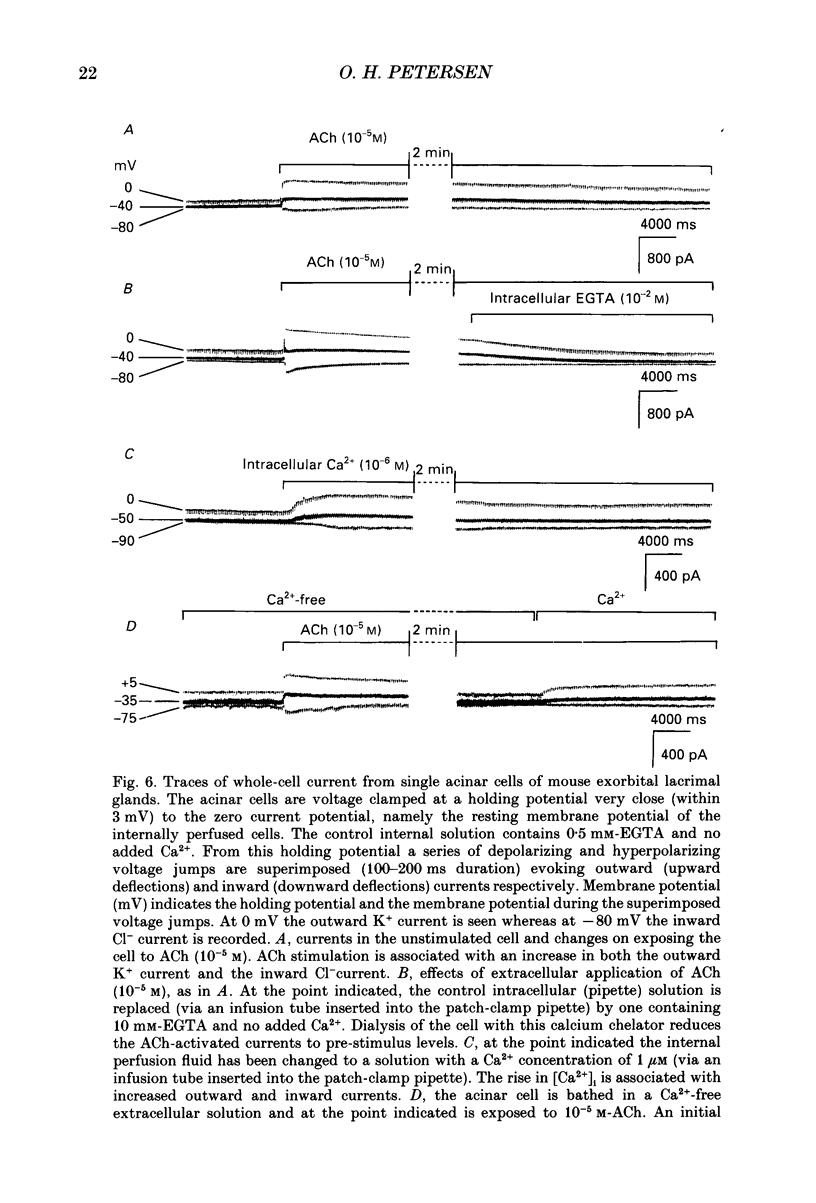




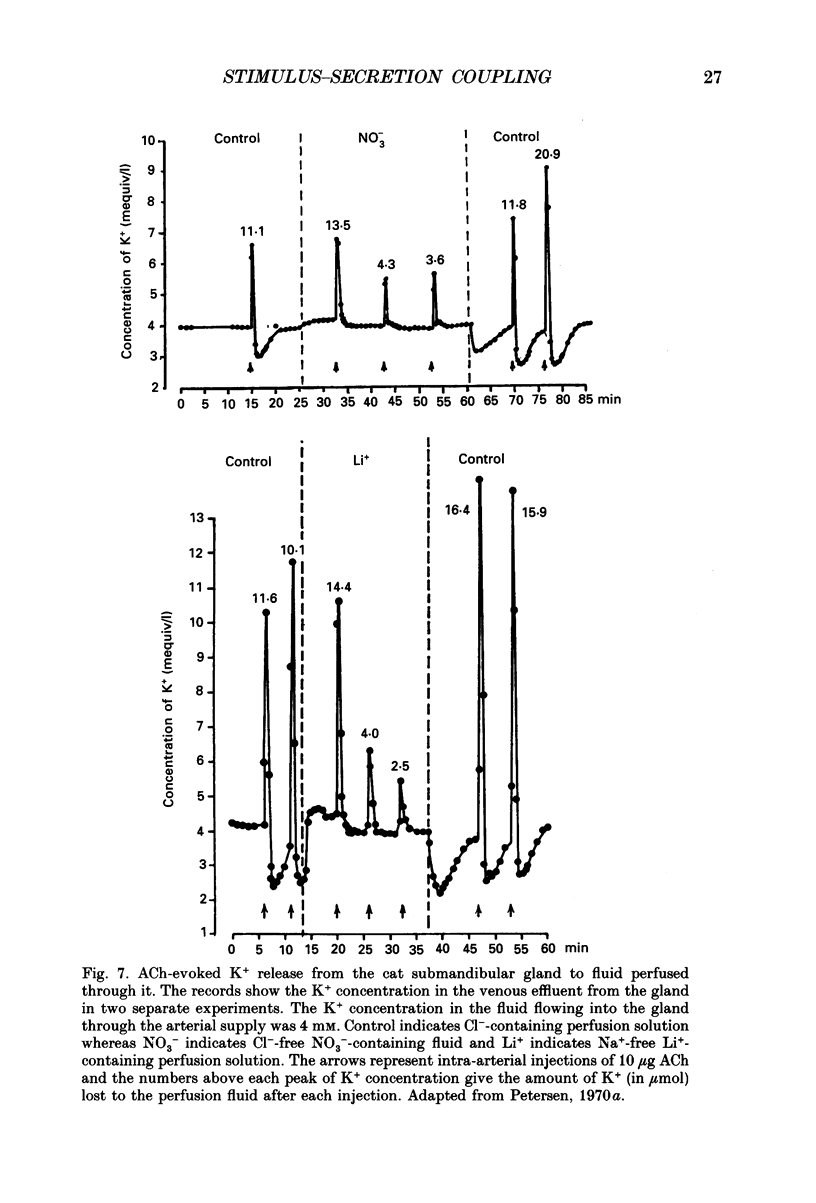
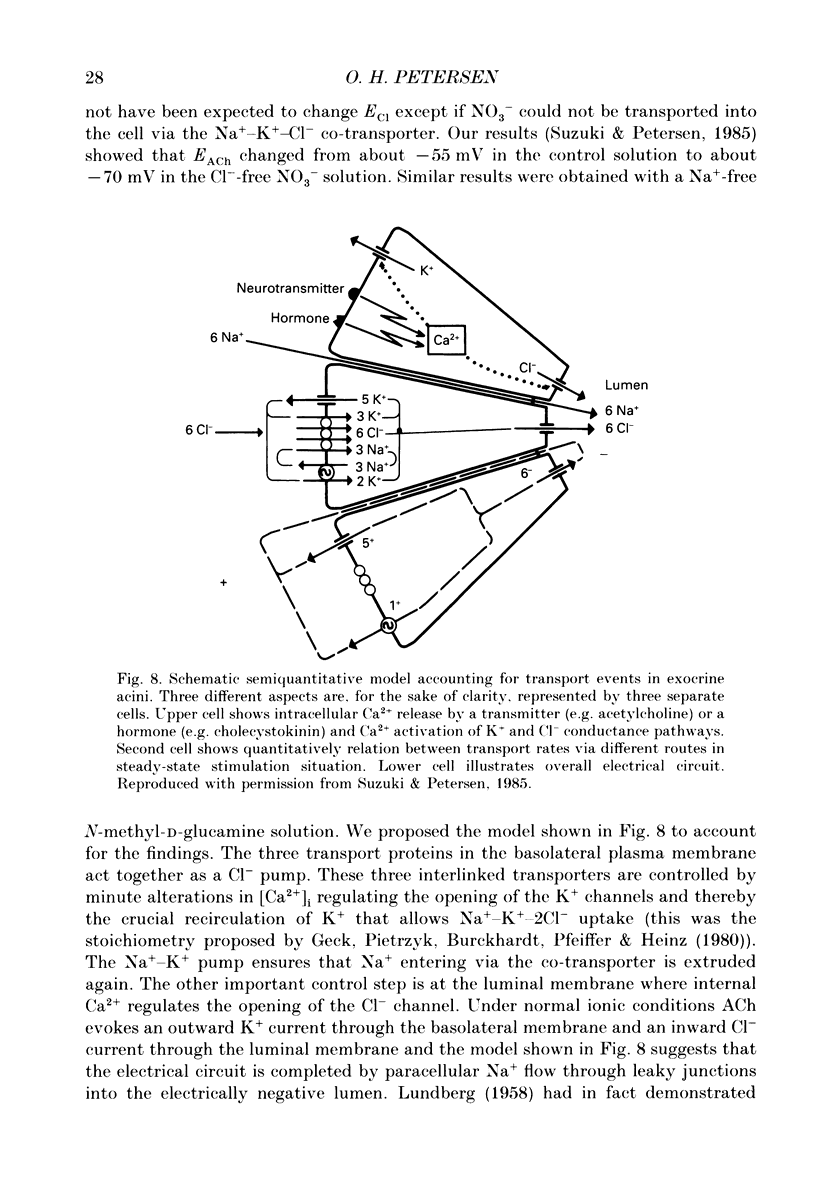















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., Welsh M. J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991 Jul 12;253(5016):202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Argent B. E., Case R. M., Scratcherd T. Stimulation of amylase secretion from the perfused cat pancreas by potassium and other alkali metal ions. J Physiol. 1971 Aug;216(3):611–624. doi: 10.1113/jphysiol.1971.sp009543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGEN A. S. The secretion of potassium in saliva. J Physiol. 1956 Apr 27;132(1):20–39. doi: 10.1113/jphysiol.1956.sp005500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Cobbold P. H., Cuthbertson K. S. Spatial and temporal aspects of cell signalling. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):325–343. doi: 10.1098/rstb.1988.0080. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate-induced membrane potential oscillations in Xenopus oocytes. J Physiol. 1988 Sep;403:589–599. doi: 10.1113/jphysiol.1988.sp017266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Lindley B. D., Prince W. T. Membrane permeability changes during stimulation of isolated salivary glands of Calliphora by 5-hydroxytryptamine. J Physiol. 1975 Jan;244(3):549–567. doi: 10.1113/jphysiol.1975.sp010812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bundgaard M., Møller M., Poulsen J. H. Localization of sodium pump sites in cat salivary glands. J Physiol. 1977 Dec;273(1):339–353. doi: 10.1113/jphysiol.1977.sp012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Clausen T. The relationship between calcium exchange and enzyme secretion in the isolated rat pancreas. J Physiol. 1973 Nov;235(1):75–102. doi: 10.1113/jphysiol.1973.sp010379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield A. R. Chloride dependence of frusemide- and phloretin-sensitive passive sodium and potassium fluxes in human red cells. J Physiol. 1981 Mar;312:435–444. doi: 10.1113/jphysiol.1981.sp013636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Cook D. I., Poronnik P., Young J. A. Characterization of a 25-pS nonselective cation channel in a cultured secretory epithelial cell line. J Membr Biol. 1990 Mar;114(1):37–52. doi: 10.1007/BF01869383. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Pancreatic acinar cells: measurement of membrane potential and miniature depolarization potentials. J Physiol. 1972 Aug;225(1):1–13. doi: 10.1113/jphysiol.1972.sp009926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehlinger-Kremer M., Zeuzem S., Schulz I. Interaction of caffeine-, IP3- and vanadate-sensitive Ca2+ pools in acinar cells of the exocrine pancreas. J Membr Biol. 1991 Jan;119(1):85–100. doi: 10.1007/BF01868543. [DOI] [PubMed] [Google Scholar]
- Dissing S., Nauntofte B. Na+ transport properties of isolated rat parotid acini. Am J Physiol. 1990 Dec;259(6 Pt 1):G1044–G1055. doi: 10.1152/ajpgi.1990.259.6.G1044. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. The influence of calcium on the secretory response of the submaxillary gland to acetylcholine or to noradrenaline. J Physiol. 1963 Mar;165(3):528–541. doi: 10.1113/jphysiol.1963.sp007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich B. E., Watras J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988 Dec 8;336(6199):583–586. doi: 10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Latorre R., Miller C. Multi-ion conduction and selectivity in the high-conductance Ca++-activated K+ channel from skeletal muscle. Biophys J. 1986 Dec;50(6):1025–1034. doi: 10.1016/S0006-3495(86)83546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Calcium-dependent chloride currents in isolated cells from rat lacrimal glands. J Physiol. 1986 Sep;378:437–460. doi: 10.1113/jphysiol.1986.sp016229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. G., Marty A. Potentiation of muscarinic and alpha-adrenergic responses by an analogue of guanosine 5'-triphosphate. