Abstract
1. Patch-clamp methods were used to investigate the areal density and spatial location of cyclic GMP-activated channels in the surface membrane of salamander rod outer segments. 2. The density of active channels (i.e. channels able to respond to cyclic GMP) in patches excised from outer segments was determined from the number of active channels, N, and the membrane area, A. N was estimated from the current induced by a saturating concentration of cyclic GMP, while A was estimated from the electrical capacitance of the patch. 3. In patches excised from forty-one isolated outer segments prepared in the light the active channel density varied over a remarkable range: 0.34-629 microns-2, with a mean of 166 microns-2. Density was not correlated with patch area in this or any of the conditions studied. 4. The spatial distribution of open channels on the outer segment of a transducing rod was measured by recording the local dark current at various positions with a loose-patch electrode. The apparent density of open channels varied by only about +/- 50% around the circumference of the outer segment and up and down its length. This indicates that the wide range of densities in excised patches did not result from sampling a non-uniform spatial distribution of channels. 5. Patches excised from sixteen dark-adapted whole cells with healthy appearances and saturating light responses of normal size had active channel densities of 1.1-200 microns-2, with a mean of 60 microns-2. Patches from twenty light-adapted whole cells had similar densities. Many densities from the whole cells were much lower than expected. This, and the wide variation in densities, suggests that obtaining a patch often lowered the density of active channels. The number of channels in a patch was quite stable from 1 s to 30 min after excision, ruling out progressive denaturation or adsorption of channels to the glass as a cause for this effect. 6. The mean active channel density in patches excised from whole cells was lower with calcium present in the external solution than with calcium absent (80 vs. 152 microns-2, n = 36 and 30 respectively). 