Abstract
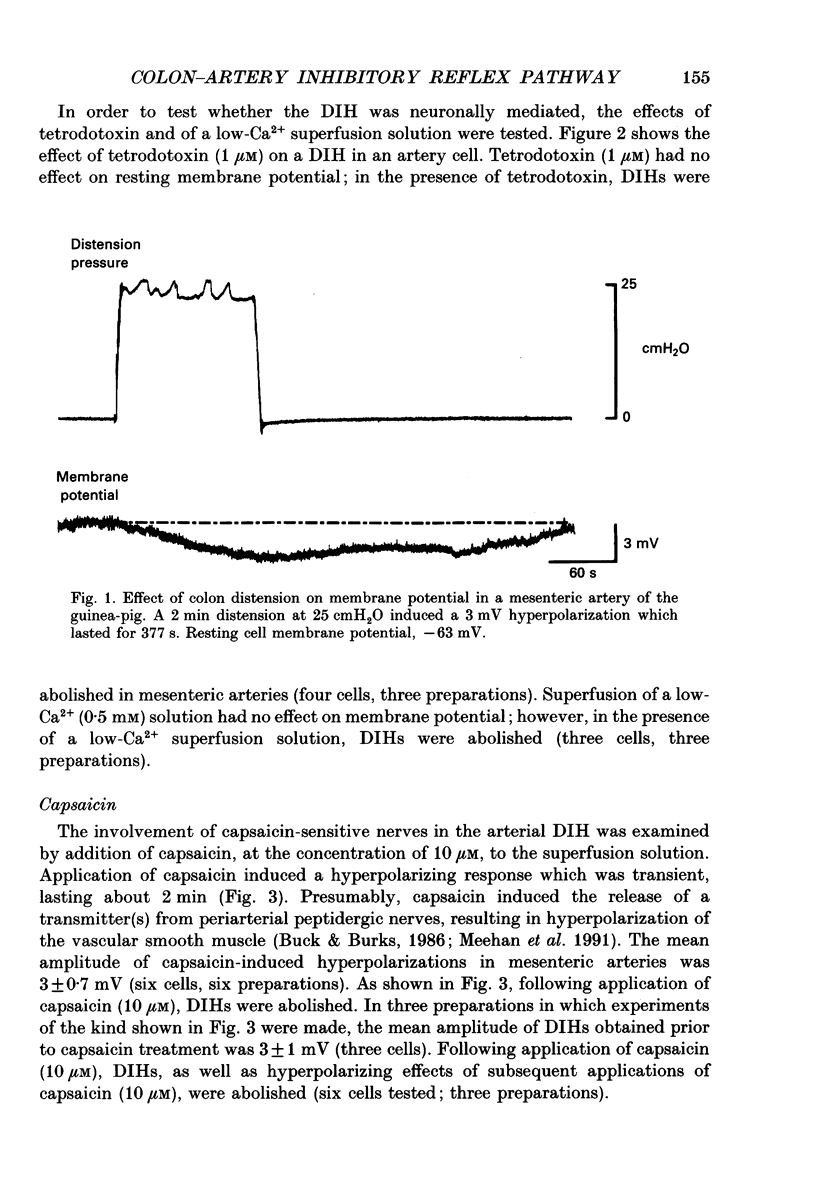
1. The present in vitro study examined the effect of distension of the distal colon on membrane potential in the inferior mesenteric artery of the guinea-pig. 2. Distension of the distal colon up to an intraluminal pressure of 25 cmH2O induced a hyperpolarization in the inferior mesenteric artery. The average amplitude of hyperpolarizations induced by 2 min distensions of the colon was 3 mV and their average duration was 268 s. 3. Distension-induced hyperpolarizations (DIHs) were abolished in the presence of tetrodotoxin or a low-Ca2+ (0.5 mM) superfusion solution. 4. Superfusion of capsaicin (10 microM) induced slow hyperpolarizing responses in mesenteric arteries. Following application of capsaicin (10 microM), DIHs were abolished. 5. These findings provide strong evidence that mesenteric arteries receive an inhibitory, capsaicin-sensitive sensory innervation from the distal colon which is activated during periods of colon distension to induce hyperpolarization of the arterial smooth muscle. This extramural inhibitory reflex pathway may play a physiological role in co-ordinating mesenteric blood flow with changes in gut motility.