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4099–4103. doi: 10.1073/pnas.83.11.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley P. M., Fuller C. M., Gallacher D. V. Potassium uptake in the mouse submandibular gland is dependent on chloride and sodium and abolished by piretanide. J Physiol. 1986 Sep;378:97–108. doi: 10.1113/jphysiol.1986.sp016209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I. A patch-clamp study of potassium channels and whole-cell currents in acinar cells of the mouse lacrimal gland. J Physiol. 1984 May;350:179–195. doi: 10.1113/jphysiol.1984.sp015195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I., Petersen O. H. Acetylcholine stimulates a Ca2+-dependent C1- conductance in mouse lacrimal acinar cells. Pflugers Arch. 1985 Mar;403(3):328–330. doi: 10.1007/BF00583609. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Melvin J. E. Activation of salivary secretion: coupling of cell volume and [Ca2+]i in single cells. Science. 1989 Jun 30;244(4912):1582–1585. doi: 10.1126/science.2500708. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Roifman C. M., Wong D. Activation of calcium oscillations by thapsigargin in parotid acinar cells. J Biol Chem. 1991 Feb 15;266(5):2778–2782. [PubMed] [Google Scholar]
- Foskett J. K., Wong D. Free cytoplasmic Ca2+ concentration oscillations in thapsigargin-treated parotid acinar cells are caffeine- and ryanodine-sensitive. J Biol Chem. 1991 Aug 5;266(22):14535–14538. [PubMed] [Google Scholar]
- Foskett J. K. [Ca2+]i modulation of Cl- content controls cell volume in single salivary acinar cells during fluid secretion. Am J Physiol. 1990 Dec;259(6 Pt 1):C998–1004. doi: 10.1152/ajpcell.1990.259.6.C998. [DOI] [PubMed] [Google Scholar]
- Frömter E., Diamond J. Route of passive ion permeation in epithelia. Nat New Biol. 1972 Jan 5;235(53):9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- Gallacher D. V., Hanley M. R., Petersen O. H., Roberts M. L., Squire-Pollard L. G., Yule D. I. Substance P and bombesin elevate cytosolic Ca2+ by different molecular mechanisms in a rat pancreatic acinar cell line. J Physiol. 1990 Jul;426:193–207. doi: 10.1113/jphysiol.1990.sp018133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V., Maruyama Y., Petersen O. H. Patch-clamp study of rubidium and potassium conductances in single cation channels from mammalian exocrine acini. Pflugers Arch. 1984 Aug;401(4):361–367. doi: 10.1007/BF00584336. [DOI] [PubMed] [Google Scholar]
- Gallacher D. V., Morris A. P. A patch-clamp study of potassium currents in resting and acetylcholine-stimulated mouse submandibular acinar cells. J Physiol. 1986 Apr;373:379–395. doi: 10.1113/jphysiol.1986.sp016054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V., Morris A. P. The receptor-regulated calcium influx in mouse submandibular acinar cells is sodium dependent: a patch-clamp study. J Physiol. 1987 Mar;384:119–130. doi: 10.1113/jphysiol.1987.sp016446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V., Petersen O. H. Electrophysiology of mouse parotid acini: effects of electrical field stimulation and ionophoresis of neurotransmitters. J Physiol. 1980 Aug;305:43–57. doi: 10.1113/jphysiol.1980.sp013348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geck P., Pietrzyk C., Burckhardt B. C., Pfeiffer B., Heinz E. Electrically silent cotransport on Na+, K+ and Cl- in Ehrlich cells. Biochim Biophys Acta. 1980 Aug 4;600(2):432–447. doi: 10.1016/0005-2736(80)90446-0. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L., House C. R., Silinsky E. M. Conductance changes associated with the secretory potential in the cockroach salivary gland. J Physiol. 1974 Feb;236(3):723–731. doi: 10.1113/jphysiol.1974.sp010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L., House C. R. Stimulus-response coupling in gland cells. Annu Rev Biophys Bioeng. 1980;9:55–80. doi: 10.1146/annurev.bb.09.060180.000415. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Greenwell J. R., Garton A. J., Argent B. E. Regulation of maxi-K+ channels on pancreatic duct cells by cyclic AMP-dependent phosphorylation. J Membr Biol. 1990 May;115(3):203–215. doi: 10.1007/BF01868636. [DOI] [PubMed] [Google Scholar]
