Abstract
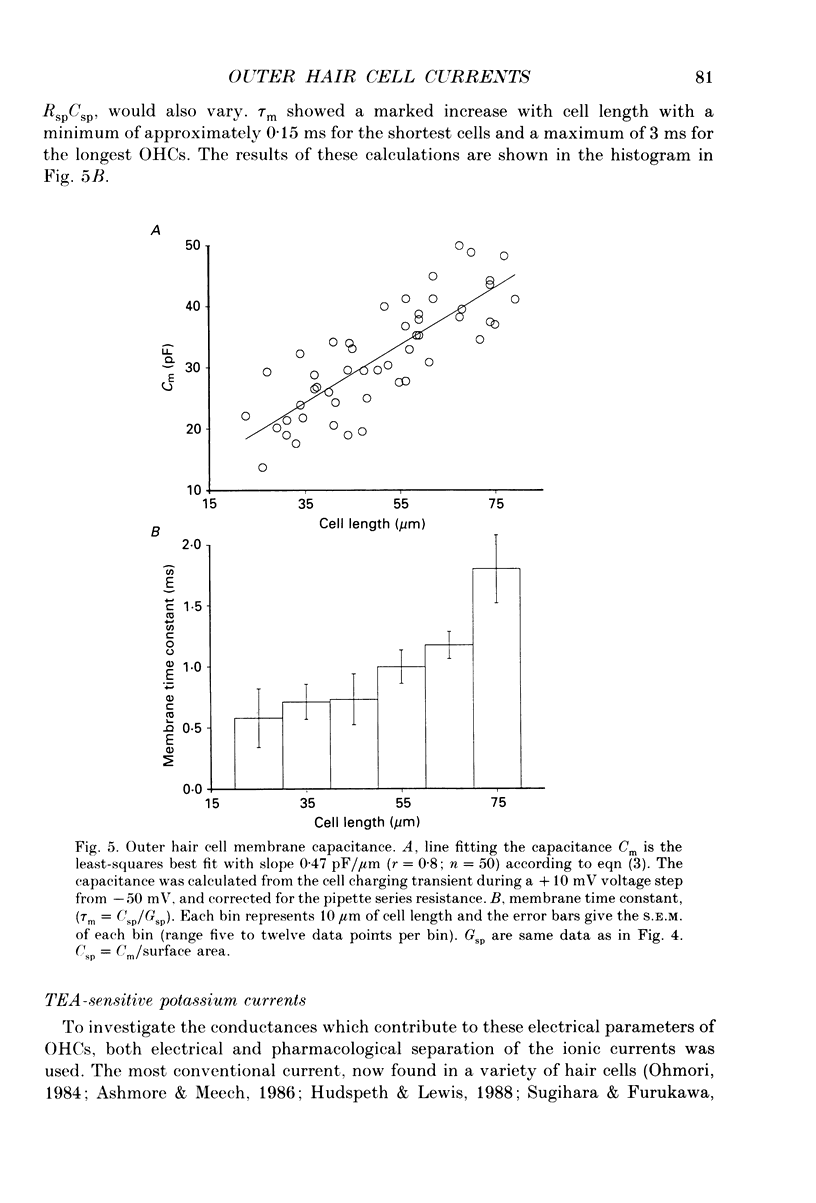
1. Whole-cell currents were measured in outer hair cells isolated from each turn of the organ of Corti of the guinea-pig. 2. The slope input conductances at -70 mV of the cells ranged from 3.6 to 51 nS depending on the length of the cell. Shorter cells from the basal turns of the cochlea had the highest values. The membrane time constant of the cells varied from 3 to 0.2 ms from the apex to the base. 3. Irrespective of the position of the cells along the cochlea, three distinct currents were found. Each type of current was found in approximately the same proportion in all cells. 4. An outward K+ current was present which activated at potentials more positive than -35 mV. The current was sensitive to tetraethylammonium (30 mM), quinidine (100 microM) and nifedipine (50 microM). It could be removed by replacing external Ca2+ with Ba2+ or Mg2+. The current was also removed by substituting Nai+ or Csi+ for Ki+ pipette solution. This outwardly rectifying current appears similar to the calcium-activated K+ current described in other hair cells. 5. The main current present at membrane potentials from -90 mV to -50 mV was a second voltage-activated K+ current. It was 50% activated at -80 mV, and relaxed with a time constant of 20-40 ms on hyperpolarization to -120 mV. Near rest the kinetics were essentially time-dependent , but depended upon the external K+ concentration. The current was blocked by 5 mM external Cs+. 6. This current was highly selective for K+. Measured from reversal of the tail currents, the permeability ratio PK:PNa was approximately 30:1. Depolarization of the cell, presumed to lead to an elevation of intracellular calcium, produced a prolonged activation of the current. 7. A third current found in the cells was a cation current. By external ion replacement, the selectivity sequence was determined to be Ca2+ greater than Na+ approximately equal to K+ greater than choline+ greater than NMDG+ (respective permeabilities relative to Na: 2.9, 1.0, 0.99, 0.63 and 0.37). This current was reduced by external Ba2+ (3 mM) and by nifedipine (50 microM). The activation of this current appeared to depend upon raised levels of Cai2+. 8. These currents account for reported in vivo properties of cochlear outer hair cells as cells permeable to potassium at large negative resting potentials. The consequences for sound detection in the cochlea are briefly discussed.
