Abstract
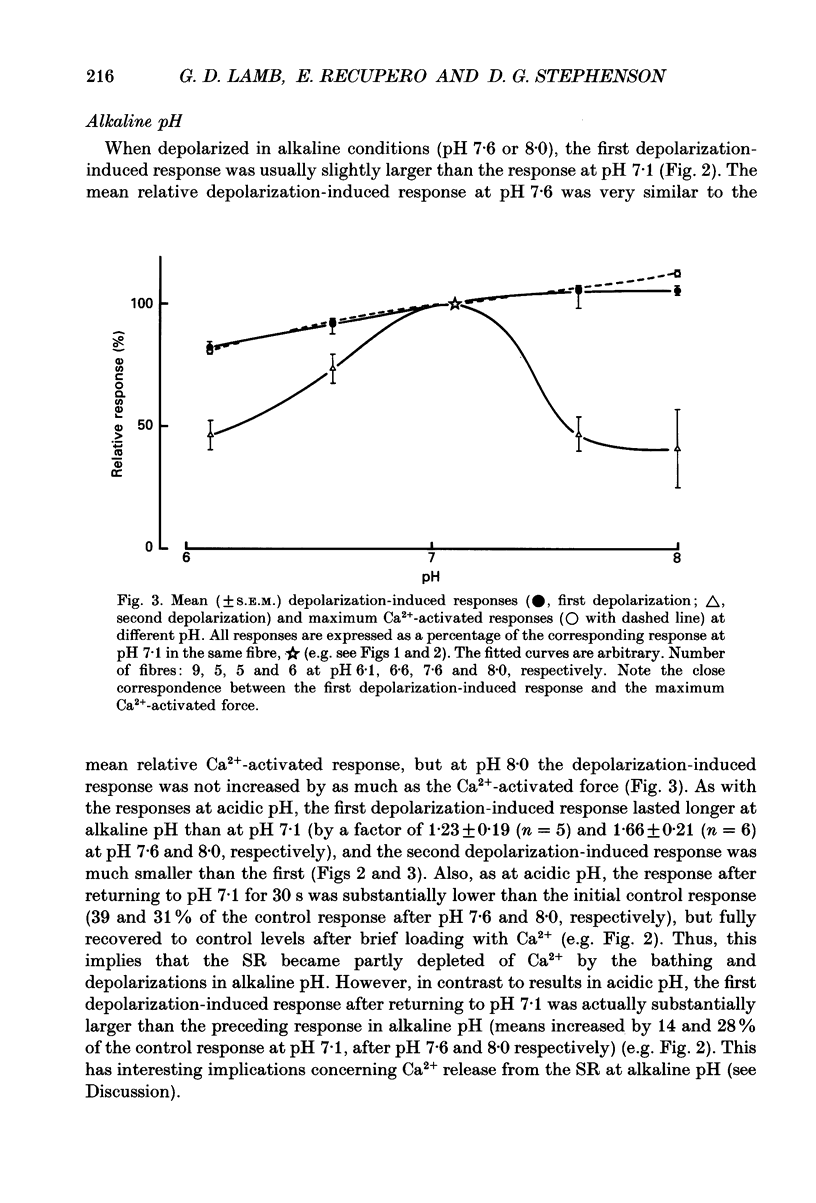
1. The effect of pH on excitation-contraction coupling in skeletal muscle of the toad was examined using a skinned fibre preparation which gives ready access to the intracellular environment while still allowing stimulation of Ca2+ release by the normal voltage-sensor mechanism. 2. In each fibre, depolarization-induced responses (produced by changing the ions in the bathing solution) were examined first at pH 7.1, and then at another pH between 6.1 and 8.0. At all pH levels examined, the first depolarization elicited a large response which was slightly greater (pH 7.6 and 8.0) or smaller (pH 6.6 and 6.1) than that at pH 7.1. The size of the first depolarization-induced response varied with pH in almost exactly the same manner as did the maximum Ca(2+)-activated response. The duration of the depolarization-induced response at all other pH levels was longer than at pH 7.1. 3. Repeated depolarizations (30 s or more apart) produced similar responses at pH 7.1, but at all other pH levels examined the second and subsequent responses became progressively smaller. The reasons for this decrease were different at low and high pH. 4. Examination of the size of the depolarization-induced response after reloading the depleted sarcoplasmic reticulum (SR) with Ca2+ in solutions at various pH levels, indicated that the SR Ca2+ pump operated more poorly at pH 6.6 and 6.1 than at pH 7.1. This can account for the wide and successively smaller depolarization-induced responses at acidic pH. 5. Examination of the size of the depolarization-induced response after 5 min bathing without stimulation in a very low [Ca2+] solution (-log10[Ca2+] (pCa) greater than 8.0, 1 mM-BAPTA (1,2-bis(O-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid), indicated that the sarcoplasmic reticulum (SR) lost far more Ca2+ at pH 7.6 and 8.0 than at pH 7.1 or 6.1. This indicates that SR is 'leakier' at alkaline pH. 6. Exposure of fibres heavily loaded with Ca2+ to solutions at pH 8.0 invariably induced a large response within 1-6 s. This response was unaffected by inactivation of the voltage sensors and was blocked completely by 2 microM-Ruthenium Red. This response and the 'leak' at alkaline pH are consistent with previous studies of the pH dependence of Ca(2+)-activated opening of single Ca2+ release channels in lipid bilayers. 7. These findings indicate that depolarization can induce massive Ca2+ release at both acidic and alkaline pH, provided that the SR is normally loaded with Ca2+, and give further insight into the intracellular events underlying fatigue in skeletal muscle.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Lee J. A., Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. J Physiol. 1989 Aug;415:433–458. doi: 10.1113/jphysiol.1989.sp017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley C. C., Moisescu D. G. Effect of changing the composition of the bathing solutions upon the isometric tension-pCa relationship in bundles of crustacean myofibrils. J Physiol. 1977 Sep;270(3):627–652. doi: 10.1113/jphysiol.1977.sp011972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. E., Al-Shawi M. K. Inhibition of sarcoplasmic reticulum Ca2+-ATPase by Mg2+ at high pH. J Biol Chem. 1988 Feb 5;263(4):1886–1892. [PubMed] [Google Scholar]