- Gray M. A., Pollard C. E., Harris A., Coleman L., Greenwell J. R., Argent B. E. Anion selectivity and block of the small-conductance chloride channel on pancreatic duct cells. Am J Physiol. 1990 Nov;259(5 Pt 1):C752–C761. doi: 10.1152/ajpcell.1990.259.5.C752. [DOI] [PubMed] [Google Scholar]
- Gray P. T. Oscillations of free cytosolic calcium evoked by cholinergic and catecholaminergic agonists in rat parotid acinar cells. J Physiol. 1988 Dec;406:35–53. doi: 10.1113/jphysiol.1988.sp017367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger R., Schlatter E., Gögelein H. Cl- -channels in the apical cell membrane of the rectal gland "induced" by cAMP. Pflugers Arch. 1985 Apr;403(4):446–448. doi: 10.1007/BF00589260. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E. Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch. 1981 Nov;392(1):92–94. doi: 10.1007/BF00584588. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey A. M., Macintosh F. C. Calcium and synaptic transmission in a sympathetic ganglion. J Physiol. 1940 Jan 15;97(3):408–416. doi: 10.1113/jphysiol.1940.sp003818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Maruyama Y., Matsumoto O., Nishiyama A. Activation of Ca2+-dependent Cl- and K+ conductances in rat and mouse parotid acinar cells. Jpn J Physiol. 1985;35(6):933–944. doi: 10.2170/jjphysiol.35.933. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Acetylcholine-like effects of intracellular calcium application in pancreatic acinar cells. Nature. 1977 Jul 14;268(5616):147–149. doi: 10.1038/268147a0. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Amino acids evoke short-latency membrane conductance increase in pancreatic acinar cells. Nature. 1980 Jan 31;283(5746):492–494. doi: 10.1038/283492a0. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Determination of acetylcholine null potential in mouse pancreatic acinar cells. Nature. 1976 Oct 28;263(5580):784–786. doi: 10.1038/263784a0. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Dissociation between stimulant-evoked acinar membrane resistance change and amylase secretion in the mouse parotid gland. J Physiol. 1981 May;314:79–84. doi: 10.1113/jphysiol.1981.sp013691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Electrical coupling and uncoupling of exocrine acinar cells. J Cell Biol. 1978 Nov;79(2 Pt 1):533–545. doi: 10.1083/jcb.79.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. In vitro action of bombesin on amylase secretion, membrane potential, and membrane resistance in rat and mouse pancreatic acinar cells. A comparison with other secretagogues. J Clin Invest. 1978 Jan;61(1):41–46. doi: 10.1172/JCI108923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Membrane potential, resistance, and intercellular communication in the lacrimal gland: effects of acetylcholine and adrenaline. J Physiol. 1978 Feb;275:507–520. doi: 10.1113/jphysiol.1978.sp012204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Pancreatic acinar cells: localization of acetylcholine receptors and the importance of chloride and calcium for acetylcholine-evoked depolarization. J Physiol. 1977 Aug;269(3):723–733. doi: 10.1113/jphysiol.1977.sp011925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Pancreatic acinar cells: the acetylcholine equilibrium potential and its ionic dependency. J Physiol. 1977 Aug;269(3):735–751. doi: 10.1113/jphysiol.1977.sp011926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauch P., Petersen O. H., Läuger P. Electrogenic properties of the sodium-alanine cotransporter in pancreatic acinar cells: I. Tight-seal whole-cell recordings. J Membr Biol. 1986;94(2):99–115. doi: 10.1007/BF01871191. [DOI] [PubMed] [Google Scholar]
