Abstract
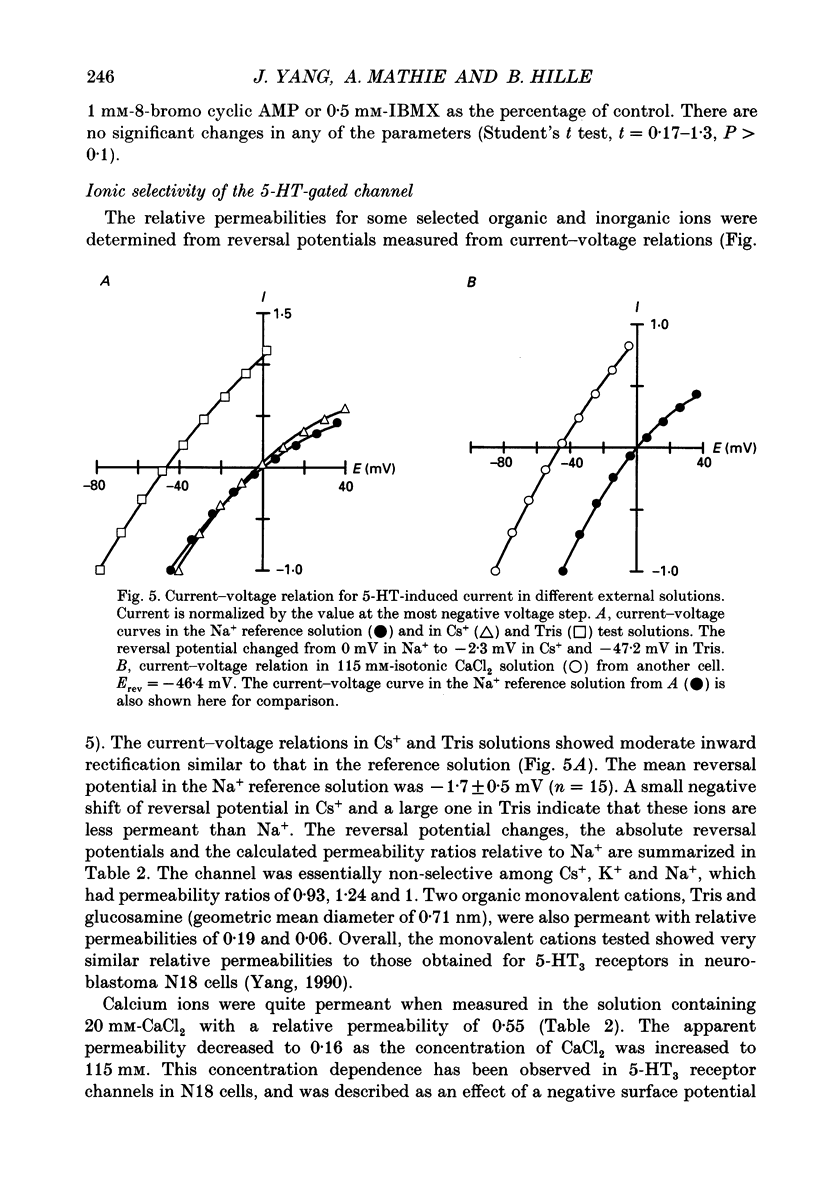
1. Whole-cell and single-channel voltage-clamp techniques were used to record the 5-HT3 receptor-mediated currents in neurons freshly dissociated from rat superior cervical ganglia. 2. Whole-cell currents elicited by brief pressure ejection of 5-HT (10 microM) reversed at -4.5 mV when extracellular and intracellular solutions mainly contained NaCl and CsCl. The peak current-voltage relation showed modest inward rectification that was fully developed within less than 2 ms of the applied voltage step. 3. With prolonged application of 5-HT (10 microM) using a fast perfusion system, the response desensitized in two phases with fast and slow time constants of 0.57 and 6.0 s at -74 mV. The time constants showed little voltage dependence; however, the relative amplitude of the two components was significantly dependent on voltage. The time course of desensitization was not affected by agents that increase the levels of intracellular cyclic AMP. 4. The relative permeability of the channel was determined from reversal potential changes. The channel passed small cations non-selectively, with permeability ratios (PX/PNa) of 0.93 and 1.24 for Cs+ and K+. The organic cations Tris and glucosamine were measurably permeant with permeability ratios of 0.19 and 0.06. Ca2+ was fairly permeant with a relative permeability of 0.55 in 20 mM solution and of 0.16 when the concentration of CaCl2 was increased to 115 mM. No permeability was detected for Cl-. 5. Fluctuation analysis of the whole-cell current revealed an apparent single-channel current of approximately 0.18 pA at -74 mV. 6. 5-HT-activated single-channel currents were recorded in excised outside-out patches. When 5-HT (10 microM) was delivered by pressure ejection, channel openings appeared rapidly with a delay of 28 ms. The unitary current was about approximately 0.80 pA at -74 mV. The channel activity induced by bath perfusion of 5-HT (0.8 microM) was significantly reduced by 100 nM of the 5-HT3 receptor-specific antagonists 3-tropanyl-3,5-dichlorobenzoate (MDL 72222) or 3-tropanyl-indole-3-carboxylate (ICS 205-930). 7. The single-channel current-voltage relation was non-linear, with moderate inward rectification similar to that of the whole-cell current. The chord conductance of the channel decreased with membrane depolarization from 14.6 pS at -104 mV to only 9.9 pS at -54 mV. Open-time distributions consisted of two components with mean time constants of 0.45 and 2.8 ms at -104 mV. Burst-length distributions were also made up of two components with time constants of 0.45 and 4.6 ms.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernheim L., Beech D. J., Hille B. A diffusible second messenger mediates one of the pathways coupling receptors to calcium channels in rat sympathetic neurons. Neuron. 1991 Jun;6(6):859–867. doi: 10.1016/0896-6273(91)90226-p. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Howe J. R., Ogden D. C. Noise and single channels activated by excitatory amino acids in rat cerebellar granule neurones. J Physiol. 1988 Jun;400:189–222. doi: 10.1113/jphysiol.1988.sp017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V., Surprenant A., North R. A. 5-HT3 receptors are membrane ion channels. Nature. 1989 Jun 29;339(6227):706–709. doi: 10.1038/339706a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- GERTNER S. B., PASSONEN M. K., GIARMAN N. J. Studies concerning the presence of 5-hydroxytryptamine (serotonin) in the perfusate from the superior cervical ganglion. J Pharmacol Exp Ther. 1959 Dec;127:268–275. [PubMed] [Google Scholar]