Full text
PDF
























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Art J. J., Fettiplace R. Variation of membrane properties in hair cells isolated from the turtle cochlea. J Physiol. 1987 Apr;385:207–242. doi: 10.1113/jphysiol.1987.sp016492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F. A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J Physiol. 1987 Jul;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F., Meech R. W. Ionic basis of membrane potential in outer hair cells of guinea pig cochlea. Nature. 1986 Jul 24;322(6077):368–371. doi: 10.1038/322368a0. [DOI] [PubMed] [Google Scholar]
- Ashmore J. F., Ohmori H. Control of intracellular calcium by ATP in isolated outer hair cells of the guinea-pig cochlea. J Physiol. 1990 Sep;428:109–131. doi: 10.1113/jphysiol.1990.sp018203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell W. E., Bader C. R., Bertrand D., de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985 Jan 11;227(4683):194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Cody A. R., Russell I. J. The response of hair cells in the basal turn of the guinea-pig cochlea to tones. J Physiol. 1987 Feb;383:551–569. doi: 10.1113/jphysiol.1987.sp016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979 Oct 25;281(5733):675–677. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- Correia M. J., Christensen B. N., Moore L. E., Lang D. G. Studies of solitary semicircular canal hair cells in the adult pigeon. I. Frequency- and time-domain analysis of active and passive membrane properties. J Neurophysiol. 1989 Oct;62(4):924–934. doi: 10.1152/jn.1989.62.4.924. [DOI] [PubMed] [Google Scholar]
- Dallos P., Santos-Sacchi J., Flock A. Intracellular recordings from cochlear outer hair cells. Science. 1982 Nov 5;218(4572):582–584. doi: 10.1126/science.7123260. [DOI] [PubMed] [Google Scholar]
- Davis H. An active process in cochlear mechanics. Hear Res. 1983 Jan;9(1):79–90. doi: 10.1016/0378-5955(83)90136-3. [DOI] [PubMed] [Google Scholar]
- Fuchs P. A., Evans M. G. Potassium currents in hair cells isolated from the cochlea of the chick. J Physiol. 1990 Oct;429:529–551. doi: 10.1113/jphysiol.1990.sp018271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V., Morris A. P. A patch-clamp study of potassium currents in resting and acetylcholine-stimulated mouse submandibular acinar cells. J Physiol. 1986 Apr;373:379–395. doi: 10.1113/jphysiol.1986.sp016054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins J. E., Jr Cytoarchitectural basis of the cochlear transducer. Cold Spring Harb Symp Quant Biol. 1965;30:147–157. doi: 10.1101/sqb.1965.030.01.017. [DOI] [PubMed] [Google Scholar]
- Housley G. D., Norris C. H., Guth P. S. Electrophysiological properties and morphology of hair cells isolated from the semicircular canal of the frog. Hear Res. 1989 Apr;38(3):259–276. doi: 10.1016/0378-5955(89)90070-1. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J., Lewis R. S. Kinetic analysis of voltage- and ion-dependent conductances in saccular hair cells of the bull-frog, Rana catesbeiana. J Physiol. 1988 Jun;400:237–274. doi: 10.1113/jphysiol.1988.sp017119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. O. Active and nonlinear cochlear biomechanics and the role of outer-hair-cell subsystem in the mammalian auditory system. Hear Res. 1986;22:105–114. doi: 10.1016/0378-5955(86)90088-2. [DOI] [PubMed] [Google Scholar]
- Kros C. J., Crawford A. C. Potassium currents in inner hair cells isolated from the guinea-pig cochlea. J Physiol. 1990 Feb;421:263–291. doi: 10.1113/jphysiol.1990.sp017944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond J., Krnjevic K. Hypoxic changes in hippocampal neurons. J Neurophysiol. 1989 Jul;62(1):1–14. doi: 10.1152/jn.1989.62.1.1. [DOI] [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Akaike N., Kimitsuki T., Komune S., Arima T. ATP-induced current in isolated outer hair cells of guinea pig cochlea. J Neurophysiol. 1990 May;63(5):1068–1074. doi: 10.1152/jn.1990.63.5.1068. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Kakehata S., Akaike N., Komune S., Takasaka T., Uemura T. Calcium channel in isolated outer hair cells of guinea pig cochlea. Neurosci Lett. 1991 Apr 15;125(1):81–84. doi: 10.1016/0304-3940(91)90136-h. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Studies of ionic currents in the isolated vestibular hair cell of the chick. J Physiol. 1984 May;350:561–581. doi: 10.1113/jphysiol.1984.sp015218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M., Jacobs R. A., Hudspeth A. J. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990 Nov;10(11):3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell I. J., Cody A. R., Richardson G. P. The responses of inner and outer hair cells in the basal turn of the guinea-pig cochlea and in the mouse cochlea grown in vitro. Hear Res. 1986;22:199–216. doi: 10.1016/0378-5955(86)90096-1. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Intracellular studies of hair cells in the mammalian cochlea. J Physiol. 1978 Nov;284:261–290. doi: 10.1113/jphysiol.1978.sp012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J Physiol. 1983 May;338:179–206. doi: 10.1113/jphysiol.1983.sp014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J., Dilger J. P. Whole cell currents and mechanical responses of isolated outer hair cells. Hear Res. 1988 Sep 15;35(2-3):143–150. doi: 10.1016/0378-5955(88)90113-x. [DOI] [PubMed] [Google Scholar]
- Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol. 1987 Jun;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A. Dimensions of the cochlear stereocilia in man and the guinea pig. Hear Res. 1984 Jan;13(1):89–98. doi: 10.1016/0378-5955(84)90099-6. [DOI] [PubMed] [Google Scholar]