- Kasai H., Augustine G. J. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature. 1990 Dec 20;348(6303):735–738. doi: 10.1038/348735a0. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A. Electrophysiology of salivary glands. Physiol Rev. 1958 Jan;38(1):21–40. doi: 10.1152/physrev.1958.38.1.21. [DOI] [PubMed] [Google Scholar]
- Larsen E. H., Novak I., Pedersen P. S. Cation transport by sweat ducts in primary culture. Ionic mechanism of cholinergically evoked current oscillations. J Physiol. 1990 May;424:109–131. doi: 10.1113/jphysiol.1990.sp018058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugier R., Petersen O. H. Effects of intracellular EGTA injection on stimulant-evoked membrane potential and resistance changes in pancreatic acinar cells. Pflugers Arch. 1980 Jul;386(2):147–152. doi: 10.1007/BF00584202. [DOI] [PubMed] [Google Scholar]
- Laugier R., Petersen O. H. Pancreatic acinar cells: electrophysiological evidence for stimulant-evoked increase in membrane calcium permeability in the mouse. J Physiol. 1980 Jun;303:61–72. doi: 10.1113/jphysiol.1980.sp013270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgaroli A., Fesce R., Meldolesi J. Spontaneous [Ca2+]i fluctuations in rat chromaffin cells do not require inositol 1,4,5-trisphosphate elevations but are generated by a caffeine- and ryanodine-sensitive intracellular Ca2+ store. J Biol Chem. 1990 Feb 25;265(6):3005–3008. [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Marty A., Tan Y. P. The initiation of calcium release following muscarinic stimulation in rat lacrimal glands. J Physiol. 1989 Dec;419:665–687. doi: 10.1113/jphysiol.1989.sp017892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y. Agonist-induced changes in cell membrane capacitance and conductance in dialysed pancreatic acinar cells of rats. J Physiol. 1988 Dec;406:299–313. doi: 10.1113/jphysiol.1988.sp017381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama Y., Gallacher D. V., Petersen O. H. Voltage and Ca2+-activated K+ channel in baso-lateral acinar cell membranes of mammalian salivary glands. Nature. 1983 Apr 28;302(5911):827–829. doi: 10.1038/302827a0. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Nishiyama A., Izumi T., Hoshimiya N., Petersen O. H. Ensemble noise and current relaxation analysis of K+ current in single isolated salivary acinar cells from rat. Pflugers Arch. 1986 Jan;406(1):69–72. doi: 10.1007/BF00582956. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Petersen O. H. Cholecystokinin activation of single-channel currents is mediated by internal messenger in pancreatic acinar cells. Nature. 1982 Nov 4;300(5887):61–63. doi: 10.1038/300061a0. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Petersen O. H. Control of K+ conductance by cholecystokinin and Ca2+ in single pancreatic acinar cells studied by the patch-clamp technique. J Membr Biol. 1984;79(3):293–298. doi: 10.1007/BF01871068. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Petersen O. H., Flanagan P., Pearson G. T. Quantification of Ca2+-activated K+ channels under hormonal control in pig pancreas acinar cells. Nature. 1983 Sep 15;305(5931):228–232. doi: 10.1038/305228a0. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Petersen O. H. Single calcium-dependent cation channels in mouse pancreatic acinar cells. J Membr Biol. 1984;81(1):83–87. doi: 10.1007/BF01868812. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Petersen O. H. Voltage clamp study of stimulant-evoked currents in mouse pancreatic acinar cells. Pflugers Arch. 1983 Sep;399(1):54–62. doi: 10.1007/BF00652522. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Peterson O. H. Single-channel currents in isolated patches of plasma membrane from basal surface of pancreatic acini. Nature. 1982 Sep 9;299(5879):159–161. doi: 10.1038/299159a0. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H. Pancreatic acinar cells: ionic dependence of the membrane potential and acetycholine-induced depolarization. J Physiol. 1973 Jun;231(2):283–295. doi: 10.1113/jphysiol.1973.sp010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H., Williams J. A. Pancreatic acinar cells: acetylcholine-induced membrane depolarization, calcium efflux and amylase release. J Physiol. 1973 Nov;234(3):689–701. doi: 10.1113/jphysiol.1973.sp010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless M., Nishiyama A., Petersen O. H., Philpott H. G. Mouse pancreatic acinar cells: voltage-clamp study of acetylcholine-evoked membrane current. J Physiol. 1981 Sep;318:57–71. doi: 10.1113/jphysiol.1981.sp013850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. Cell signaling by second messenger waves. Cell. 1991 Feb 22;64(4):675–678. doi: 10.1016/0092-8674(91)90496-l. [DOI] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Fuller C. M., Scott J. Cholinergic receptor-regulation of potassium channels and potassium transport in human submandibular acinar cells. J Dent Res. 1987 Feb;66(2):541–546. doi: 10.1177/00220345870660022601. [DOI] [PubMed] [Google Scholar]
- Morris A. P., Gallacher D. V., Irvine R. F., Petersen O. H. Synergism of inositol trisphosphate and tetrakisphosphate in activating Ca2+-dependent K+ channels. Nature. 1987 Dec 17;330(6149):653–655. doi: 10.1038/330653a0. [DOI] [PubMed] [Google Scholar]
- Muallem S., Pandol S. J., Beeker T. G. Hormone-evoked calcium release from intracellular stores is a quantal process. J Biol Chem. 1989 Jan 5;264(1):205–212. [PubMed] [Google Scholar]
- Nauntofte B., Poulsen J. H. Effects of Ca2+ and furosemide on Cl- transport and O2 uptake in rat parotid acini. Am J Physiol. 1986 Aug;251(2 Pt 1):C175–C185. doi: 10.1152/ajpcell.1986.251.2.C175. [DOI] [PubMed] [Google Scholar]
- Nielsen S. P., Petersen O. H. Transport of calcium in the perfused submandibular gland of the cat. J Physiol. 1972 Jun;223(3):685–697. doi: 10.1113/jphysiol.1972.sp009869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Kagayama M. Biphasic secretory potentials in cat and rabbit submaxillary glands. Experientia. 1973 Feb 15;29(2):161–163. doi: 10.1007/BF01945449. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Membrane potential and resistance measurement in acinar cells from salivary glands in vitro: effect of acetylcholine. J Physiol. 1974 Oct;242(1):173–188. doi: 10.1113/jphysiol.1974.sp010700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: ionic dependence of acetylcholine-induced membrane potential and resistance change. J Physiol. 1975 Jan;244(2):431–465. doi: 10.1113/jphysiol.1975.sp010807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: membrane potential and resistance change evoked by acetylcholine. J Physiol. 1974 Apr;238(1):145–158. doi: 10.1113/jphysiol.1974.sp010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- O'Doherty J., Stark R. J., Crane S. J., Brugge K. L. Changes in cytosolic calcium during cholinergic and adrenergic stimulation of the parotid salivary gland. Pflugers Arch. 1983 Aug;398(3):241–246. doi: 10.1007/BF00657159. [DOI] [PubMed] [Google Scholar]
- O'Doherty J., Youmans S. J., Armstrong W. M., Stark R. J. Calcium regulation during stimulus-secretion coupling: continuous measurement of intracellular calcium activities. Science. 1980 Jul 25;209(4455):510–513. doi: 10.1126/science.7394518. [DOI] [PubMed] [Google Scholar]
- Osipchuk Y. V., Wakui M., Yule D. I., Gallacher D. V., Petersen O. H. Cytoplasmic Ca2+ oscillations evoked by receptor stimulation, G-protein activation, internal application of inositol trisphosphate or Ca2+: simultaneous microfluorimetry and Ca2+ dependent Cl- current recording in single pancreatic acinar cells. EMBO J. 1990 Mar;9(3):697–704. doi: 10.1002/j.1460-2075.1990.tb08162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen P. S. Cholinergic-induced oscillating transepithelial short-circuit current in cultured human sweat duct cells. Acta Physiol Scand. 1990 Mar;138(3):359–368. doi: 10.1111/j.1748-1716.1990.tb08858.x. [DOI] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Petersen C. C., Petersen O. H. Receptor-activated cytoplasmic Ca2+ spikes in communicating clusters of pancreatic acinar cells. FEBS Lett. 1991 Jun 17;284(1):113–116. doi: 10.1016/0014-5793(91)80774-w. [DOI] [PubMed] [Google Scholar]