7. We conclude that copies of the channel protein were present at a density of at least 650 microns-2 in the surface membrane of the outer segment and that the distribution of channels was fairly uniform on a 1 micron scale.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
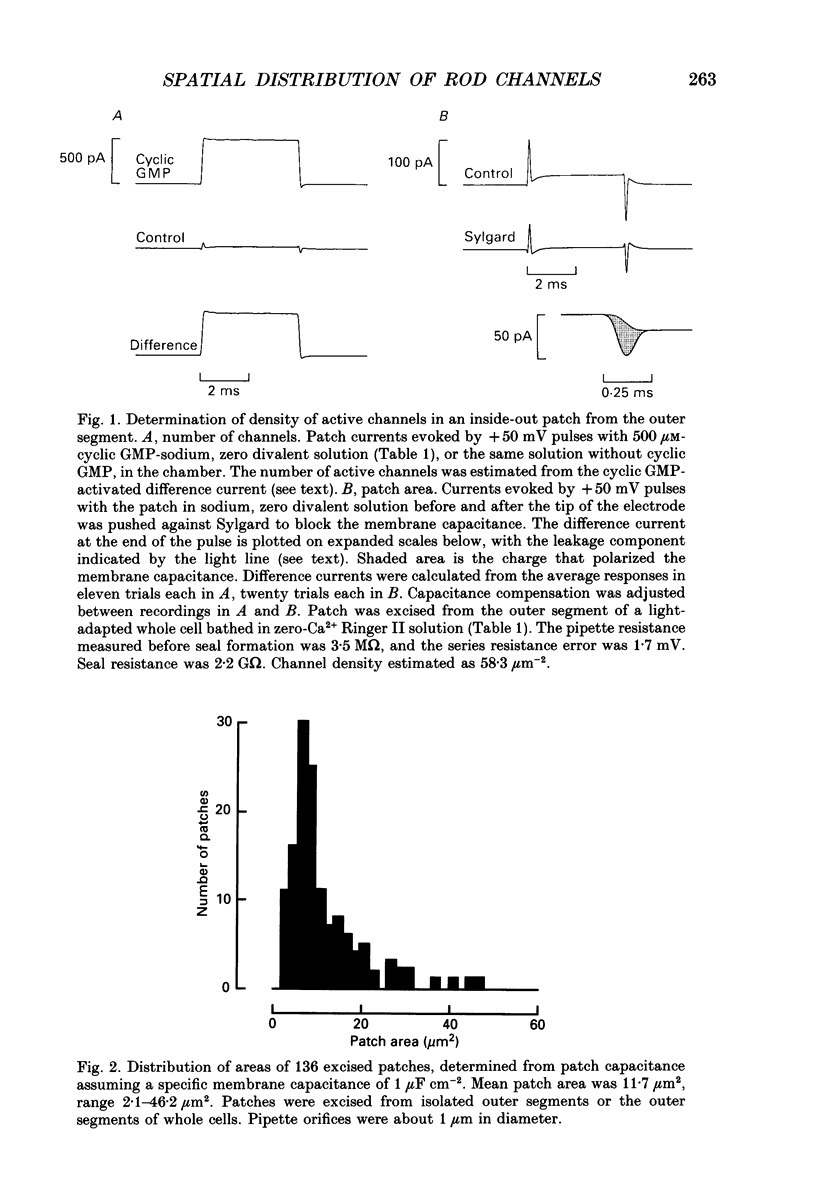
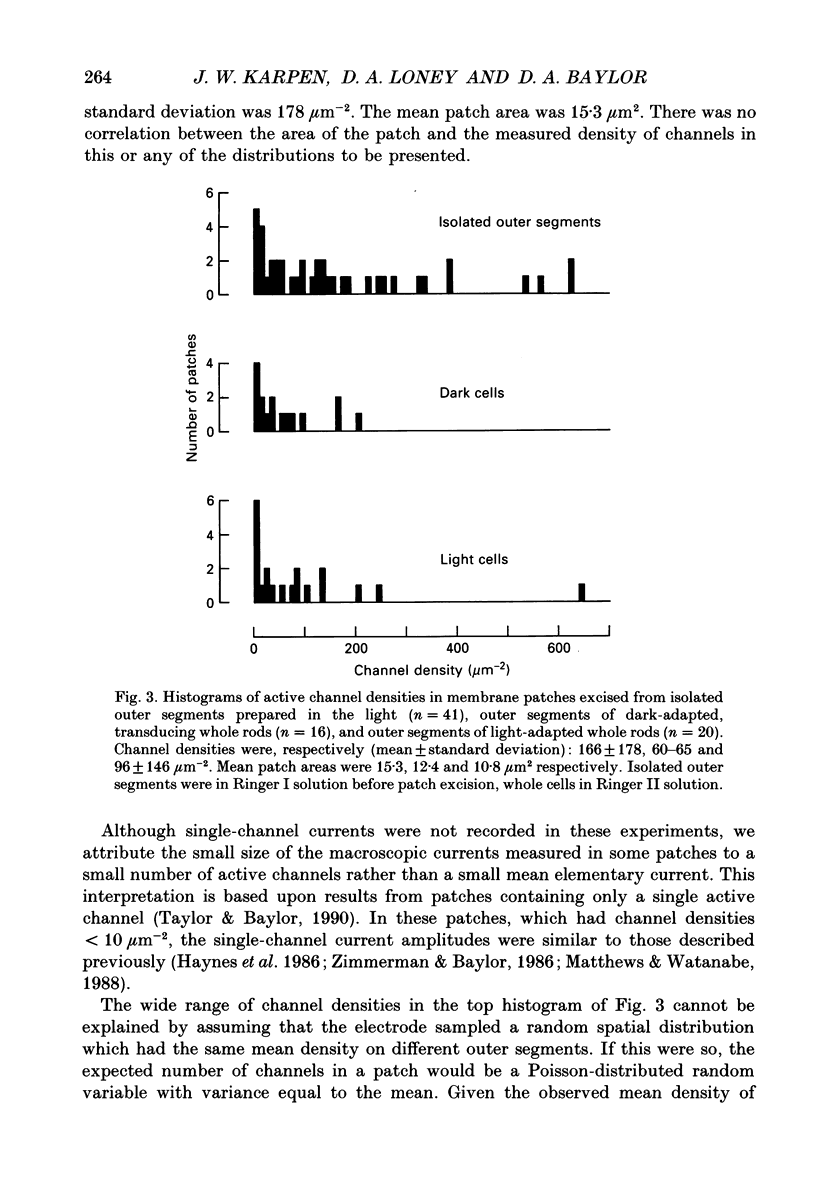
PDF




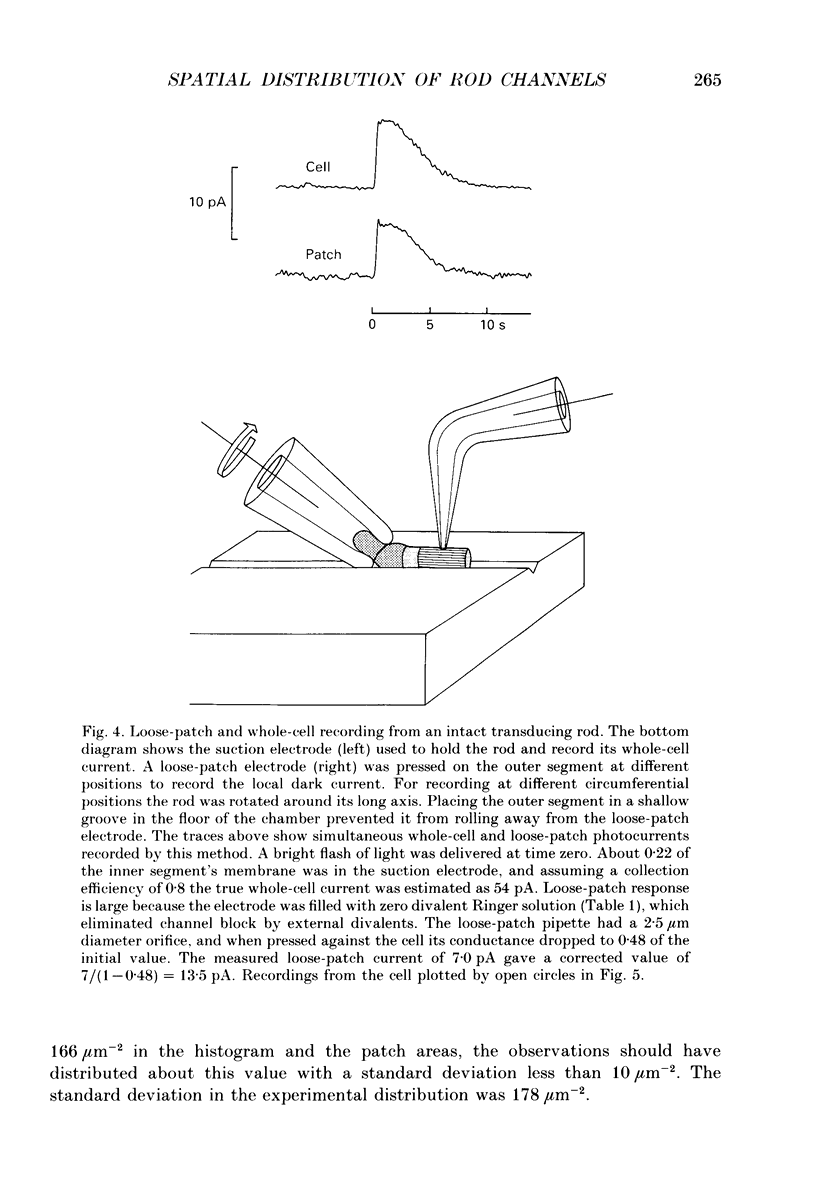
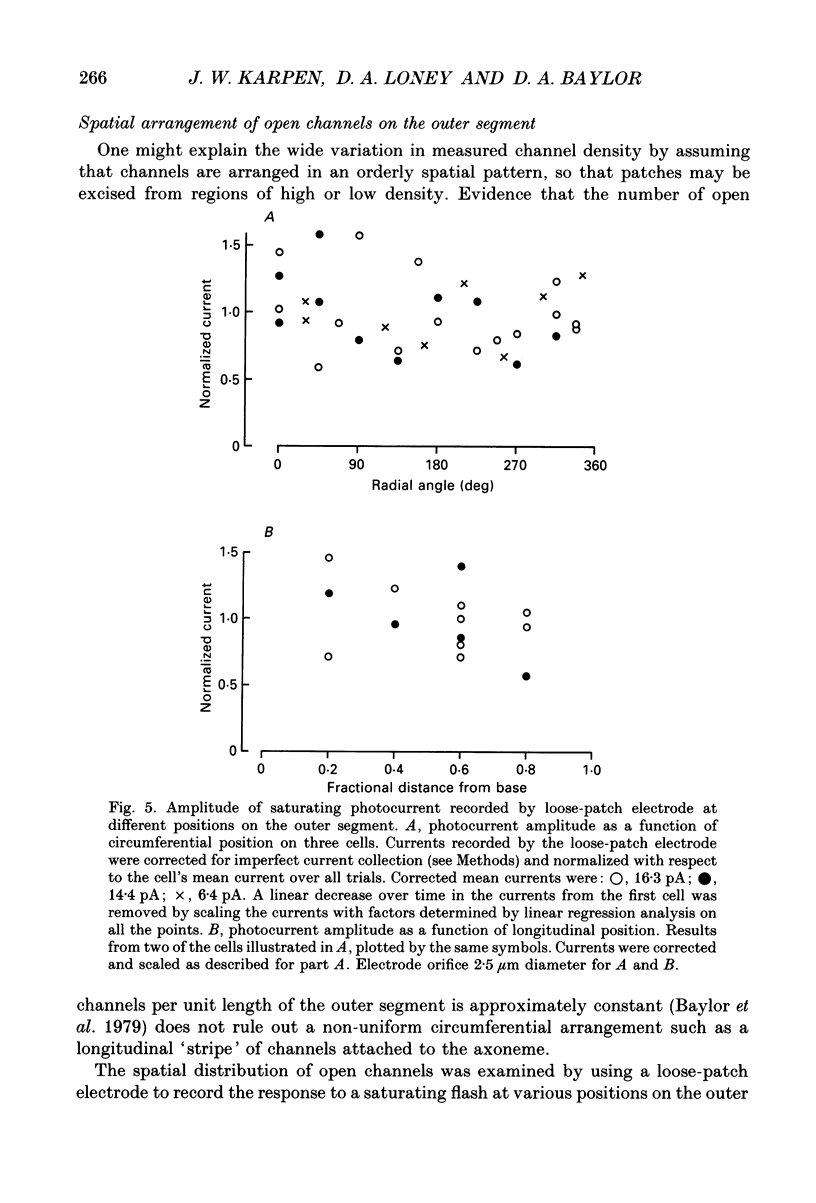






Selected References
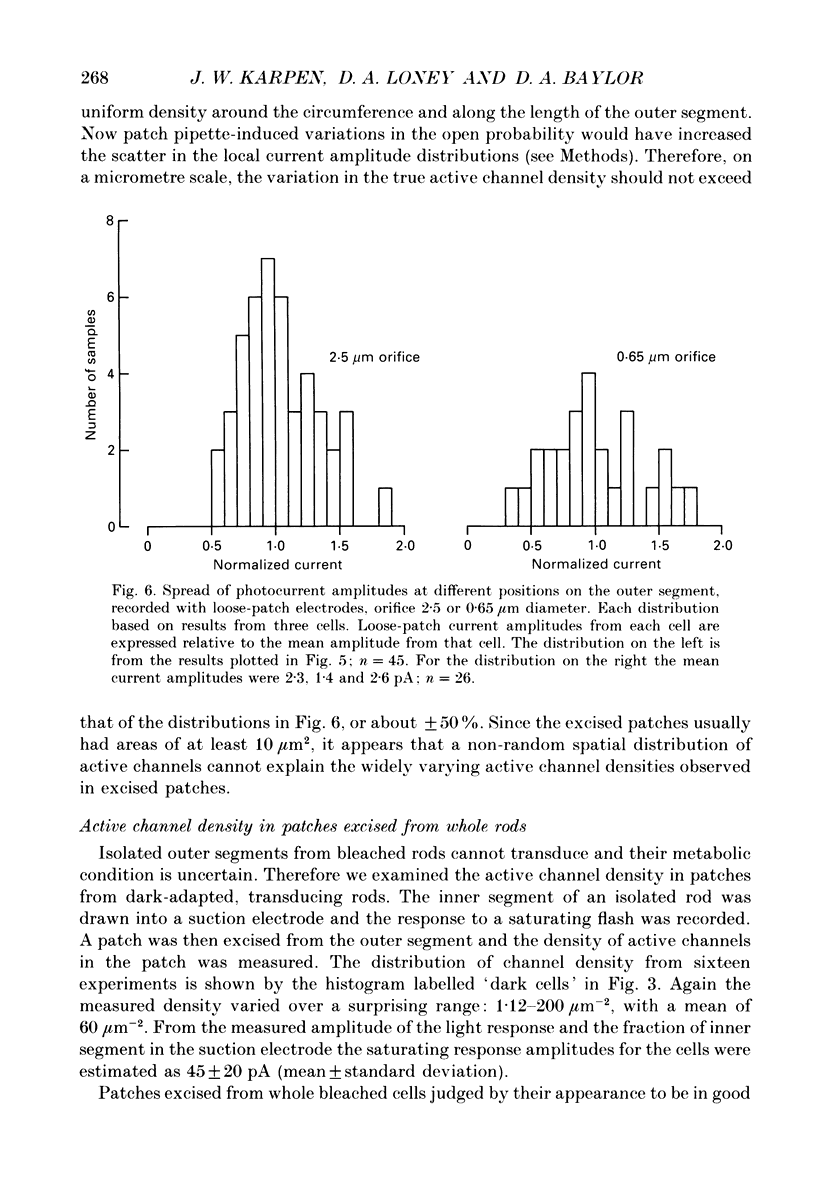
These references are in PubMed. This may not be the complete list of references from this article.
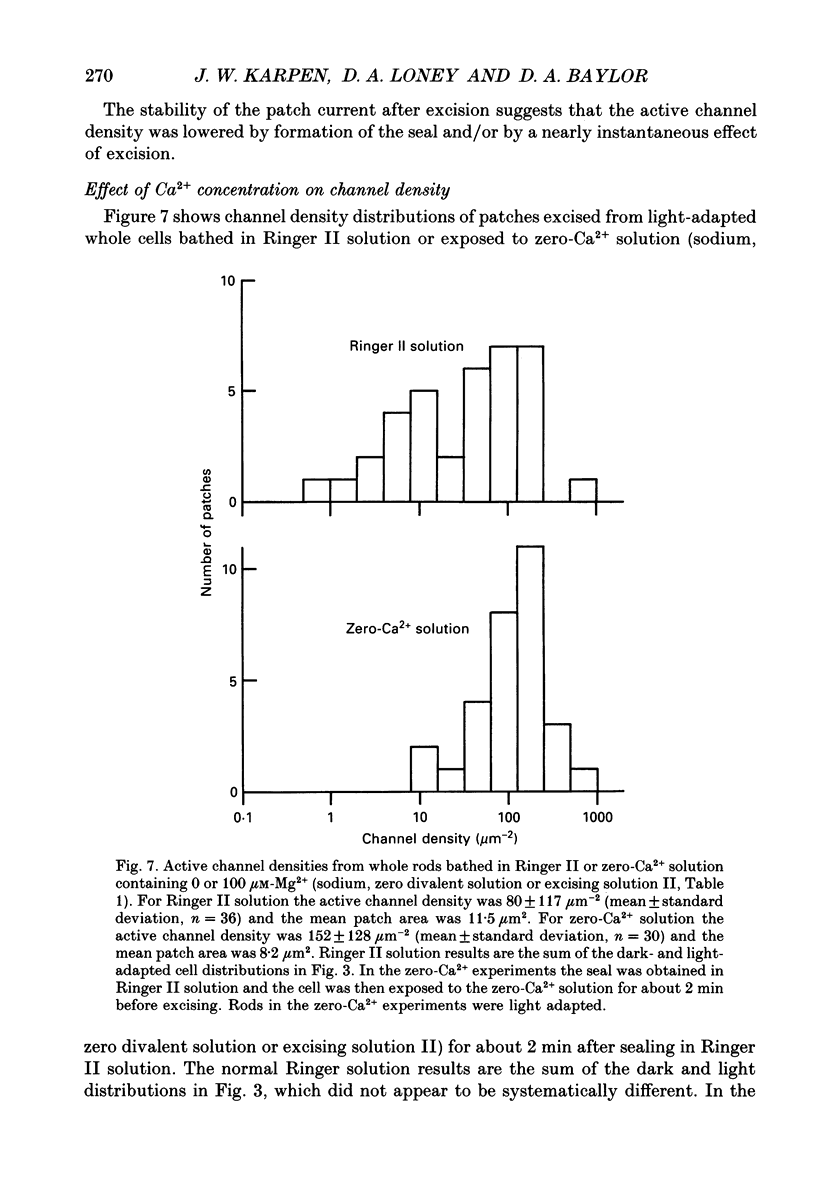
- Bauer P. J. Evidence for two functionally different membrane fractions in bovine retinal rod outer segments. J Physiol. 1988 Jul;401:309–327. doi: 10.1113/jphysiol.1988.sp017164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J. Electrical properties of the light-sensitive conductance of rods of the salamander Ambystoma tigrinum. J Physiol. 1986 Feb;371:115–145. doi: 10.1113/jphysiol.1986.sp015964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodoia R. D., Detwiler P. B. Patch-clamp recordings of the light-sensitive dark noise in retinal rods from the lizard and frog. J Physiol. 