- Hadjiconstantinou M., Potter P. E., Neff N. H. Trans-synaptic modulation via muscarinic receptors of serotonin-containing small intensely fluorescent cells of superior cervical ganglion. J Neurosci. 1982 Dec;2(12):1836–1839. doi: 10.1523/JNEUROSCI.02-12-01836.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Higashi H., Nishi S. 5-Hydroxytryptamine receptors of visceral primary afferent neurones on rabbit nodose ganglia. J Physiol. 1982 Feb;323:543–567. doi: 10.1113/jphysiol.1982.sp014091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron. 1990 Nov;5(5):555–567. doi: 10.1016/0896-6273(90)90211-w. [DOI] [PubMed] [Google Scholar]
- Ifune C. K., Steinbach J. H. Rectification of acetylcholine-elicited currents in PC12 pheochromocytoma cells. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4794–4798. doi: 10.1073/pnas.87.12.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland S. J., Jordan C. C. Pharmacological characterization of 5-hydroxytryptamine-induced hyperpolarization of the rat superior cervical ganglion. Br J Pharmacol. 1987 Oct;92(2):417–427. doi: 10.1111/j.1476-5381.1987.tb11338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick G. J., Jones B. J., Tyers M. B. Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature. 1987 Dec 24;330(6150):746–748. doi: 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- Lambert J. J., Peters J. A., Hales T. G., Dempster J. The properties of 5-HT3 receptors in clonal cell lines studied by patch-clamp techniques. Br J Pharmacol. 1989 May;97(1):27–40. doi: 10.1111/j.1476-5381.1989.tb11920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi A., Foppen F. H., Saavedra J. M., Levi-Montalcini R., Kopin I. J. Gas chromatographic-mass spectrometric assay of serotonin in rat superior cervical ganglia. Effects of nerve growth factor and 6-hydroxydopamine. Brain Res. 1977 Sep 16;133(2):354–357. doi: 10.1016/0006-8993(77)90771-5. [DOI] [PubMed] [Google Scholar]
- Mathie A., Colquhoun D., Cull-Candy S. G. Rectification of currents activated by nicotinic acetylcholine receptors in rat sympathetic ganglion neurones. J Physiol. 1990 Aug;427:625–655. doi: 10.1113/jphysiol.1990.sp018191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Neijt H. C., Plomp J. J., Vijverberg H. P. Kinetics of the membrane current mediated by serotonin 5-HT3 receptors in cultured mouse neuroblastoma cells. J Physiol. 1989 Apr;411:257–269. doi: 10.1113/jphysiol.1989.sp017572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Cheshire S. H., Gilbert M. J. Evidence that the 5-HT3 receptors of the rat, mouse and guinea-pig superior cervical ganglion may be different. Br J Pharmacol. 1991 Mar;102(3):615–620. doi: 10.1111/j.1476-5381.1991.tb12221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Gilbert M. J. 5-Hydroxytryptamine evokes three distinct responses on the rat superior cervical ganglion in vitro. Eur J Pharmacol. 1989 Mar 21;162(2):197–205. doi: 10.1016/0014-2999(89)90282-3. [DOI] [PubMed] [Google Scholar]