- Petersen C. C., Toescu E. C., Petersen O. H. Different patterns of receptor-activated cytoplasmic Ca2+ oscillations in single pancreatic acinar cells: dependence on receptor type, agonist concentration and intracellular Ca2+ buffering. EMBO J. 1991 Mar;10(3):527–533. doi: 10.1002/j.1460-2075.1991.tb07979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C. C., Toescu E. C., Potter B. V., Petersen O. H. Inositol triphosphate produces different patterns of cytoplasmic Ca2+ spiking depending on its concentration. FEBS Lett. 1991 Nov 18;293(1-2):179–182. doi: 10.1016/0014-5793(91)81181-7. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Calcium dependence of bombesin-evoked pancreatic amylase secretion [proceedings]. J Physiol. 1978 Dec;285:30P–31P. [PubMed] [Google Scholar]
- Petersen O. H. Calcium-activated potassium channels and fluid secretion by exocrine glands. Am J Physiol. 1986 Jul;251(1 Pt 1):G1–13. doi: 10.1152/ajpgi.1986.251.1.G1. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Findlay I. Electrophysiology of the pancreas. Physiol Rev. 1987 Jul;67(3):1054–1116. doi: 10.1152/physrev.1987.67.3.1054. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Findlay I., Iwatsuki N., Singh J., Gallacher D. V., Fuller C. M., Pearson G. T., Dunne M. J., Morris A. P. Human pancreatic acinar cells: studies of stimulus-secretion coupling. Gastroenterology. 1985 Jul;89(1):109–117. doi: 10.1016/0016-5085(85)90751-6. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Formation of saliva and potassium transport in the perfused cat submandibular gland. J Physiol. 1971 Jul;216(1):129–142. doi: 10.1113/jphysiol.1971.sp009513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Gallacher D. V. Electrophysiology of pancreatic and salivary acinar cells. Annu Rev Physiol. 1988;50:65–80. doi: 10.1146/annurev.ph.50.030188.000433. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y. Calcium-activated potassium channels and their role in secretion. Nature. 1984 Feb 23;307(5953):693–696. doi: 10.1038/307693a0. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y., Graf J., Laugier R., Nishiyama A., Pearson G. T. Ionic currents across pancreatic acinar cell membranes and their role in fluid secretion. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):151–166. doi: 10.1098/rstb.1981.0179. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y. What is the mechanism of the calcium influx to pancreatic acinar cells evoked by secretagogues? Pflugers Arch. 1983 Jan;396(1):82–84. doi: 10.1007/BF00584703. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Matthews E. K. The effect of pancreozymin and acetylcholine on the membrane potential of the pancreatic acinar cells. Experientia. 1972 Sep 15;28(9):1037–1038. doi: 10.1007/BF01918657. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Membrane potential measurement in mouse salivary gland cells. Experientia. 1973 Feb 15;29(2):160–161. doi: 10.1007/BF01945448. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Philpott H. G. Mouse pancreatic acinar cells: the anion selectivity of the acetylcholine-opened chloride pathway. J Physiol. 1980 Sep;306:481–492. doi: 10.1113/jphysiol.1980.sp013409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Poulsen J. H. Secretory potentials, potassium transport and secretion in the cat submandibular gland during perfusion with sulphate Locke's solution. Experientia. 1968 Sep 15;24(9):919–920. doi: 10.1007/BF02138654. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Some factors influencing stimulation-induced release of potassium from the cat submandibular gland to fluid perfused through the gland. J Physiol. 1970 Jun;208(2):431–447. doi: 10.1113/jphysiol.1970.sp009129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H. The dependence of the transmembrane salivary secretory potential on the external potassium and sodium concentration. J Physiol. 1970 Sep;210(1):205–215. doi: 10.1113/jphysiol.1970.sp009204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Ueda N. Pancreatic acinar cells: the role of calcium in stimulus-secretion coupling. J Physiol. 1976 Jan;254(3):583–606. doi: 10.1113/jphysiol.1976.sp011248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Wakui M. Oscillating intracellular Ca2+ signals evoked by activation of receptors linked to inositol lipid hydrolysis: mechanism of generation. J Membr Biol. 1990 Nov;118(2):93–105. doi: 10.1007/BF01868467. [DOI] [PubMed] [Google Scholar]
- Peterson O. H., Iwatsuki N. The role of calcium in pancreatic acinar cell stimulus-secretion coupling: an electrophysiological approach. Ann N Y Acad Sci. 1978 Apr 28;307:599–617. doi: 10.1111/j.1749-6632.1978.tb41984.x. [DOI] [PubMed] [Google Scholar]
- Philpott H. G., Petersen O. H. Extracellular but not intracellular application of peptide hormones activates pancreatic acinar cells. Nature. 1979 Oct 25;281(5733):684–686. doi: 10.1038/281684a0. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Chanson M., Trautmann A. Calcium and secretagogues-induced conductances in rat exocrine pancreas. Pflugers Arch. 1988 Jan;411(1):53–57. doi: 10.1007/BF00581646. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Jacob R. Calcium oscillations in non-excitable cells. Trends Neurosci. 1989 Feb;12(2):43–46. doi: 10.1016/0166-2236(89)90133-1. [DOI] [PubMed] [Google Scholar]
- Roberts M. L., Iwatsuki N., Petersen O. H. Parotid acinar cells: ionic dependence of acetylcholine-evoked membrane potential changes. Pflugers Arch. 1978 Sep 6;376(2):159–167. doi: 10.1007/BF00581579. [DOI] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of a cell junction and the local cytoplasmic free ionized calcium concentration: a study with aequorin. J Membr Biol. 1976 Aug 27;28(1):87–119. doi: 10.1007/BF01869692. [DOI] [PubMed] [Google Scholar]
- Saito Y., Ozawa T., Hayashi H., Nishiyama A. The effect of acetylcholine on chloride transport across the mouse lacrimal gland acinar cell membranes. Pflugers Arch. 1987 Jul;409(3):280–288. doi: 10.1007/BF00583477. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Gallacher D. V. Extracellular ATP activates receptor-operated cation channels in mouse lacrimal acinar cells to promote calcium influx in the absence of phosphoinositide metabolism. FEBS Lett. 1990 May 7;264(1):130–134. doi: 10.1016/0014-5793(90)80782-e. [DOI] [PubMed] [Google Scholar]
- Schramm M., Selinger Z. The function of alpha- and beta-adrenergic receptors and a cholinergic receptor in the secretory cell of rat parotid gland. Adv Cytopharmacol. 1974;2:29–32. [PubMed] [Google Scholar]
- Selinger Z., Eimerl S., Schramm M. A calcium ionophore simulating the action of epinephrine on the alpha-adrenergic receptor. Proc Natl Acad Sci U S A. 1974 Jan;71(1):128–131. doi: 10.1073/pnas.71.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell W. A., Young J. A. Secretion of electrolytes by the pancreas of the anaestetized rat. J Physiol. 1975 Nov;252(2):379–396. doi: 10.1113/jphysiol.1975.sp011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaje L. H., Poulsen J. H., Ussing H. H. Evidence from O2 uptake measurements for Na+ -K+ -2 Cl- co-transport in the rabbit submandibular gland. Pflugers Arch. 1986 May;406(5):492–496. doi: 10.1007/BF00583372. [DOI] [PubMed] [Google Scholar]