1985 Oct;367:183–216. doi: 10.1113/jphysiol.1985.sp015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretta A., Saibil H. Visualization of cyclic nucleotide binding sites in the vertebrate retina by fluorescence microscopy. J Cell Biol. 1989 Apr;108(4):1517–1522. doi: 10.1083/jcb.108.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. J., Molday L. L., Reid D., Kaupp U. B., Molday R. S. The cGMP-gated channel of bovine rod photoreceptors is localized exclusively in the plasma membrane. J Biol Chem. 1989 Apr 25;264(12):6996–6999. [PubMed] [Google Scholar]
- Falk G., Fatt P. Passive electrical properties of rod outer segments. J Physiol. 1968 Oct;198(3):627–646. doi: 10.1113/jphysiol.1968.sp008630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P., Attwell D. Kinetics of light-sensitive channels in vertebrate photoreceptors. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):379–388. doi: 10.1098/rspb.1985.0007. [DOI] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., Matthews H. R. External and internal actions in the response of salamander retinal rods to altered external calcium concentration. J Physiol. 1988 Sep;403:473–494. doi: 10.1113/jphysiol.1988.sp017259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Watanabe S. Activation of single ion channels from toad retinal rod inner segments by cyclic GMP: concentration dependence. J Physiol. 1988 Sep;403:389–405. doi: 10.1113/jphysiol.1988.sp017255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Watanabe S. Properties of ion channels closed by light and opened by guanosine 3',5'-cyclic monophosphate in toad retinal rods. J Physiol. 1987 Aug;389:691–715. doi: 10.1113/jphysiol.1987.sp016678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton P. A. Light response of vertebrate photoreceptors. Physiol Rev. 1990 Jul;70(3):847–883. doi: 10.1152/physrev.1990.70.3.847. [DOI] [PubMed] [Google Scholar]
- Molday L. L., Cook N. J., Kaupp U. B., Molday R. S. The cGMP-gated cation channel of bovine rod photoreceptor cells is associated with a 240-kDa protein exhibiting immunochemical cross-reactivity with spectrin. J Biol Chem. 1990 Oct 25;265(30):18690–18695. [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Matthews G. Regional distribution of cGMP-activated ion channels in the plasma membrane of the rod photoreceptor. J Neurosci. 1988 Jul;8(7):2334–2337. doi: 10.1523/JNEUROSCI.08-07-02334.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S., Molday R. S. A spectrin-like protein in retinal rod outer segments. Biochemistry. 1986 Oct 7;25(20):6294–6300. doi: 10.1021/bi00368a069. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Karpen J. W., Baylor D. A. Hindered diffusion in excised membrane patches from retinal rod outer segments. Biophys J. 1988 Aug;54(2):351–355. doi: 10.1016/S0006-3495(88)82966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. L., Yamanaka G., Eckstein F., Baylor D. A., Stryer L. Interaction of hydrolysis-resistant analogs of cyclic GMP with the phosphodiesterase and light-sensitive channel of retinal rod outer segments. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8813–8817. doi: 10.1073/pnas.82.24.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]