- Peters J. A., Hales T. G., Lambert J. J. Divalent cations modulate 5-HT3 receptor-induced currents in N1E-115 neuroblastoma cells. Eur J Pharmacol. 1988 Jul 14;151(3):491–495. doi: 10.1016/0014-2999(88)90550-x. [DOI] [PubMed] [Google Scholar]
- Peters J. A., Malone H. M., Lambert J. J. Antagonism of 5-HT3 receptor mediated currents in murine N1E-115 neuroblastoma cells by (+)-tubocurarine. Neurosci Lett. 1990 Mar 2;110(1-2):107–112. doi: 10.1016/0304-3940(90)90796-c. [DOI] [PubMed] [Google Scholar]
- Shao X. M., Yakel J. L., Jackson M. B. Differentiation of NG108-15 cells alters channel conductance and desensitization kinetics of the 5-HT3 receptor. J Neurophysiol. 1991 Mar;65(3):630–638. doi: 10.1152/jn.1991.65.3.630. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A., Crist J. Electrophysiological characterization of functionally distinct 5-hydroxytryptamine receptors on guinea-pig submucous plexus. Neuroscience. 1988 Jan;24(1):283–295. doi: 10.1016/0306-4522(88)90331-4. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Dichter M., Morad M. Quisqualate activates a rapidly inactivating high conductance ionic channel in hippocampal neurons. Science. 1989 Mar 17;243(4897):1474–1477. doi: 10.1126/science.2467378. [DOI] [PubMed] [Google Scholar]
- Vanner S., Surprenant A. Effects of 5-HT3 receptor antagonists on 5-HT and nicotinic depolarizations in guinea-pig submucosal neurones. Br J Pharmacol. 1990 Apr;99(4):840–844. doi: 10.1111/j.1476-5381.1990.tb13017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhofstad A. A., Steinbusch H. W., Penke B., Varga J., Joosten H. W. Serotonin-immunoreactive cells in the superior cervical ganglion of the rat. Evidence for the existence of separate serotonin- and catecholamine-containing small ganglionic cells. Brain Res. 1981 May 11;212(1):39–49. doi: 10.1016/0006-8993(81)90030-5. [DOI] [PubMed] [Google Scholar]
- Wallis D. I., North R. A. The action of 5-hydroxytryptamine on single neurones of the rabbit superior cervical ganglion. Neuropharmacology. 1978 Dec;17(12):1023–1028. doi: 10.1016/0028-3908(78)90028-x. [DOI] [PubMed] [Google Scholar]
- Yakel J. L., Jackson M. B. 5-HT3 receptors mediate rapid responses in cultured hippocampus and a clonal cell line. Neuron. 1988 Sep;1(7):615–621. doi: 10.1016/0896-6273(88)90111-0. [DOI] [PubMed] [Google Scholar]
- Yakel J. L., Shao X. M., Jackson M. B. Activation and desensitization of the 5-HT3 receptor in a rat glioma x mouse neuroblastoma hybrid cell. J Physiol. 1991 May;436:293–308. doi: 10.1113/jphysiol.1991.sp018551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakel J. L., Shao X. M., Jackson M. B. The selectivity of the channel coupled to the 5-HT3 receptor. Brain Res. 1990 Nov 12;533(1):46–52. doi: 10.1016/0006-8993(90)91793-g. [DOI] [PubMed] [Google Scholar]
- Yakel J. L., Trussell L. O., Jackson M. B. Three serotonin responses in cultured mouse hippocampal and striatal neurons. J Neurosci. 1988 Apr;8(4):1273–1285. doi: 10.1523/JNEUROSCI.08-04-01273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. Ion permeation through 5-hydroxytryptamine-gated channels in neuroblastoma N18 cells. J Gen Physiol. 1990 Dec;96(6):1177–1198. doi: 10.1085/jgp.96.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]