- Speksnijder J. E., Miller A. L., Weisenseel M. H., Chen T. H., Jaffe L. F. Calcium buffer injections block fucoid egg development by facilitating calcium diffusion. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6607–6611. doi: 10.1073/pnas.86.17.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Petersen O. H. Patch-clamp study of single-channel and whole-cell K+ currents in guinea pig pancreatic acinar cells. Am J Physiol. 1988 Sep;255(3 Pt 1):G275–G285. doi: 10.1152/ajpgi.1988.255.3.G275. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Petersen O. H. The effect of Na+ and Cl- removal and of loop diuretics on acetylcholine-evoked membrane potential changes in mouse lacrimal acinar cells. Q J Exp Physiol. 1985 Jul;70(3):437–445. doi: 10.1113/expphysiol.1985.sp002927. [DOI] [PubMed] [Google Scholar]
- Taylor C. W., Merritt J. E., Putney J. W., Jr, Rubin R. P. Effects of Ca2+ on phosphoinositide breakdown in exocrine pancreas. Biochem J. 1986 Sep 15;238(3):765–772. doi: 10.1042/bj2380765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepikin A. V., Kostyuk P. G., Snitsarev V. A., Belan P. V. Extrusion of calcium from a single isolated neuron of the snail Helix pomatia. J Membr Biol. 1991 Jul;123(1):43–47. doi: 10.1007/BF01993961. [DOI] [PubMed] [Google Scholar]
- Trautmann A., Marty A. Activation of Ca-dependent K channels by carbamoylcholine in rat lacrimal glands. Proc Natl Acad Sci U S A. 1984 Jan;81(2):611–615. doi: 10.1073/pnas.81.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. J., George J. N., Baum B. J. Evidence for a Na+/K+/Cl- cotransport system in basolateral membrane vesicles from the rabbit parotid. J Membr Biol. 1986;94(2):143–152. doi: 10.1007/BF01871194. [DOI] [PubMed] [Google Scholar]
- Ussing H. H., Eskesen K. Mechanism of isotonic water transport in glands. Acta Physiol Scand. 1989 Jul;136(3):443–454. doi: 10.1111/j.1748-1716.1989.tb08686.x. [DOI] [PubMed] [Google Scholar]
- Wakui M., Kase H., Petersen O. H. Cytoplasmic Ca2+ signals evoked by activation of cholecystokinin receptors: Ca(2+)-dependent current recording in internally perfused pancreatic acinar cells. J Membr Biol. 1991 Nov;124(2):179–187. doi: 10.1007/BF01870462. [DOI] [PubMed] [Google Scholar]
- Wakui M., Osipchuk Y. V., Petersen O. H. Receptor-activated cytoplasmic Ca2+ spiking mediated by inositol trisphosphate is due to Ca2(+)-induced Ca2+ release. Cell. 1990 Nov 30;63(5):1025–1032. doi: 10.1016/0092-8674(90)90505-9. [DOI] [PubMed] [Google Scholar]
- Wakui M., Petersen O. H. Cytoplasmic Ca2+ oscillations evoked by acetylcholine or intracellular infusion of inositol trisphosphate or Ca2+ can be inhibited by internal Ca2+. FEBS Lett. 1990 Apr 24;263(2):206–208. doi: 10.1016/0014-5793(90)81374-w. [DOI] [PubMed] [Google Scholar]
- Wakui M., Potter B. V., Petersen O. H. Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature. 1989 May 25;339(6222):317–320. doi: 10.1038/339317a0. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Anion selectivity in biological systems. Physiol Rev. 1977 Jan;57(1):109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]
- Yoshimura H., Imai Y. Studies on the secretory potential of acinal cells of the dog submaxillary gland and its ionic dependency. Jpn J Physiol. 1967 Jun;17(3):280–293. doi: 10.2170/jjphysiol.17.280. [DOI] [PubMed] [Google Scholar]
- Yule D. I., Gallacher D. V. Oscillations of cytosolic calcium in single pancreatic acinar cells stimulated by acetylcholine. FEBS Lett. 1988 Nov 7;239(2):358–362. doi: 10.1016/0014-5793(88)80951-7. [DOI] [PubMed] [Google Scholar]
- Yule D. I., Lawrie A. M., Gallacher D. V. Acetylcholine and cholecystokinin induce different patterns of oscillating calcium signals in pancreatic acinar cells. Cell Calcium. 1991 Feb-Mar;12(2-3):145–151. doi: 10.1016/0143-4160(91)90016-8. [DOI] [PubMed] [Google Scholar]
- von Tscharner V., Prod'hom B., Baggiolini M., Reuter H. Ion channels in human neutrophils activated by a rise in free cytosolic calcium concentration. 1986 Nov 27-Dec 3Nature. 324(6095):369–372. doi: 10.1038/324369a0. [DOI] [PubMed] [Google Scholar]