Full text
PDF

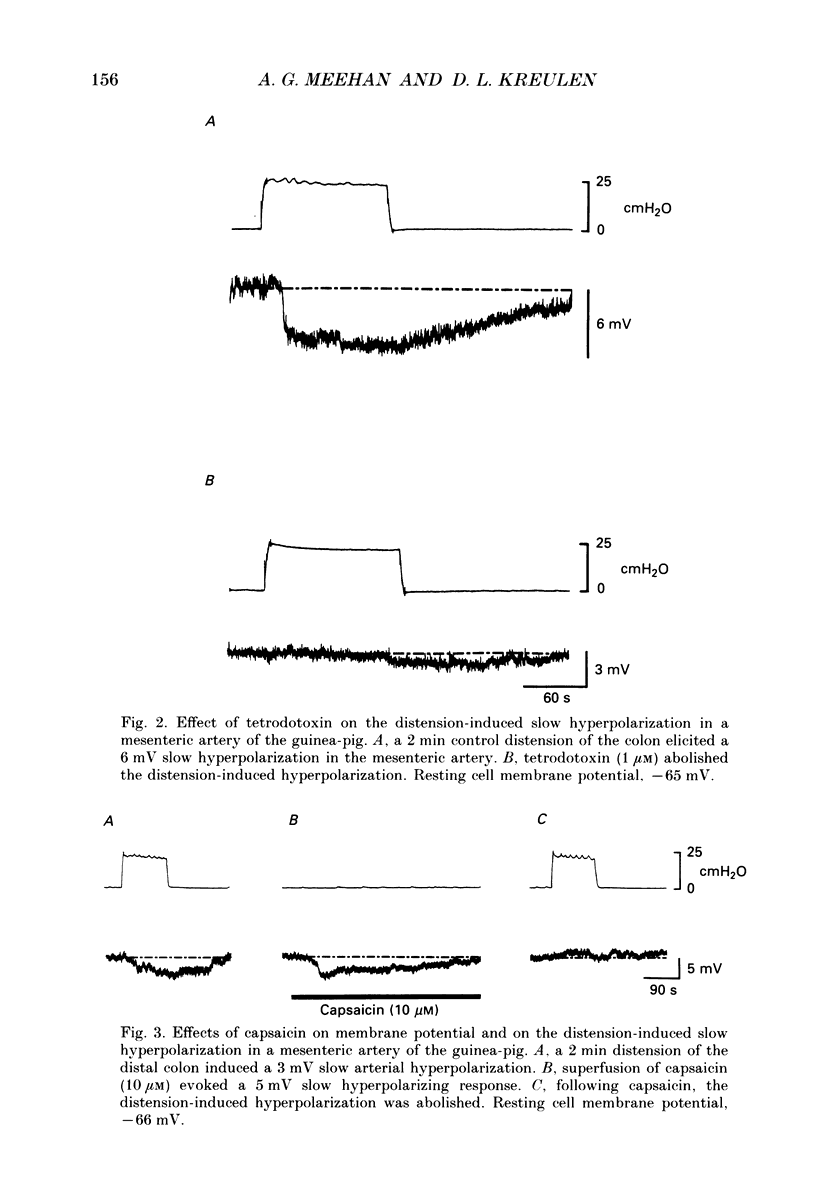



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss W. M. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. J Physiol. 1901 Feb 28;26(3-4):173–209. doi: 10.1113/jphysiol.1901.sp000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Szolcsányi J. Sensory neuron-specific actions of capsaicin: mechanisms and applications. Trends Pharmacol Sci. 1990 Aug;11(8):330–333. doi: 10.1016/0165-6147(90)90237-3. [DOI] [PubMed] [Google Scholar]
- Buck S. H., Burks T. F. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986 Sep;38(3):179–226. [PubMed] [Google Scholar]
- Crowcroft P. J., Holman M. E., Szurszewski J. H. Excitatory input from the distal colon to the inferior mesenteric ganglion in the guinea-pig. J Physiol. 1971 Dec;219(2):443–461. doi: 10.1113/jphysiol.1971.sp009671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard C. J., Hökfelt T., Schultzberg M., Lundberg J. M., Terenius L., Dockray G. J., Goldstein M. Origin of peptide-containing fibers in the inferior mesenteric ganglion of the guinea-pig: immunohistochemical studies with antisera to substance P, enkephalin, vasoactive intestinal polypeptide, cholecystokinin and bombesin. Neuroscience. 1983 May;9(1):191–211. doi: 10.1016/0306-4522(83)90056-8. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Papka R. E., Della N. G., Costa M., Eskay R. L. Substance P-like immunoreactivity in nerves associated with the vascular system of guinea-pigs. Neuroscience. 1982 Feb;7(2):447–459. doi: 10.1016/0306-4522(82)90279-2. [DOI] [PubMed] [Google Scholar]
- Han S. P., Naes L., Westfall T. C. Inhibition of periarterial nerve stimulation-induced vasodilation of the mesenteric arterial bed by CGRP (8-37) and CGRP receptor desensitization. Biochem Biophys Res Commun. 1990 Apr 30;168(2):786–791. doi: 10.1016/0006-291x(90)92390-l. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Takasaki K., Saito A., Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988 Sep 8;335(6186):164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- Kreulen D. L. Activation of mesenteric arteries and veins by preganglionic and postganglionic nerves. Am J Physiol. 1986 Dec;251(6 Pt 2):H1267–H1275. doi: 10.1152/ajpheart.1986.251.6.H1267. [DOI] [PubMed] [Google Scholar]
- Kreulen D. L., Peters S. Non-cholinergic transmission in a sympathetic ganglion of the guinea-pig elicited by colon distension. J Physiol. 1986 May;374:315–334. doi: 10.1113/jphysiol.1986.sp016081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N. Antidromic action: Part I. J Physiol. 1923 Aug 16;57(6):428–446. doi: 10.1113/jphysiol.1923.sp002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T. Nocifensor System of Nerves. Br Med J. 1937 Feb 27;1(3973):431–435. doi: 10.1136/bmj.1.3973.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi C. A., Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol. 1988;19(1):1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- Meehan A. G., Hottenstein O. D., Kreulen D. L. Capsaicin-sensitive nerves mediate inhibitory junction potentials and dilatation in guinea-pig mesenteric artery. J Physiol. 1991 Nov;443:161–174. doi: 10.1113/jphysiol.1991.sp018828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Huang Y., Brayden J. E., Hescheler J., Standen N. B. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990 Apr 19;344(6268):770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- Peters S., Kreulen D. L. A slow EPSP in mammalian inferior mesenteric ganglion persists after in vivo capsaicin. Brain Res. 1984 Jun 11;303(1):186–189. doi: 10.1016/0006-8993(84)90227-0. [DOI] [PubMed] [Google Scholar]