- Curtin N. A. Effects of carbon dioxide and tetanus duration on relaxation of frog skeletal muscle. J Muscle Res Cell Motil. 1986 Jun;7(3):269–275. doi: 10.1007/BF01753560. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. M., Bers D. M. The effect of temperature and ionic strength on the apparent Ca-affinity of EGTA and the analogous Ca-chelators BAPTA and dibromo-BAPTA. Biochim Biophys Acta. 1987 Aug 13;925(2):133–143. doi: 10.1016/0304-4165(87)90102-4. [DOI] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Calcium release in skinned muscle fibres of the toad by transverse tubule depolarization or by direct stimulation. J Physiol. 1990 Apr;423:495–517. doi: 10.1113/jphysiol.1990.sp018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Control of calcium release and the effect of ryanodine in skinned muscle fibres of the toad. J Physiol. 1990 Apr;423:519–542. doi: 10.1113/jphysiol.1990.sp018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Effect of Mg2+ on the control of Ca2+ release in skeletal muscle fibres of the toad. J Physiol. 1991 Mar;434:507–528. doi: 10.1113/jphysiol.1991.sp018483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. A., Westerblad H., Allen D. G. Changes in tetanic and resting [Ca2+]i during fatigue and recovery of single muscle fibres from Xenopus laevis. J Physiol. 1991 Feb;433:307–326. doi: 10.1113/jphysiol.1991.sp018427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J., Westerblad H. Force decline due to fatigue and intracellular acidification in isolated fibres from mouse skeletal muscle. J Physiol. 1991 Mar;434:307–322. doi: 10.1113/jphysiol.1991.sp018471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Fill M., Knudson C. M., Campbell K. P., Coronado R. Ryanodine receptor of skeletal muscle is a gap junction-type channel. Science. 1988 Oct 7;242(4875):99–102. doi: 10.1126/science.2459777. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- MacLennan D. H., Wong P. T. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1231–1235. doi: 10.1073/pnas.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J. M., Allard Y., Mainwood G. W. Is the change in intracellular pH during fatigue large enough to be the main cause of fatigue? Can J Physiol Pharmacol. 1986 Jun;64(6):764–767. doi: 10.1139/y86-130. [DOI] [PubMed] [Google Scholar]
- Robertson S. P., Kerrick W. G. The effects of pH on Ca2+-activated force in frog skeletal muscle fibers. Pflugers Arch. 1979 May 15;380(1):41–45. doi: 10.1007/BF00582610. [DOI] [PubMed] [Google Scholar]
- Rousseau E., Pinkos J. pH modulates conducting and gating behaviour of single calcium release channels. Pflugers Arch. 1990 Feb;415(5):645–647. doi: 10.1007/BF02583520. [DOI] [PubMed] [Google Scholar]
- Shigekawa M., Dougherty J. P., Katz A. M. Reaction mechanism of Ca2+-dependent ATP hydrolysis by skeletal muscle sarcoplasmic reticulum in the absence of added alkali metal salts. I. Characterization of steady state ATP hydrolysis and comparison with that in the presence of KCl. J Biol Chem. 1978 Mar 10;253(5):1442–1450. [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]


